Metabolic bone disease concerns a broad spectrum of conditions related to reduced bone density. Metabolic bone disease has been linked to chronic inflammatory diseases, such as ulcerative colitis. This study examines the prevalence of metabolic bone disease in ulcerative colitis patients and explores possible clinical predictors.
MethodThe authors performed a retrospective study involving children and adolescents with confirmed ulcerative colitis between January 2013 and December 2018. Bone density was evaluated through a dual-energy X-ray absorptiometry scan of the spine and total body. Osteoporosis was defined as a bone mineral density Z-score of <−2 and osteopenia as a Z-score of between −1.0 and −2.
ResultsA total of 37 patients were included in this analysis, with a mean age of 13.4±3.9 years and a mean duration of illness of 2.1±2.4 years. Using lumbar spine Z-scores and total body Z-scores, osteoporosis and osteopenia were identified by dual-energy X-ray absorptiometry scan measurements in 11 patients (29.7%) and 15 patients (40.5%), and in ten patients (27%) and 13 patients (35%), respectively. Lumbar spine Z-scores were significantly positively associated with male gender (B=2.02; p=0.0001), and negatively associated with the presence of extraintestinal manifestations (B=−1.51, p=0.009) and the use of biologics (B=−1.33, p=0.004). However, total body Z-scores were positively associated with body mass index Z-scores (B=0.26, p=0.004) and duration of illness in years (B=0.35, p=0.003).
ConclusionsMetabolic bone disease is very common in this cohort of Saudi Arabian children and adolescents with ulcerative colitis and its occurrence appears to increase in female patients who suffer from extraintestinal manifestations.
Ulcerative colitis (UC) is a chronic inflammatory condition known for its relapsing and remitting course that exclusively affects the large intestine.1 UC has clinical characteristics that differ from the other main subtype of inflammatory bowel disease (IBD), Crohn's disease (CD), which can involve any part of the gastrointestinal tract. Patients with UC tend to complain of bloody diarrhea and tenesmus, and are less likely to have abdominal pain and malnutrition. Moreover, UC typically demonstrates continuous inflammation of the colonic mucosa, and almost always involves the rectum on endoscopy, whereas CD is known to be a patchy disease that often does not concern the rectum.2 There are also a number of other diagnostic differences between the two diseases, with correspondingly diverse therapeutic strategies. In recent years, treatment of IBD has evolved to encompass more personalized approaches that not only focus on treating symptoms, but address objective markers of inflammation and determinants of quality of life.3 Furthermore, one of the main objectives of treatment in children and adolescents is fulfilling the ability to thrive normally.4
The management of UC currently utilizes more personalized approaches based on multidisciplinary team interactions.5 Extraintestinal manifestations (EIMs) can occur in up to 70% of patients with IBD. EIMs include musculoskeletal complaints such as arthralgia and arthritis, sacroiliitis, and ankylosing spondylitis.6 EIMs can also involve the skin, eyes, and hepatobiliary tract, and involve micro- and macro-nutrient deficiencies, such as vitamin D deficiency.6 In adults, concerns regarding fertility, employment, and social productivity are often addressed.7 However, for both children and adolescents, growth is a primary concern.4,8 The ability to grow adequately requires an intact pituitary-hypothalamic axis and a healthy musculoskeletal system that reacts properly to hormonal stimulation. In the presence of chronic inflammation, both of these elements are affected, and hence younger patients with chronic inflammatory conditions are susceptible to growth failure.5,9 Tight control of the disease through highly effective therapies in addition to frequent surveillance for malnutrition or metabolic bone disease (MBD) is necessary; otherwise, these derangements can go unnoticed and ultimately result in growth failure.8 Many inflammatory conditions, including IBD, have been associated with MBD, including osteopenia and osteoporosis.5,9,10 The mechanisms behind this association include the effect of circulating pro-inflammatory cytokines, enhanced osteoclastic activity, the use of corticosteroids, and vitamin D deficiency.10–17 This association carries many implications owing to the potential for increased morbidity and a significant impact on quality of life, especially in younger patients who often fail to grow adequately.18 Thus, the ability to predict the presence of MBD in patients with IBD is an important part of the clinical assessment of these patients in order to facilitate optimal treatment. In children, some studies have suggested that patients with IBD are more prone to developing MBD than healthy controls.19 In the study by Gupta et al., lumbar spine bone mineral density (BMD) Z-score was reduced for CD patients as compared to controls, but there was a similar BMD Z-score between UC patients and controls. There is currently limited data evaluating MBD in pediatric IBD patients.20
Since the prevalence of MBD in UC is not as well studied as MBD in CD,8 this study was conducted to report on the prevalence of osteoporosis and osteopenia in a cohort of Saudi Arabian children and adolescents diagnosed with UC, and to identify possible predictors of low BMD based on dual-energy X-ray absorptiometry (DEXA) scanning.
Materials and methodsStudy participantsThe authors conducted a retrospective analysis of all patients diagnosed with UC at King Abdulaziz University Hospital and treated in the same hospital between January 2013 and December 2018. The diagnosis of UC was based on conventional clinical, endoscopic, and histological criteria.21 All data concerning demographics, disease characteristics, history of surgical resections, laboratory results at the time of the DEXA scan, and prescribed medications were collected and entered into a standard data entry sheet. C-reactive protein (CRP) was determined by the nephelometry method, serum albumin by using bromocresol purple (BCP), serum calcium by the o-cresolphthalein complexone method, serum phosphate by the molybdate ultraviolet method, and 25-hyroxyvitamin D and parathyroid hormone (PTH) by using the immunoassay method.
BMD measurementsBMD was measured using DEXA (Hologic, QDR 4500; Hologic Inc. – Bedford, Massachusetts, United States) scans at the radiology department at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. The results were expressed as BMD Z-scores from different regions of the musculoskeletal system, including femoral neck, lumbar spine (L1-4), and total body (excluding the head). Lumbar spine and total body scans have been more commonly utilized for BMD evaluation in children,22 hence it was decided to use them in this study. Femoral head Z-score was not used in the analysis of BMD given that previous literature has suggested that this region is not reflective of actual BMD in children.23 Osteoporosis was defined as a BMD Z-score of <−2 and osteopenia as a Z-score of between −1.0 and −2, which is in accordance with the guidelines of the International Society for Clinical Densitometry (ISCD).24
Anthropometric measurementsAt the time of the DEXA scan, the patients’ weight (in kg) and height (in cm) were measured with a digital weighing scale (which was calibrated daily) and a standard wall-fixed stadiometer. Following this, the body mass index (BMI) was calculated.
Using the anthropometric statistical software program Epi Info (Centers for Disease Control and Prevention – Atlanta, GA, United States), weight and height measurements were converted into standard deviations (SDs) and Z-scores.
Statistical analysisBaseline characteristics of the cohort were summarized. Frequencies with percentages were used to present categorical data, and means with SDs or medians with interquartile ranges (IQRs), according to the distribution, were used for numerical variables. Univariate analysis was performed using the chi-squared test or Fisher's exact test to compare categorical variables, and Student's t-test or the Mann–Whitney U-test were used to compare summary statistics of numerical variables, according to the distribution. The authors used pairwise correlation to examine the linear relationship between the BMD Z-scores of the total body and the lumbar spine scans. Pearson's correlation coefficient (r) was used to express the degree of correlation. A backward and forward stepwise regression elimination model was used to identify predictors of total and lumbar spine Z-scores. Statistical significance was set at a p-value of <0.05. Statistical analysis was conducted using Stata 2011. (Stata Statistical Software: Release 12; StataCorp LP – College Station, TX, United States)..
Ethical considerationApproval for this study was granted by the Ethical Research Committee of the Biomedical Ethics Unit at King Abdulaziz University Hospital, Jeddah (Reference No. 801-12) prior to its commencement. Standard ethical considerations were followed in accordance with the Declaration of Helsinki.
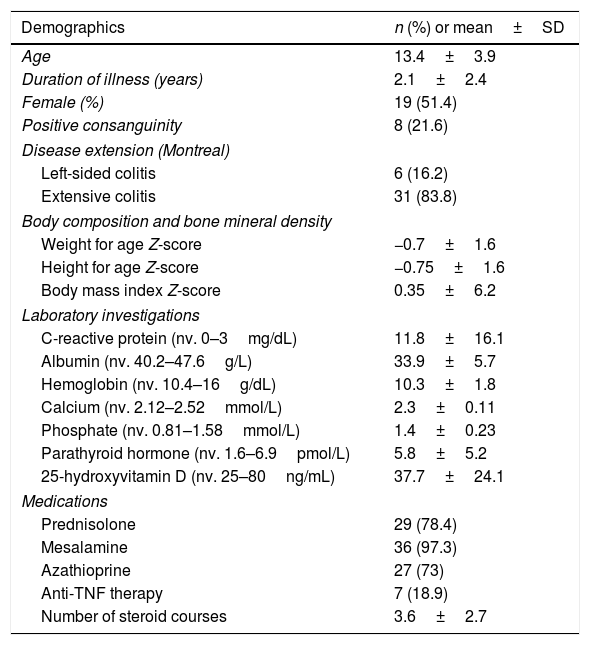
ResultsBaseline characteristicsA total of 37 patients were included in this analysis with a mean age of 13.4±3.9 years and a mean duration of illness of 2.1±2.4 years. The majority of patients were females (n=19, 51.4%) and eight patients (21.6%) reported positive parenteral consanguinity. The vast majority had extensive colitis (n=31, 83.8%) and the mean BMI Z-score was 0.35±6.2. Three patients reported EIMs (8%) and 29 patients (78.4%) reported using corticosteroids, with an average number of courses of 3.6±2.7. Mesalamine derivatives were used by most patients (n=36, 97.3%) and anti-tumor necrosis factor (TNF) therapy by only seven patients (18.9%). Anti-TNF therapies used included infliximab (n=1) and adalimumab (n=6). Twenty-seven patients (73%) reported using AZA as a therapy. Baseline characteristics are summarized in Table 1.
Baseline characteristics of the study cohort at the time of osteoporosis screening (n=37).
| Demographics | n (%) or mean±SD |
|---|---|
| Age | 13.4±3.9 |
| Duration of illness (years) | 2.1±2.4 |
| Female (%) | 19 (51.4) |
| Positive consanguinity | 8 (21.6) |
| Disease extension (Montreal) | |
| Left-sided colitis | 6 (16.2) |
| Extensive colitis | 31 (83.8) |
| Body composition and bone mineral density | |
| Weight for age Z-score | −0.7±1.6 |
| Height for age Z-score | −0.75±1.6 |
| Body mass index Z-score | 0.35±6.2 |
| Laboratory investigations | |
| C-reactive protein (nv. 0–3mg/dL) | 11.8±16.1 |
| Albumin (nv. 40.2–47.6g/L) | 33.9±5.7 |
| Hemoglobin (nv. 10.4–16g/dL) | 10.3±1.8 |
| Calcium (nv. 2.12–2.52mmol/L) | 2.3±0.11 |
| Phosphate (nv. 0.81–1.58mmol/L) | 1.4±0.23 |
| Parathyroid hormone (nv. 1.6–6.9pmol/L) | 5.8±5.2 |
| 25-hydroxyvitamin D (nv. 25–80ng/mL) | 37.7±24.1 |
| Medications | |
| Prednisolone | 29 (78.4) |
| Mesalamine | 36 (97.3) |
| Azathioprine | 27 (73) |
| Anti-TNF therapy | 7 (18.9) |
| Number of steroid courses | 3.6±2.7 |
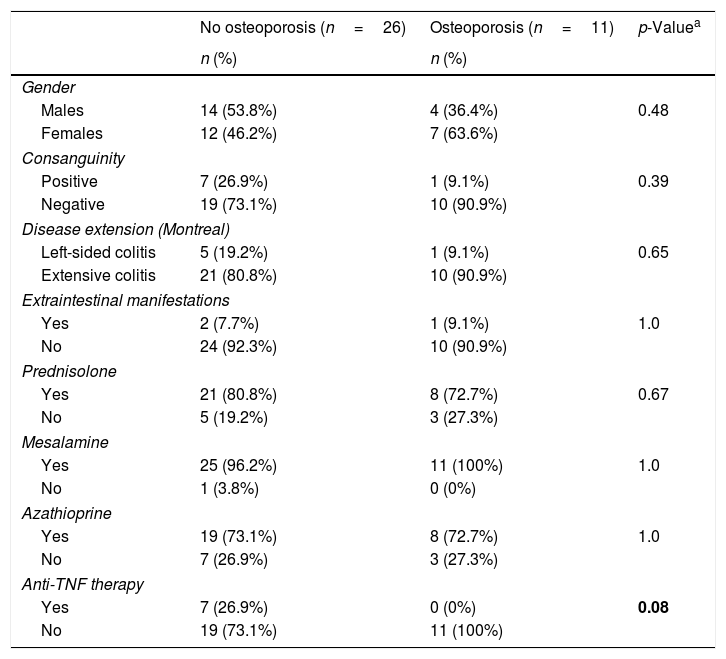
Using lumbar spine Z-scores and total body Z-scores, osteoporosis and osteopenia were identified in 11 patients (29.7%) and 15 patients (40.5%), and in ten patients (27%) and 13 patients (35%), respectively. More patients without osteoporosis (n=7/26, 26.9%) were treated with anti-TNF therapies compared to patients with osteoporosis (0/11, 0%) (p=0.056). Aside from these, no significant differences were detected between the two groups (Table 2).
Bivariate analysis of patients with and without osteoporosis according to lumbar spine Z-scores.
| No osteoporosis (n=26) | Osteoporosis (n=11) | p-Valuea | |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender | |||
| Males | 14 (53.8%) | 4 (36.4%) | 0.48 |
| Females | 12 (46.2%) | 7 (63.6%) | |
| Consanguinity | |||
| Positive | 7 (26.9%) | 1 (9.1%) | 0.39 |
| Negative | 19 (73.1%) | 10 (90.9%) | |
| Disease extension (Montreal) | |||
| Left-sided colitis | 5 (19.2%) | 1 (9.1%) | 0.65 |
| Extensive colitis | 21 (80.8%) | 10 (90.9%) | |
| Extraintestinal manifestations | |||
| Yes | 2 (7.7%) | 1 (9.1%) | 1.0 |
| No | 24 (92.3%) | 10 (90.9%) | |
| Prednisolone | |||
| Yes | 21 (80.8%) | 8 (72.7%) | 0.67 |
| No | 5 (19.2%) | 3 (27.3%) | |
| Mesalamine | |||
| Yes | 25 (96.2%) | 11 (100%) | 1.0 |
| No | 1 (3.8%) | 0 (0%) | |
| Azathioprine | |||
| Yes | 19 (73.1%) | 8 (72.7%) | 1.0 |
| No | 7 (26.9%) | 3 (27.3%) | |
| Anti-TNF therapy | |||
| Yes | 7 (26.9%) | 0 (0%) | 0.08 |
| No | 19 (73.1%) | 11 (100%) | |
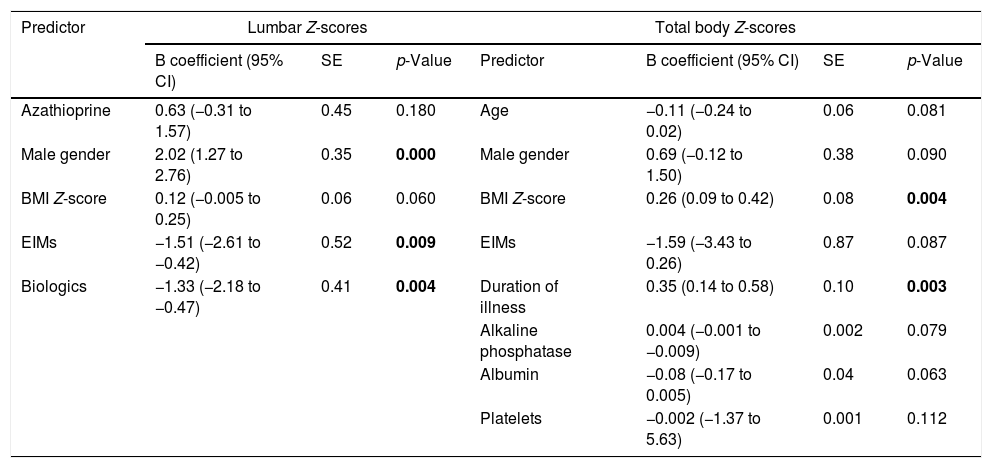
Using the stepwise regression elimination model, lumbar spine Z-scores were significantly positively associated with male gender (B coef.=2.02; 95% CI=1.27 to 2.76, p=0.0001), and negatively associated with the presence of EIMs (B coef.=−1.51, 95% CI=−2.61 to −0.42, p=0.009) and the use of biologics (B coef.=−1.33, 95% CI=−2.18 to −0.47, p=0.004). However, total body Z-scores were positively associated with BMI Z-scores (B coef.=0.26, 95% CI=−0.09 to 0.42, p=0.004), and duration of illness in years (B coef.=0.35, 95% CI=0.14 to 0.58, p=0.003; Table 3).
Final predictive models according to stepwise elimination selection model for lumbar and total body Z-scores.
| Predictor | Lumbar Z-scores | Total body Z-scores | |||||
|---|---|---|---|---|---|---|---|
| B coefficient (95% CI) | SE | p-Value | Predictor | B coefficient (95% CI) | SE | p-Value | |
| Azathioprine | 0.63 (−0.31 to 1.57) | 0.45 | 0.180 | Age | −0.11 (−0.24 to 0.02) | 0.06 | 0.081 |
| Male gender | 2.02 (1.27 to 2.76) | 0.35 | 0.000 | Male gender | 0.69 (−0.12 to 1.50) | 0.38 | 0.090 |
| BMI Z-score | 0.12 (−0.005 to 0.25) | 0.06 | 0.060 | BMI Z-score | 0.26 (0.09 to 0.42) | 0.08 | 0.004 |
| EIMs | −1.51 (−2.61 to −0.42) | 0.52 | 0.009 | EIMs | −1.59 (−3.43 to 0.26) | 0.87 | 0.087 |
| Biologics | −1.33 (−2.18 to −0.47) | 0.41 | 0.004 | Duration of illness | 0.35 (0.14 to 0.58) | 0.10 | 0.003 |
| Alkaline phosphatase | 0.004 (−0.001 to −0.009) | 0.002 | 0.079 | ||||
| Albumin | −0.08 (−0.17 to 0.005) | 0.04 | 0.063 | ||||
| Platelets | −0.002 (−1.37 to 5.63) | 0.001 | 0.112 | ||||
EIMs, extraintestinal manifestations; BMI, body mass index.
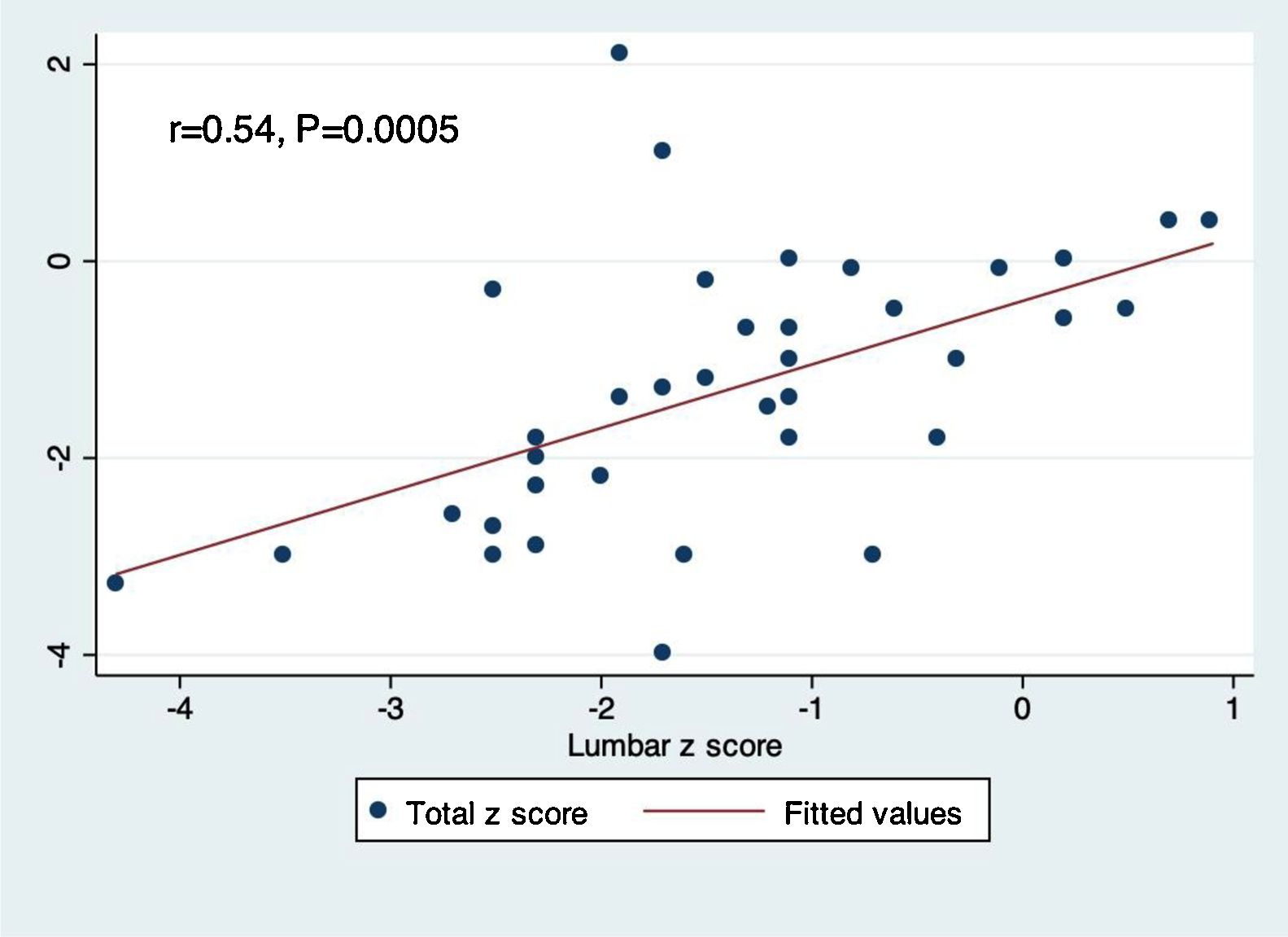
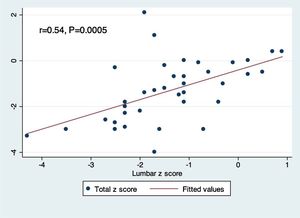
Pairwise correlation between lumbar and total body Z-scores demonstrated a positive linear relationship between the two measurements (r=0.54, p=0.0005; Fig. 1).
DiscussionGrowth failure is a common complication among children with IBD that occurs more frequently in CD compared to UC.12 A meta-analysis of seven cross-sectional and cohort studies reported a significant association between adulthood IBD and osteoporosis, with a pooled OR of 1.32 (95% CI, 1.2 to 1.4).18 Previous studies have reported high prevalence rates of MBD in children and adolescents suffering from CD17 but similar studies for UC are sparse.16 A Spanish study that included a large cohort of IBD patients (n=612 patients, 58.6% had CD) reported that male gender, menopausal women, and patients with UC to be at risk for osteoporosis, whereas older age, more than three IBD-related hospitalizations, and previous steroid treatment were associated with MBD in general.10 For this reason, the present study specifically examined the prevalence of MBD in a Saudi Arabian cohort of patients with UC. However, prevalence of MBD is site-dependent, and therefore varies according to the type of score used (t-score or Z-score).11 A previous study by Ismail et al. examined the prevalence of MBD in 95 adult patients with IBD and reported a prevalence rate of osteopenia of 44.2%, and osteoporosis of 30.5%, based on measurements made by both lumbar spine and proximal femur Z-scores. Higher Z-scores were associated with BMI, age, and calcium supplementation.14 Another study that examined a number of EIMs in 120 Indian patients with IBD reported a prevalence rate of osteoporosis or osteopenia of 50%, and higher rates of EIMs for females, Hindu religion, severe disease, and steroid usage.25 Furthermore, data from a previous retrospective analysis of patients with intestinal failure reported an MBD rate of 50% and good agreement between total body and lumbar Z-scores.15 It is noted that BMD measurements of different parts of the skeleton can give varying results, since bone loss is not a homogenous process.26 This may be related to the fact that lumbar spine mainly consists of trabecular bone, whereas the bone of the total body consists of 80% cortical bone.26 It was found that the rate of MBD in the present cohort, of osteoporosis (29.7%) and osteopenia (40.5%) according to lumbar spine Z-scores in adults with IBD, is broadly in line with previous reports.14
Osteoporosis and osteopenia are commonly associated with female gender, corticosteroid use, surgical resections, and vitamin D deficiency in adults with IBD.13,27–29 The present study sought to identify predictors of MBD in children and adolescents with UC. A stepwise regression elimination model using both lumbar spine and total body Z-scores as dependent variables identified male gender (B coef.=2.02; 95% CI=1.27 to 2.76, p=0.0001), the presence of EIMs (B coef.=−1.51, 95% CI=−2.61 to −0.42, p=0.009), and the use of biologics (B coef.=−1.33, 95% CI=−2.18 to −0.47, p=0.004) to be positively and negatively associated with lumbar spine Z-scores, respectively. Total body Z-scores were positively associated with BMI Z-scores (B coef.=0.26, 95% CI=−0.09–0.42, p=0.004), and duration of illness in years (B coef.=0.35, 95% CI=0.14 to 0.58, p=0.003). The propensity for females to develop MBD is in line with previously published literature.16,22 The negative association between higher Z-scores and other EIMs and the use of biologics could be a surrogate marker of severity, since patients with EIMs and those with UC who require biologics are more likely to have severe disease, which predisposes to MBD. A possible explanation of why patients with longer duration of disease tend to have higher Z-scores is that as patients grow older, they automatically have higher Z-scores.
Previous data from Rajaei et al. has suggested that total body Z-scores underestimate site-specific DEXA scan measurements.30 The present results support this notion, since osteopenia defined by total body Z-scores was calculated to be 27%, but was 29.7% according to the lumbar spine Z-scores. A study by Moreira et al. revealed good inter-rater reliability between DEXA scan measurements from various anatomical sites.31 However, to the authors’ knowledge, no studies have directly correlated total body and lumbar spinal Z-scores. Based on the present analysis, a moderate linear correlation exists between these two types of measurements (r=0.54, p=0.0005).
It is noteworthy that the current study is limited by its retrospective design, short duration of follow-up, and small sample size. The authors therefore recommend interpreting these results with caution.
In conclusion, MBD is very common in children with UC and its occurrence was found to be greater in female patients who suffer from EIMs. Future studies could focus on establishing more reliable predictive models for MBD in child and adolescent patients with UC.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge Dr. Trevor Rawbone, Cardiff, UK for the English proofreading of the manuscript.