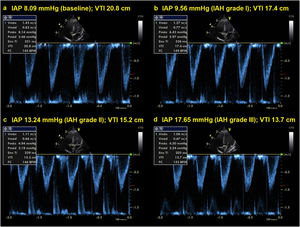
To evaluate the influence of intra-abdominal pressure on the cardiac index (CI) at different intra-abdominal hypertension grades achieved when performing an abdominal compression maneuver (ACM). Evaluating the effectiveness of the ACM in distending the left internal jugular vein (LIJV).
MethodsProspective observational study conducted in the PICU of a quaternary care teaching hospital. Participants underwent the ACM and the IAP was measured with an indwelling urinary catheter. At each IAH grade reached during the ACM, the CI was measured by transthoracic echocardiography and the LIJV cross-sectional area (CSA) was determined by ultrasonography.
ResultsTwenty-four children were included (median age and weight of 3.5 months and 6.37kg, respectively). The median CI observed at baseline and during IAH grades I, II, III, and IV were 3.65L/min/m2 (IQR 3.12−4.03), 3.38L/min/m2 (IQR 3.04−3.73), 3.16L/min/m2 (IQR 2.70−3.53), 2.89L/min/m2 (IQR 2.38−3.22), and 2.42L/min/m2 (IQR 1.91−2.79), respectively. A 25% increase in the LIJV CSA area was achieved in 14 participants (58%) during the ACM.
ConclusionThe ACM significantly increases IAP, causing severe reversible impairment in the cardiovascular system and is effective in distending the LIJV in just over half of the subjects. Even low levels of HIA can result in significant cardiac dysfunction in children. Therefore, health professionals should be aware of the negative hemodynamic repercussions caused by the increased IAP.
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are associated with high morbidity and mortality rates in critically ill children.1 The prevalence of IAH appears to be high, with rates of up to 43.9% reported in children admitted to the PICU.2 Nevertheless, IAH and ACS are conditions poorly recognized by pediatric health care providers.3
The main risk factors for increased intra-abdominal pressure (IAP) in children include: (1) diminished abdominal wall compliance (e.g., gastroschisis, omphalocele, and abdominal surgery with tight closure); (2) increased intraluminal contents (e.g. fecal impaction and Hirschsprung disease); (3) increased abdominal contents (e.g. visceromegaly, intra-abdominal tumors, and ascites); and (4) capillary leak and fluid resuscitation (e.g., systemic inflammatory response syndrome and sepsis).4 In addition, critically ill children may experience acute IAP changes during usual daily PICU practice situations, such as peritoneal dialysis, prone ventilation, or during the abdominal compression maneuver (ACM).5
Despite the lack of evidence supporting its effectiveness, the ACM is empirically used during central venous catheterization in order to maximize the internal jugular vein (IJV) cross-sectional area (CSA), and thus improve the success rate of the procedure.6,7 Recently, the ACM was proposed as a reliable method to predict fluid responsiveness in children with acute circulatory failure regardless of their ventilation status.6 Some pediatric studies evaluated the efficacy of ACM in increasing the IJV CSA, but the physiological consequences of the maneuver were not assessed.7,8 The transient increase in IAP caused by the ACM provides an interesting opportunity to study the immediate effects of IAH on the cardiovascular system. In fact, few studies have evaluated the hemodynamic repercussions of increased IAP in children.1,9 This knowledge would be valuable since IAH may result in multi-organ failure at lower thresholds than those defined for adults.1
In this study, we sought to evaluate the influence of IAP on the cardiac index (CI) at different IAH grades achieved during the ACM. We also aimed to evaluate the effectiveness of the ACM in increasing the left IJV (LIJV) CSA.
MethodsThe study was a prospective observational cohort conducted in the PICU of the Clinics Hospital of the State University of Campinas (UNICAMP), São Paulo, Brazil; a quaternary care academic teaching hospital with an annual PICU census of more than 600 children. Participants were enrolled from September 2019 to December 2019 as a convenience sample, with recruitment determined by the researcher’s availability. The study was approved by Research Ethics Committee (registration number 13424719.5.0000.5404) of UNICAMP, and registered with the Brazilian Clinical Trials Registry (RBR-3mr2vm). Written informed consent was obtained from the legal guardians of all participants.
Participant inclusion and exclusion criteriaAll mechanically ventilated patients aged between 28 days and 14 years were evaluated for eligibility. Patients were included in the study if they met the following criteria: invasive mechanical ventilation with a tidal volume of 10mL/kg of predicted body weight and positive end-expiratory pressure ≤ 6cm H20; urinary catheter use; normal blood pressure, heart rate (HR), and respiratory rate for their age.10 Exclusion criteria were: IAP>10mmHg; peritoneal dialysis; previous IJV thrombosis; central venous catheter in the LIJV; cervical or abdominal skin lesions; vasoactive drug use: dobutamine at doses > 5 mcg/kg/min; epinephrine or norepinephrine at doses > 0.1 mcg/kg/min; or milrinone at doses > 0.25 mcg/kg/min.
Data collection and study proceduresParticipants were placed in the elevated at a 30° horizontal dorsal decubitus position. The bladder method was used for IAP measurements as recommended by the World Society of the Abdominal Compartment Syndrome (WSACS).9 After clamping the urine collection bag tube, 1mL/kg of sterile saline was instilled into the bladder, with a minimal optimal volume of 3mL and a maximum of 25mL. The IAP was determined using a measuring tape (cm) zeroed at the level where the mid-axillary line crosses the iliac crest and a Y-set water column system connected to the sample port of the urine collection bag tube. Pressure readings taken in cmH2O were converted into mmHg and graded according to the WSACS IAH grading system: grade I (10−12mmHg); grade II (13−15mmHg); grade III (16−19mmHg); and grade IV (≥ 20mmHg).1
Intra-abdominal pressure and all dependent variables were assessed immediately before and at the end of the ACM. A single member of the researcher team performed all of the ACM by positioning their hand against the right upper quadrant of the abdomen to provide a sustained measurable compression force to the undersurface of the liver. If the IAP value observed at the end of the ACM was equal or higher than IAH grade II, then a progressive abdominal decompression was performed immediately and IAH grade and the dependent variables were assessed again. In the case of hemodynamic or respiratory instability, the exam was immediately terminated.
Dependent variables were assessed by performing the ACM two times.
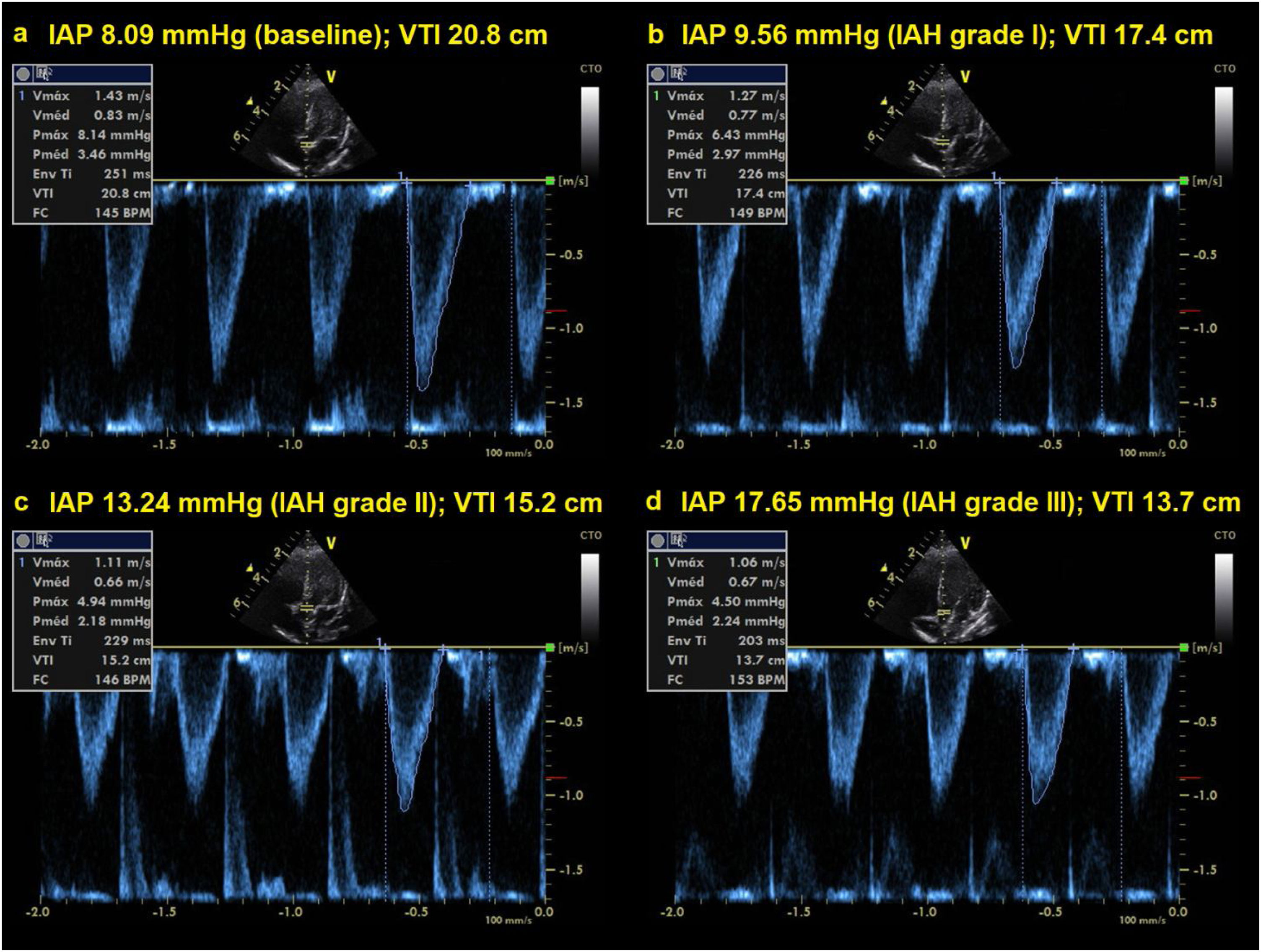
Firstly, hemodynamic variables were assessed by transthoracic echocardiography using the same ultrasound machine equipped with a phased array probe (3.5−5MHz). Using the parasternal long-axis view in 2D mode, the left ventricular outflow tract diameter was recorded and its surface was calculated as follows:
An apical five-chamber view was obtained and a 2mm pulsed wave doppler gate positioned 1cm proximal to the aortic valve. Here, velocity-time integral (VTI) was recorded for at least 10s. Heart rate (HR) and aortic VTI were recorded in each echocardiographic assessment. Mean VTI over one respiratory cycle was used to calculate stroke volume index (SVi) in order to account for variation in VTI due to cardiorespiratory interactions. SVi was calculated as the left ventricular outflow tract surface multiplied by the mean VTI divided by the body surface area (square meters). Cardiac output data was indexed by body surface area and expressed as cardiac index (CI).
Secondly, LIJV CSA was determined and stored using Healthcare Vivid Q (CA, USA) ultrasound machine equipped with a linear probe (5–13MHz). In each patient, the ultrasound probe was held for at least 10s in a stable position perpendicular to the skin immediately cephalad to the clavicle with the head rotated approximately 10° to the contralateral side. The CSA of each LIJV was measured using a planimeter. The LIJV was chosen due to its challenging site to cannulate and, therefore, the results of this study could be more useful for clinical practice. In addition, as the vast majority of the eligible subjects had their right IJV cannulated during the enrollment, the bandages would prevent the assessment of this site.
All ultrasound examinations were performed by a qualified pediatric ultrasound instructor at the Brazilian Society of Intensive Care, with 6 years of experience in pediatric point-of-care ultrasound.
OutcomesThe outcomes were assessed during and at the end of the ACM and included HR, aortic VTI, SVi, CI, and IAP. Based on a previous pediatric study,7 participants who achieved a 25% increase in the LIJV CSA during the ACM were categorized as responders, while those who did not were categorized as non-responders.
Statistical analysisNormality of the data distribution was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous variables were asymmetrically distributed and are thus described as medians and interquartile range (IQR). Categorical variables were expressed as absolute values and associated percentages. The Mann-Whitney U-test was used to perform comparisons between responders and non-responders. In the case of categorical data, Fisher’s exact and chi-square tests were used accordingly. Since the data did not follow a normal distribution, differences between baseline and multiple observations of variables were analyzed using Friedman’s test. Post-hoc analysis for Friedman’s test was performed according to Conover.11 The Wilcoxon signed-rank test was used to compare two measurements on the same subject, when necessary. Significance was defined as p<0.05.
The sample size required was calculated using mean IJV CSA and SD reported in previous pediatric studies and assuming that a clinically significant increase in LIJV CSA should be of at least 25%.7,12 The sample size calculation showed that 24 patients were required to obtain a statistical power of 90% with a two-tailed type I error of 0.05.
Statistical analysis was performed using MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium) and IBM SPSS Statistics for Windows version 22.0 (Armonk, NY: IBM Corp).
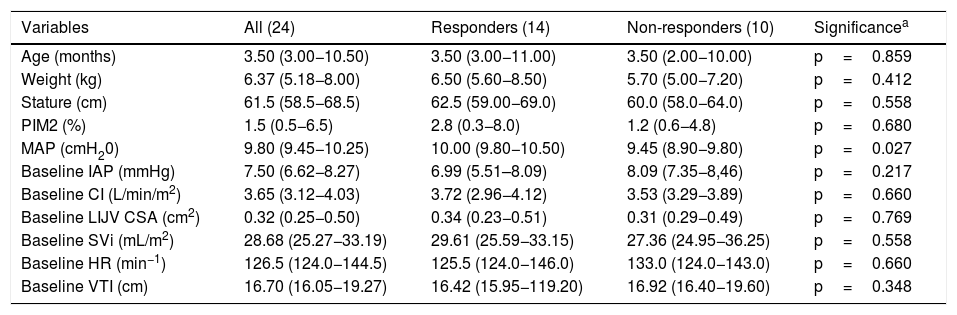
ResultsTwenty-four patients were enrolled in the study, of which fifteen were male (62.5%) and nine were female (37.5%). Acute respiratory failure was the most prevalent diagnosis upon admission (21/24), followed by sepsis (3/24). The median Pediatric index of mortality (PIM) 2 score was 1.5 (IQR 0.5–6.5). The median age, weight, and stature were 3.5 months (IQR 3.0–10.5), 6.37kg (IQR 5.18–8.00), and 61.5cm (IQR 58.5–68.5), respectively. Pressure-controlled ventilation mode was used on all participants and the median mean airway pressure (MAP) was 9.80cm H2O (IQR 9.45–10.25). Demographic characteristics of the study population are shown in Table 1.
Participants’ characteristics in responder and non-responder groups. Values are median and interquartile range.
| Variables | All (24) | Responders (14) | Non-responders (10) | Significancea |
|---|---|---|---|---|
| Age (months) | 3.50 (3.00−10.50) | 3.50 (3.00−11.00) | 3.50 (2.00−10.00) | p=0.859 |
| Weight (kg) | 6.37 (5.18−8.00) | 6.50 (5.60−8.50) | 5.70 (5.00−7.20) | p=0.412 |
| Stature (cm) | 61.5 (58.5−68.5) | 62.5 (59.00−69.0) | 60.0 (58.0−64.0) | p=0.558 |
| PIM2 (%) | 1.5 (0.5−6.5) | 2.8 (0.3−8.0) | 1.2 (0.6−4.8) | p=0.680 |
| MAP (cmH20) | 9.80 (9.45−10.25) | 10.00 (9.80−10.50) | 9.45 (8.90−9.80) | p=0.027 |
| Baseline IAP (mmHg) | 7.50 (6.62−8.27) | 6.99 (5.51–8.09) | 8.09 (7.35−8,46) | p=0.217 |
| Baseline CI (L/min/m2) | 3.65 (3.12−4.03) | 3.72 (2.96−4.12) | 3.53 (3.29−3.89) | p=0.660 |
| Baseline LIJV CSA (cm2) | 0.32 (0.25−0.50) | 0.34 (0.23−0.51) | 0.31 (0.29−0.49) | p=0.769 |
| Baseline SVi (mL/m2) | 28.68 (25.27−33.19) | 29.61 (25.59−33.15) | 27.36 (24.95−36.25) | p=0.558 |
| Baseline HR (min−1) | 126.5 (124.0−144.5) | 125.5 (124.0−146.0) | 133.0 (124.0−143.0) | p=0.660 |
| Baseline VTI (cm) | 16.70 (16.05−19.27) | 16.42 (15.95−119.20) | 16.92 (16.40−19.60) | p=0.348 |
MAP, mean airway pressure; IAP, intra-abdominal pressure; CI, cardiac index; LJIV CSA, left internal jugular vein cross-sectional area; SVi, stroke volume index; HR, heart rate; VTI, velocity time integral.
The median baseline IAP value observed was 7.50mmHg (IQR 6.62-8.27). During the ACM, all participants experienced IAH grade III, and in 14 children the IAP reached values of IAH grade IV. The median IAP observed during the ACM was 17.65mmHg (IQR 24.00–28.00).
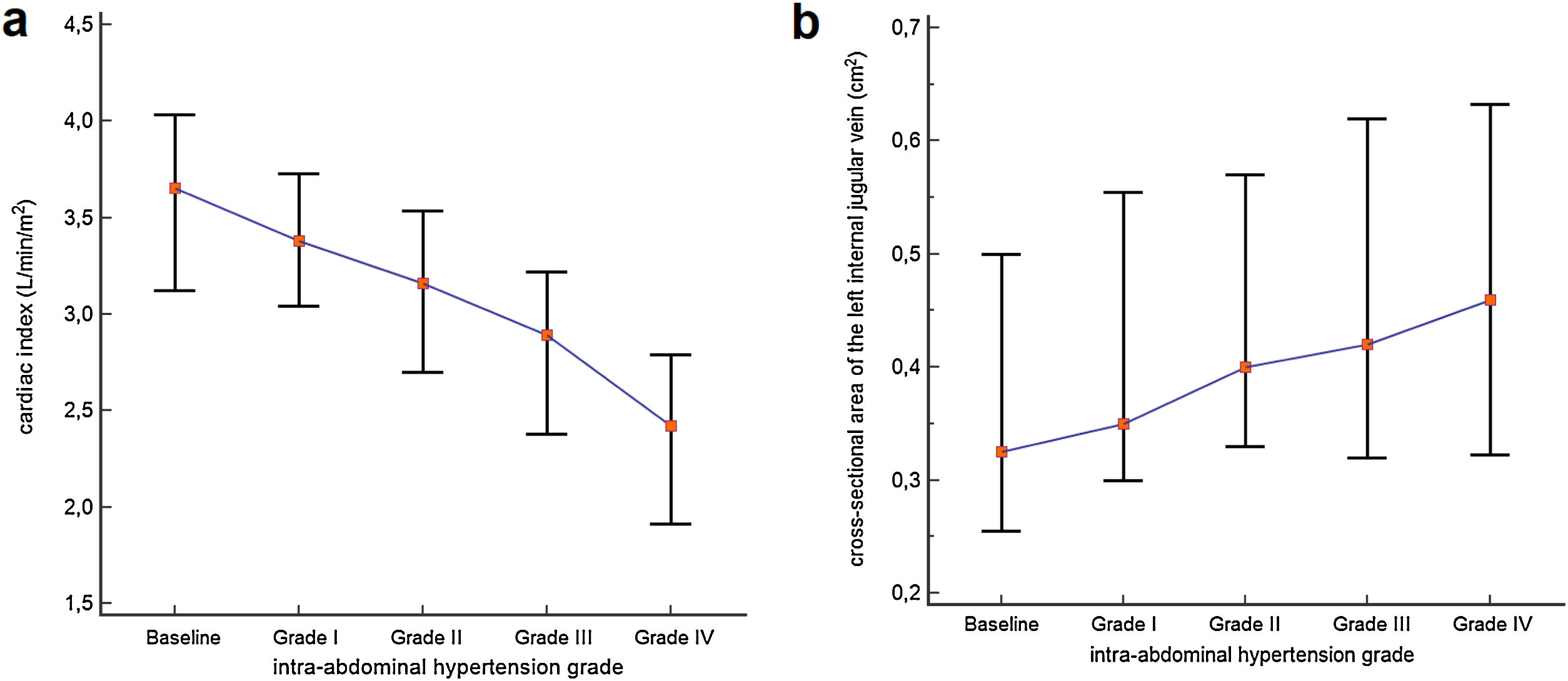
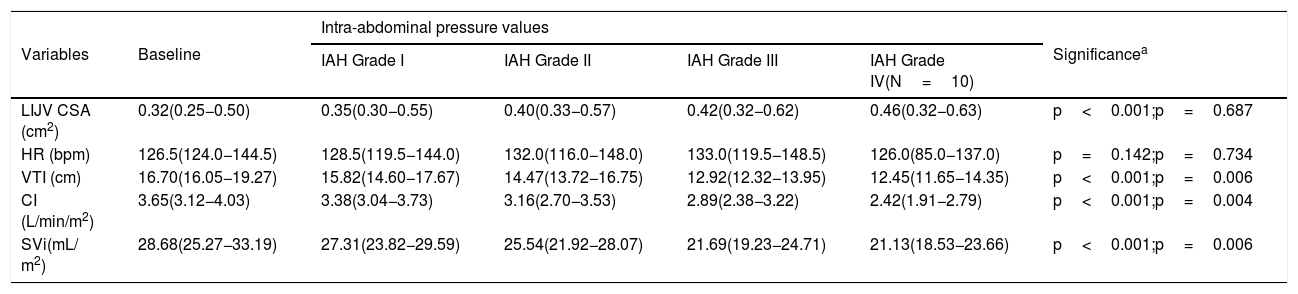
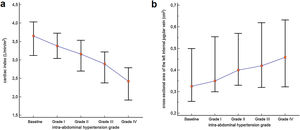
Cardiac index, SVi, VTI, and HR were significantly different between IAH grades I, II, and III (p<0.001) (Table 2). Post-hoc analysis also showed that the values observed in each IAH grade were different from each other. With the exception of HR, a progressive reduction was observed as IAH grades increased. Values observed in IAH grade IV were significantly different from those observed in IAH grade III for CI [2.84L/min/m2 (IQR 2.31–3.14) vs. 2.42L/min/m2 (IQR 1.91−2.79), p=0.004]; SVi [22.91mL (IQR 21.47–27.27) vs. 21.13mL (IQR 18.53−23.66), p=0.006]; and VTI [13.70cm (IQR 12.75–16.75) vs. 12.45cm (IQR 11.65−14.35), p=0.006]. The median heart rate observed in IAH grade IV was similar to that in IAH grade III [131.0 min−1 (IQR 89.0–144.0) vs 126.0min−1 (IQR 85.0−137.0), p=0.734]. Fig. 1 shows a significant reduction in the CI during the ACM. The CI and IJV CSA measurements during the ACM are shown as box plots in Fig. 2.
Dependent variables registered at baseline and during the abdominal compression maneuver stratified by intra-abdominal hypertension grades. Values are median and interquartile range.
| Variables | Baseline | Intra-abdominal pressure values | Significancea | |||
|---|---|---|---|---|---|---|
| IAH Grade I | IAH Grade II | IAH Grade III | IAH Grade IV(N=10) | |||
| LIJV CSA (cm2) | 0.32(0.25−0.50) | 0.35(0.30−0.55) | 0.40(0.33−0.57) | 0.42(0.32−0.62) | 0.46(0.32−0.63) | p<0.001;p=0.687 |
| HR (bpm) | 126.5(124.0−144.5) | 128.5(119.5−144.0) | 132.0(116.0−148.0) | 133.0(119.5−148.5) | 126.0(85.0−137.0) | p=0.142;p=0.734 |
| VTI (cm) | 16.70(16.05−19.27) | 15.82(14.60−17.67) | 14.47(13.72−16.75) | 12.92(12.32−13.95) | 12.45(11.65−14.35) | p<0.001;p=0.006 |
| CI (L/min/m2) | 3.65(3.12−4.03) | 3.38(3.04−3.73) | 3.16(2.70−3.53) | 2.89(2.38−3.22) | 2.42(1.91−2.79) | p<0.001;p=0.004 |
| SVi(mL/ m2) | 28.68(25.27−33.19) | 27.31(23.82−29.59) | 25.54(21.92−28.07) | 21.69(19.23−24.71) | 21.13(18.53−23.66) | p<0.001;p=0.006 |
IAH, intra-abdominal hypertension; LIJV CSA, left internal jugular vein cross-sectional area; HR, heart rate; VTI, aortic velocity time integral; CI, cardiac index; SVI, stroke volume index.
CI and LIJV CSA of the participants at baseline and at different intra-abdominal hypertension grades. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Lines outside the boxes extend from the minimum to the maximum value. (a) Cardiac indexes were significantly different within all IAH grades. (b) The IJV CSA measured were significantly different from IAH grades I, II, and III. However, the median IJV CSA of the 10 participants who experienced IAH grade IV did not differ from their median in IAH grade (p=0.687).
A 25% increase in IJV CSA during the ACM was achieved in 14 participants (responders). An example of a positive response to the ACM is illustrated in Supplementary material 1. The response was observed with IAP values of IAH grade I in 8 participants (33%), of grade II in 4 participants (17%), and of grade III in 2 participants (8%). Responders and non-responders were similar in respect to age, weight, IJV CSA, HR, SVi, VTI, and CI (Table 1). However, non-responders had lower MAP compared to responders [9.80cm H20 (IQR 9.45−10.25 vs. 10.00 cmH20 (IQR 9.80−10.50); p=0.027).
The IJV CSA measured were significantly different between IAH grades I, II, and III, with higher values as IAH grades increased (p<0.001) (Table 2). Post-hoc analysis showed that the values observed in each IAH grade were different from each other. The median IJV CSA of the 10 participants who experienced IAH grade IV did not differ from their median in IAH grade III [0.46cm2 (IQR 0.32−0.63) vs. 0.41cm2 (IQR 0.29−0.61), p=0.687).
DiscussionIn our study, we observed that the ACM was effective in significantly increasing the LIJV CSA in just over half of the subjects. However, all participants experienced a significant increase in IAP with significant negative hemodynamic repercussions. To the best of our knowledge, this is the first study to evaluate the influence of IAP on hemodynamic parameters at different IAH grades.
The negative effects of increased IAP on cardiovascular function are explained by significant changes that occur in preload, afterload, and contractility.13 Reduced cardiac output results in lower blood pressure, contributing to poor organ perfusion, which will lead to exacerbated IAH.1 This is a very dangerous vicious cycle. Sustained elevation of IAP can progress to ACS, which has been related to a mortality rate of 40-60%.14–16 Despite its importance, the level of understanding and awareness about IAH and ACS among critical care providers remains low.3 In fact, a survey involving German pediatric intensivists showed that only 20% of the respondents regularly measure IAP.3 Our results suggest that cardiac dysfunction may occur in low grades of IAH. The prompt recognition of this dysfunction could lead to an early diagnosis and treatment of ACS.
ACS in children is defined as a sustained elevation in IAP>10mmHg associated with new or worsening organ dysfunction that can be attributed to elevated IAP.1 However, ACS is often only diagnosed in children with higher levels of IAP. In the study of Beck et al., children presented a mean IAP at ACS diagnosis of 23.9±3.8 (range 17–31) mmHg, therefore, a mean value within IAH grade IV.16 Difficulties in cardiovascular assessments may have delayed the ACS diagnosis in these children. In fact, hemodynamic assessment at bedside can be challenging. Some studies have shown that clinicians failed to correctly categorize parameters of cardiac performance solely on the basis of their clinical assessment.17 In our study, a severe CI impairment was already observed when the IAP values reached IAH grade II. If the patients were in septic shock, the mortality rate would be increased due to their CI being below the goal recommended by the American College of Critical Care Medicine (3.3–6L/min/m2).18
Blood pressure monitoring is often used as a surrogate measure of overall cardiovascular function. However, this can lead to inaccurate clinical evaluations. The study of Malbrain et al. evaluated organ dysfunction in adults with IAH using the Sequential Organ Failure Assessment (SOFA) score.19 They observed that the respiratory, renal, and coagulation systems were significantly more impaired in patients with IAH when compared to those with normal IAP. However, no differences in cardiovascular SOFA scores were observed between groups. It is important to note that the cardiovascular component of the SOFA score takes into account only the mean arterial pressure and the use of vasoactive drugs. As a matter of fact, hypotension should not be expected to diagnose cardiovascular dysfunction. In the classic study of Lynch et al., the CI of piglets decreased sharply with increasing IAP and there were no changes in aortic and pulmonary artery pressures.20
Several physiological changes are involved in increased IAP, which may result in multi-organ failure, but few studies have evaluated its hemodynamic repercussions in children.1,9 Studies evaluating changes in IAP and cardiac output were conducted in patients undergoing laparoscopic elective surgery.21–26 In comparison to our study, the authors evaluated the hemodynamic repercussion of lower abdominal pressure levels (Supplementary material t2). Even so, most studies have found a reduction in cardiac output with increased intra-abdominal pressure.22–26 Only one study reported an increase in cardiac output after elevated intra-abdominal pressure.21 However, the IAP applied in this study was of only 5mmHg (a normal IAP value).
The effect of the ACM on distending the neck veins was first observed by Pasteur in 1885.27 Initially described in the context of the abdominojugular test, this technique can rapidly increase right atrial pressure in a reversible manner.28 The increase in central venous pressure (CVP) is believed to be caused by the “auto-transfusion” of blood from the compressed liver and abdominal veins, increasing venous return via the inferior vena cava.28 However, other physiological mechanisms may be involved in increasing CVP. Changes in intra-abdominal pressure (IAP) caused by the ACM are transmitted to the thoracic cavity through the diaphragm leading to elevated intra-thoracic and airway pressure.1 In addition, cephalic deviation of the diaphragm during IAH leads to direct compression of the heart, reducing cardiac compliance.29 Therefore, the pressure gradient responsible for venous return is decreased, leading to IJV congestion.
To date, there are few pediatric studies that evaluate the effectiveness of ACM in increasing the IJV CSA.7,8 In 2002, Verghese et al. conducted a study involving 84 children in which the effects of 3 maneuvers were evaluated: The Valsalva maneuver, liver compression, and the Trendelenburg position.7 In contradiction to our findings, the liver compression maneuver caused an average increase of only 6.8% in the RIJV CSA in patients under 1year of age and 14.3% in those aged 1–6 years. This may be explained by some differences between the methods of the two studies. In the study by Verghese et al., liver compression was applied through a cuff inflated to a pressure of 40mmHg enclosed within a thin abdominal binder, regardless of the age or weight of the participants, and the IAP was not measured. Thus, it is very likely that these patients underwent different levels of IAP, making it impossible to compare the results of the two studies.
It should also be pointed out that our study evaluated the LIJV, while other authors evaluated the RIJV. The internal jugular vein (IJV) is often the site of choice for central venous catheterization in children, and the left IJV (LIJV) is deemed more difficult to cannulate and carries the risk of thoracic duct injury.30 It has been shown in adults and children that the LIJV has a significantly smaller CSA than the RIJV.12 In addition, the LIJV may be less responsive to CVP-increasing maneuvers than the RIJV. In 2001, Botero et al. subjected 45 mechanically ventilated children to a 10-second breath-hold with a positive inspiratory pressure of 20 cmH20.12 The maneuver effectively dilated the RIJV, but the same did not occur with LIJV.
Our study has some limitations. Firstly, the ultrasound operator was not blind to IAP values while performing dependent variable measurements. Secondly, the ultrasound analysis is an operator-dependent method, and the intra-operator variability of measurements was not evaluated in this study. Thirdly, we were unable to analyze the abdominal perfusion pressure of participants and assess whether there is a direct correlation with other variables, such as intra-abdominal pressure. Finally, we cannot say with certainty that our results are true for other causes of increased IAP, especially for conditions with sustained IAH. Our study was not able to appreciate hemodynamic compensatory response mechanisms that may reverse the effects caused by sustained increased IAP. However, the results herein reported may inspire further research on the topic.
The ACM significantly increases IAP, causing severe reversible impairment in the cardiovascular system, and is effective in distending the LIJV in just over half of the subjects. Our results suggest that low degrees of IAH in children may result in cardiac dysfunction. Therefore, health care providers should be aware of the negative hemodynamic repercussions caused by increased IAP in critically ill children.
Trial registrationRBR-3mr2vm (registered on May 15, 2019).
Conflicts of interestThe authors declare no conflicts of interest.
Thank you to Carolina Grotta Ramos Telio for her review of the manuscript. We also thank the attending physicians, resident physicians, nursing staff, and legal guardians of the participants in this study.