To discuss the approach to patients diagnosed with growth hormone deficiency (GHD) in childhood during the transition period from puberty to adulthood, focusing on the following: (1) physiology; (2) effects of recombinant human GH (rhGH) interruption/reinstitution after adult height achievement; (3) re-evaluation of somatrotropic axis; (4) management of rhGH reinstitution, when necessary.
Source of dataNarrative review of the literature published at PubMed/MEDLINE until September 2020 including original and review articles, systematic reviews and meta-analyses.
Synthesis of dataGrowth hormone is crucial for the attainment of normal growth and for adequate somatic development, which does not end concomitantly with linear growth. Retesting adolescents who already meet the criteria that predict adult GHD with high specificity is not necessary. Patients with isolated GHD have a high likelihood of normal response to GH testing after puberty. Adolescents with confirmed GHD upon retesting should restart rhGH replacement and be monitored according to IGF-I levels, clinical parameters, and complementary exams.
ConclusionPatients with isolated idiopathic GHD in childhood are a special group who must be reevaluated for GHD as many of them have normal GH provocative tests upon retesting after puberty. Patients who confirm the persistence of GHD in the transition period should maintain rhGH replacement in order to reach an ideal peak bone mass, satisfactory body composition, lipid and glucose profiles, and quality of life.
The transition period in patients with growth hormone deficiency (GHD) is defined as a developmental stage starting in late puberty and ending with full adult maturation (the period from mid to late teens until 6–7 years after the achievement of adult height).1 Even though a growth velocity below 1−2 cm per year in an adolescent indicates that their stature growth is ending, a growth hormone (GH)-dependent somatic growth will continue for the next years and GH has important metabolic actions throughout adulthood. GH has effects on bone and lipid metabolism, body composition and quality of life (QoL), even after the achievement of adult height.2–4
Re-evaluation of the somatotropic axis in this period is required since a number of individuals with a childhood diagnosis of GHD have a normal GH secretion upon retesting in late adolescence.5,6 The diagnosis of persistent GHD is important as patients require the continuation of recombinant human growth hormone (rhGH) treatment to attain full skeletal mineralization and prevent the potential changes in body composition and lipid metabolism found in GHD adults.7
Transition is a vulnerable period when adolescents may drop out of medical care. Pediatricians should start counseling patients and caregivers early about the future transfer of care and work closely with adult endocrinologists in order to facilitate the passage to adult endocrine care services as a continuum of regular treatment. The organization of a multidisciplinary team to assist these adolescents increases the possibility of a successful transfer of care. Despite the importance of ongoing GH replacement therapy during the transition period, its implementation in clinical practice remains challenging and inconsistent.8 Pediatric endocrinologists suffer from a lack of education/communication with patients about the importance of treating GHD-subjects after complete linear growth and a lack of an effective model for transferring patients to an adult endocrinologist. On the other side, adult endocrinologists have a lack of education/awareness about the importance and long-term benefits of treating GHD-adult patients, and a lack of knowledge of how to manage patients with certain conditions, such as the history of childhood cancer.8
Thus, the aim of this review is to discuss the approach to patients with GHD diagnosed in childhood during the transition period, with the following objectives: (1) physiology of this phase; (2) effects of rhGH interruption/reinstitution after adult height achievement; (3) re-evaluation of GH-IGF-I axis; (4) management of rhGH reinstitution, when necessary.
The physiology of the transition phaseIn children with GHD, rhGH treatment is discontinued when growth velocity is below 1−2 cm/year and bone maturation is almost concluded, which corresponds to a bone age of 14–15 years in girls and 16–17 years in boys.1–3,9 However, at this stage, there is still residual growth and the body (bone and lean mass) is still developing, called somatic growth.
At puberty, boys and girls have an increase in somatotropic hormone secretion [GH and insulin-like growth factor-I (IGF-I)], with significant changes in body composition according to gender. The major action of GH on bone is mediated by IGF-I, which acts as a trophic hormone with a positive effect on growth and bone turnover through the stimulus of osteoblasts, synthesis of collagen and longitudinal bone growth.10–12 Adolescence and early adulthood is not only a period of accelerated stature growth (pubertal spurt), but it is also a critical time for acquiring bone mass, with significant increases in bone mineral content detected at the lumbar spine and femur.13 Approximately 37% of the skeletal mass is gained during pubertal maturation and peak bone mass or maximal skeletal mass likely does not occur until the mid-20s to as late as 35 years of age.14 The transition period is a “window of opportunity” for the patient with GHD to gain the benefits of rhGH replacement during the period of peak bone mass.13
Boys continue gaining lean body mass (LBM) until approximately 20 years of age, and girls, until 14 years.2 GH and sex steroids have an important contribution for the maturation of LBM and muscle strength in adolescents and young adults, even after stature growth is completed.15 The larger and more effective increase of muscles in boys occurs, in part, because of the androgenic effect and the higher sensitivity to GH and IGF-I.2
Growth hormone decreases lipogenesis and stimulates lipolysis.16,17 The gain in fat is little in boys after the end of puberty, while girls continue it until 20 years old.2 These gender dysmorphic changes occur after peak height velocity, in the presence of both declining GH secretion and IGF-I levels.2
Effects of rhGH interruption after adult height achievement (and its reinstitution, when indicated)The transition period has many peculiarities making this period different from the growing child and “mature” individuals. Studies with GHD-deficient adults demonstrated significant clinical differences between patients with GHD diagnosed in childhood from those in whom GHD started after adulthood. There is sufficient evidence in the literature to demonstrate that the use of rhGH in the transition period decreases truncal fat, increases LBM and bone mass density.18–22
The interruption of rhGH replacement in adolescents with severe GHD leads to increased cardiovascular risk factors, such as an increase in visceral obesity, decrease in LBM, increase in total cholesterol, LDL-cholesterol, and apolipoprotein B, and decrease in HDL-cholesterol.17,23–25 These findings are not present after the interruption of rhGH replacement in patients with a less severe form of GHD.22 The different responses to discontinuation of rhGH when comparing severe and non-severe GHD might explain the controversial findings in the literature reporting that discontinuation of rhGH treatment in adolescents with GHD resulted in either no effect on lipid profile or an increase in serum lipid concentrations.17 The discontinuation of rhGH causes increased levels of homocysteine, a prothrombotic factor that represents an independent risk factor for cardiovascular events; and increased levels of fasting fibrinogen, an independent risk factor for stroke and myocardial infarction.26 This interruption of rhGH replacement also leads to decreased folate and vitamin B12 levels.26
Upon discontinuation of rhGH treatment, body fat increases progressively in patients with severe childhood-onset GHD, with a halt or decline in the accrual of LBM.23 Furthermore, there is a reduction of exercise capacity and muscle strength as a consequence of the great attenuation in the LBM gain.27 Different studies have demonstrated a benefic effect of rhGH replacement on body composition in transition patients, with increments in LBM and a decrease in fat mass.21,27
Studies regarding carotid artery intima-media thickness (IMT) did not demonstrate differences between treated and untreated adolescents with GHD during the transition period22,28,29; however, endothelium-dependent vasodilator response has been demonstrated to be greater in treated GHD adolescents than both untreated subjects and controls, according to Lanes et al.28 Although an increase in IMT was not observed in GHD-adolescents, an increased IMT has been reported in untreated GHD-adults; rhGH replacement resulted in a significant decrement of IMT after 1 year and it was stabilized after 2 years of rhGH therapy.30 These findings suggest that this deleterious increased IMT increased, which represents the earliest morphologic change in the arterial wall in the process of atherogenesis, may develop in later stages of life after the transition period.
Untreated GHD-adolescents have increased epicardial adipose tissue when compared with treated subjects with GHD and to healthy controls. Moreover, epicardial adipose tissue correlates positively with BMI.28
Considering the anabolic effect of GH in bone and that peak bone mass occurs years after the end of linear growth, the rhGH interruption at the final height achievement can limit an optimal peak bone mass in adolescents with GHD.31,32 Children and adults with GHD since childhood have a lower bone mineral density (BMD) when compared to healthy subjects, and rhGH treatment increases it in both groups. Even though adolescents with GHD continue to increase lumbar BMD after discontinuation of rhGH treatment at final height, the accretion of BMD is slower, the peak bone mass is reduced and the mean values of lumbar BMD declined 2 years after its peak.33 Continued rhGH treatment for 24 months in young adults treated for childhood-onset GHD after the achievement of final height was associated with an estimated 3.5% greater increase in BMD of the lumbar spine compared with controls.34 Bone turnover markers and the rhGH treatment evaluation in children with GHD concluded that these children had a reduced bone metabolism (reduction in markers of bone formation and resorption) before the beginning of treatment, and there was a significant increase in bone markers during the first year of treatment.35 The positive effects of GH on cortical and trabecular microarchitecture after the achievement of final height has been demonstrated in some studies,36,37 which may be more relevant in predicting future risk of fractures and prevention than actual BMD in young adults with childhood-onset GHD.
Studies that evaluated QoL in adults after pediatric treatment with rhGH pointed out normal psychosocial adjustment and educational attainment,35–37 but higher levels of unemployment and underemployment, reduced marital rates, besides impaired general health perception.38–41 These alterations on the social profile of adults with GHD since childhood may be a subtle indicator of psychosocial disturbances already present in childhood, that worse with age progression and adult components of the disease.38 Adults who acquired GHD in adulthood also have a worse QoL,38 which confirms the relation between GH and psychosocial issues. There is also evidence that discontinuing rhGH treatment resulted in decreased QoL, which was improved after restarting rhGH therapy.25,42,43
Resuming, rhGH replacement therapy in patients with confirmed persistent GHD during the transition period after the achievement of final height is recommended, as most studies have reported long-term improvement in body composition, bone health, QoL, and lipid metabolism in adulthood.44
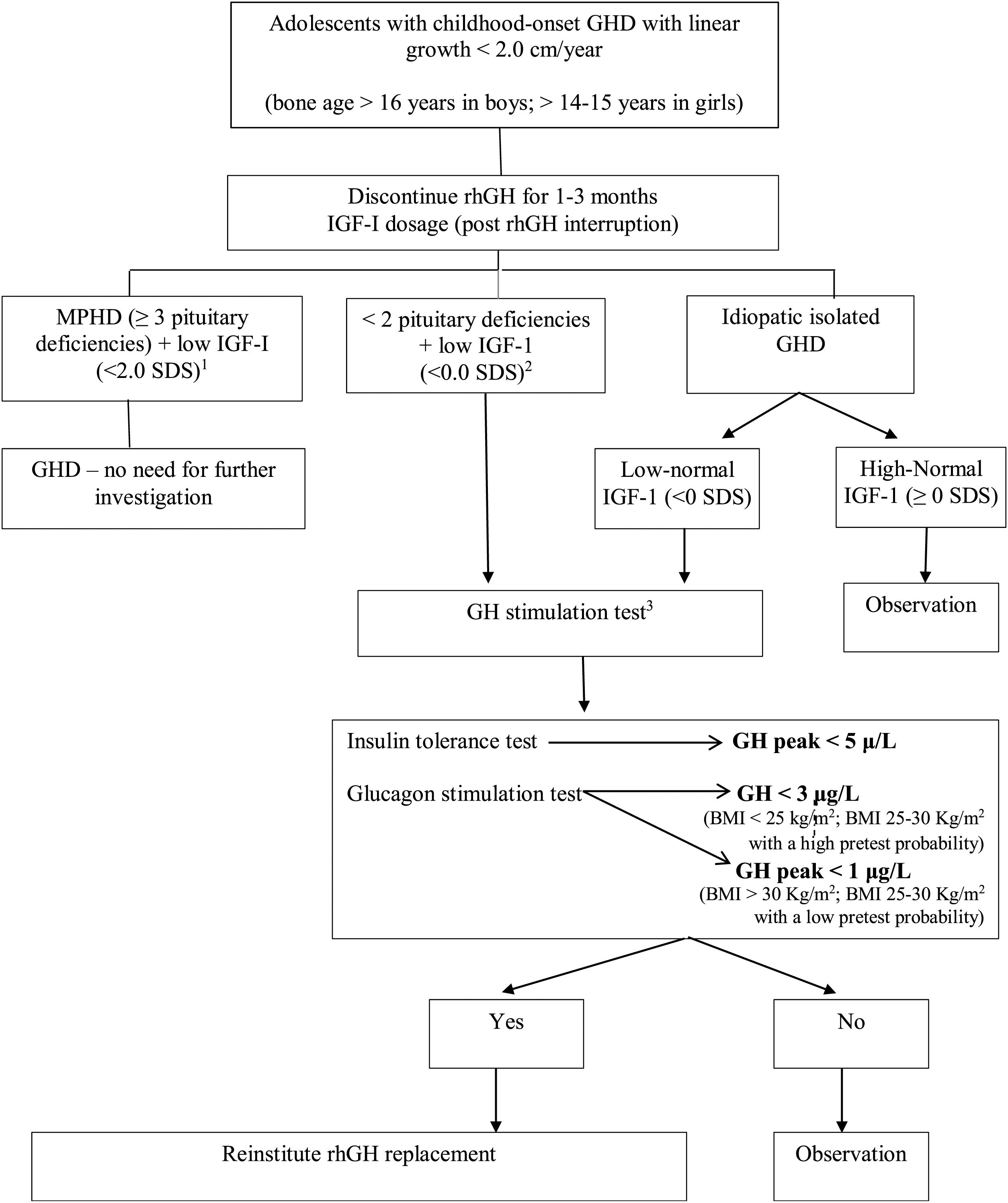
Reevaluation of GH – IGF–I axis in the transition phase: when, who and how to retestThe most frequently used criteria for interrupting rhGH use in adolescence are: growth velocity less than 2 cm/year, and bone age above 16 years in boys and 14–15 years in girls.45
Twenty to eighty-seven percent of children diagnosed as GHD, when retested at the end of stature growth were found to have normal GH secretion.2 Subjects with idiopathic isolated GHD have the highest percentage of normalization of GH upon retesting.46 The possible causes include: (1) transient or partial GHD; (2) changes in diagnostic criteria or lack of reproducibility in GH stimulation testing; (3) false-positive response at the time of diagnosis in children with short stature or pubertal delay; (4) neurosecretory dysfunction; (5) improvement in hypothalamic-pituitary function after puberty; (6) different response to stimulation test due to type of stimulation test, age, BMI, disease duration, number of pituitary hormone deficiencies and pituitary abnormalities.46
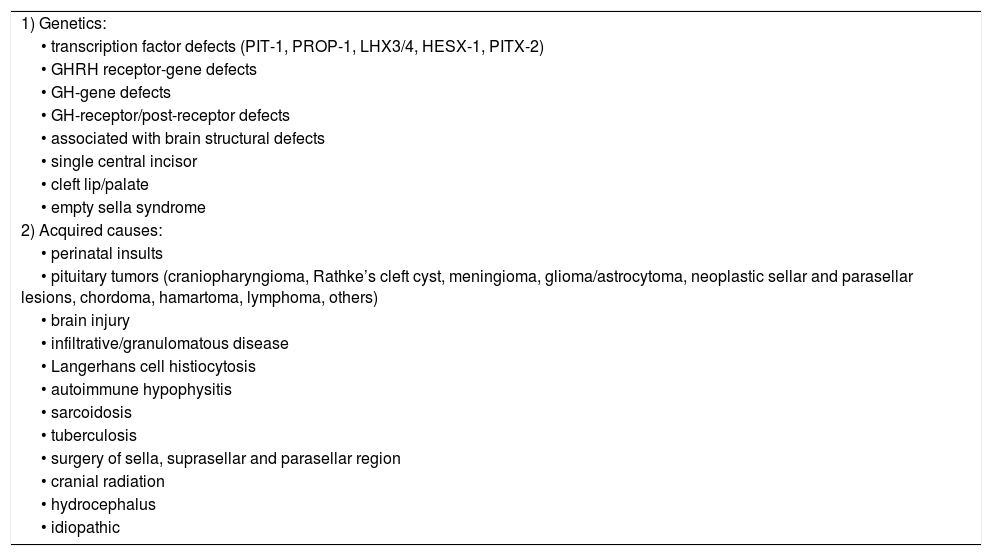
Therefore, GH stimulation testing is indicated in the appropriate clinical context of patients with a reasonable probability of GHD; and stimulation test is not required in certain patients who meet the criteria that predict adult GHD with high specificity.44 Patients with multiple (≥3) pituitary hormone deficiencies (MPHD) regardless of etiology, and low serum IGF-I (<−2.0 SDS), or GHD with a documented causal genetic mutations or specific pituitary/hypothalamic structural defect, except ectopic posterior pituitary, can be diagnosed with persistent GHD.4,44,47 These adolescents with MPHD have approximately a 100% fail rate on GH stimulation testing.9Table 1 exemplifies causes that can cause persistent GHD in this population.
Causes of persistent GHD in the transition phase. Adapted from Ref. 44.
| 1) Genetics: |
| • transcription factor defects (PIT-1, PROP-1, LHX3/4, HESX-1, PITX-2) |
| • GHRH receptor-gene defects |
| • GH-gene defects |
| • GH-receptor/post-receptor defects |
| • associated with brain structural defects |
| • single central incisor |
| • cleft lip/palate |
| • empty sella syndrome |
| 2) Acquired causes: |
| • perinatal insults |
| • pituitary tumors (craniopharyngioma, Rathke’s cleft cyst, meningioma, glioma/astrocytoma, neoplastic sellar and parasellar lesions, chordoma, hamartoma, lymphoma, others) |
| • brain injury |
| • infiltrative/granulomatous disease |
| • Langerhans cell histiocytosis |
| • autoimmune hypophysitis |
| • sarcoidosis |
| • tuberculosis |
| • surgery of sella, suprasellar and parasellar region |
| • cranial radiation |
| • hydrocephalus |
| • idiopathic |
PIT-1, pituitary transcription factor type 1; PROP-1, prophet of Pit-1; LHX3/4, LIM homeobox genes 3 and 4, HESX-1, homeobox embryonic stem cell; PITX-2, paired-like homeodomain transcription factor 2.
Patients who should be reevaluated through a GH provocative testing in the transition phase: (1) patients with idiopathic isolated GHD and low-normal (>−2 and <0 SDS) or low (<−2 SDS) serum IGF-I levels; (2) patients with GHD and deficiency of only 1 or 2 additional pituitary hormones; (3) patients with isolated GHD with pituitary hypoplasia or ectopic posterior pituitary; and (4) the previous history of cranial irradiation.9,44,47
It is mandatory the discontinuation of rhGH for 1–3 months in patients who will be retested.1,44,46 Adolescents are more likely to return for follow-up if reevaluation is done as fast as possible.48 It is noteworthy that replacement of other hormones, when other pituitary deficiencies are present, must be adequate before performing provocative testing.48 Another important piece of information is that while IGF-I levels between -2 e 0 SD does not confirm GHD or normality of GH production, requiring stimulation testing to confirm isolated GHD or GHD associated to 1−2 pituitary deficiencies,4,9 IGF-I levels in this population above 0 SD indicates normal GH secretion and indicates no need for provocative testing.
The gold-standard test for the evaluation of GH – IGF-I axis in adults is the insulin tolerance test (ITT), a test frequently used in the transition period.1,2,9,44,46–48 The counter-indications for this test are a history of seizures or epilepsy, renal or hepatic insufficiency, stroke, and ischemic cardiopathy antecedents. Alternative tests are growth hormone-releasing hormone (GHRH)-arginine test and macimorelin test (not available in Brazil); and glucagon stimulation test.44 GHRH-arginine test may be falsely normal in individuals with hypothalamic dysfunction.9 Macimorelin is an oral ghrelin mimetic that can be used as a simple, well-tolerated GH stimuli with minimal side effects, less blood draws over a shorter period of time, compared to other GH–stimulation tests; and it is anticipated that its use will increase over time.44,49 Glucagon stimulation test has become a feasible and reliable alternative to ITT after discontinuation of GHRH in the United States, with few relative contraindications, but of long duration (3–4 h).44,50–53 Clonidine provocative test is not indicated in the transition phase.
Although insulin-like growth factor binding protein-3 (IGFBP-3) measurement demonstrates a high specificity in GHD diagnosis (about 100%), its sensitivity is poor (about 30%), due to a number of pitfalls that limit reliability and usefulness in clinical practice.54
Magnetic resonance imaging (MRI) is another helpful tool to establish the diagnosis of permanent GHD. However, not all neuroradiological abnormalities detected at the time of initial diagnosis are indicative of permanent GHD, as pituitary hypoplasia and ectopic posterior pituitary.46
At the moment of rhGH interruption and GH reevaluation, the patient must have a full evaluation which includes: body composition, bone mineral density, lipid, and glucose profile, and QoL appraisal. If GHD is confirmed and rhGH replacement is reinstituted, these exams should be done periodically, as will be discussed in the next section. Fig. 1 demonstrates the evaluation of patients in the transition phase.
Reevaluation of GHD patients since childhood in the transition period. Adapted from Refs. 44 and 47.
a MPHD: multiple pituitary hormone deficiencies (congenital defects, genetic defects, organic diseases).
b Organic GHD.
c Other possible tests: GHRH-arginine (unavailable in Brazil and USA), macimorelin oral test (unavailable in Brazil).
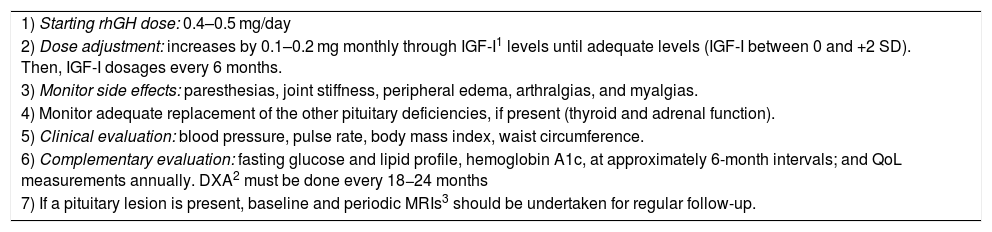
Patients with confirmed GHD in the transition period should restart rhGH replacement at a dose of 0.4−0.5 mg/day.4,9,44 Young adults may need higher rhGH doses than older adults for the same metabolic effects. The dose used prior to the interruption of rhGH can be used as a guide to the restart dose. Subcutaneous injections are administered in the evening to mimic physiologic endogenous GH secretion.44
The dose adjustment of rhGH may be done according to IGF-I levels, which must be measured every 4–8 weeks until the achievement of levels within but not exceeding the upper normal range (IGF-I between 0 and +2 SD) and subsequently, every 6 months.1,44 Dose titration must be done with increases by 0.1−0.2 mg every month until adequate levels of IGF-I.4,55
Potential side effects of rhGH replacement are related to fluid retention and include paresthesias, joint stiffness, peripheral edema, arthralgias, and myalgias. Most of these adverse reactions improve with dose reduction.4 We should remember that these patients have stopped rhGH therapy only a few months before treatment reinstitution and were used to this hormonal replacement; possible side effects were already related and well-known by patients, if previously present.
The IGF-I levels might not normalize with the reinstitution of rhGH, despite good clinical response. Some factors, such as sexual steroid replacement, can affect the basal and stimulated levels of IGF-I, since oral estrogen (and not transdermic ones) can inhibit the hepatic production of IGF-I.2 On the other hand, androgens improve the GH sensitivity, increasing the IGF-I levels, and act synergically in the protein anabolism.56 Sex steroid replacement, together with rhGH, is extremely important for the optimization of somatic development.4
It is important to emphasize that patients who refuse to reinitiate rhGH, and patients who had isolated idiopathic GHD with discordant results in IGF-I levels and GH provocative test, may be accompanied for any clinical deterioration.48 The development of GHD in patients who received cranial irradiation depends on dose and time, as GHD may take years to develop with a lower radiation dose. These patients need careful follow-up and retesting from time to time.
The adequate replacement of the other pituitary deficiencies, if present, is important. The rhGH replacement can accelerate the cortisol metabolism and may increase the risk of adrenal insufficiency in patients with subtle degrees of adrenocorticotrophic deficiency or in patients with a suboptimal replacement of glucocorticoid.4,55 Thyroid function must also be monitored in patients in rhGH therapy, since GH causes variables changes in thyroid hormone levels — the most relevant one being decreased free thyroxine (T4) levels.55
Monitoring should also include clinical evaluation (side effects, blood pressure, pulse rate, body mass index, waist circumference) and assessment of fasting glucose, hemoglobin A1c, lipid profile, serum-free T4 (and TSH, if not central hypothyroidism confirmed), and early morning cortisol (in patients not on glucocorticoid replacement) if clinically indicated, at approximately 6-month intervals, and QoL measurements annually.44,54 BMD may be reevaluated through dual-energy X-ray absorptiometry (DXA) every 18−24 months.4,55 If a pituitary lesion is present, baseline and periodic MRIs should be undertaken for regular follow-up.44
Another important point is for how long these patients should replace rhGH. If patients on rhGH replacement report significant QoL benefits and/or there are objective improvements, such as in cardiovascular risk markers, BMD, body composition, or physical activity tolerance, then rhGH treatment can be continued during adulthood.44 If there are no subjective or objective benefits of treatment after 12–18 months of therapy, interrupting rhGH replacement can be discussed with the patient.44Table 2 summarizes the follow-up of adolescents in rhGH replacement in the transition phase.
Follow up of patients in transition phase in rhGH treatment.
| 1) Starting rhGH dose: 0.4–0.5 mg/day |
| 2) Dose adjustment: increases by 0.1–0.2 mg monthly through IGF-I1 levels until adequate levels (IGF-I between 0 and +2 SD). Then, IGF-I dosages every 6 months. |
| 3) Monitor side effects: paresthesias, joint stiffness, peripheral edema, arthralgias, and myalgias. |
| 4) Monitor adequate replacement of the other pituitary deficiencies, if present (thyroid and adrenal function). |
| 5) Clinical evaluation: blood pressure, pulse rate, body mass index, waist circumference. |
| 6) Complementary evaluation: fasting glucose and lipid profile, hemoglobin A1c, at approximately 6-month intervals; and QoL measurements annually. DXA2 must be done every 18−24 months |
| 7) If a pituitary lesion is present, baseline and periodic MRIs3 should be undertaken for regular follow-up. |
IGF-I, insulin-like growth factor-I; DXA, dual-energy X-ray absorptiometry; MRIs, magnetic resonance images.
In conclusion, GH is not only crucial for the attainment of adult height, but also for adequate somatic development, which does not end concomitantly with linear growth. In this way, patients with GHD in childhood who confirm the persistence of GHD in the transition period should maintain rhGH replacement in order to reach an ideal peak bone mass, the main determinant of osteoporosis in adult life, and also satisfactory body composition, lipid and glucose profiles, and QoL. Patients with isolated idiopathic GHD in childhood are a special group who really must be reevaluated for GHD in the transition period once many of them have normal GH provocative test at this time.
Conflicts of interestAna Beatriz Winter Tavares declares no conflicts of interest. Paulo Ferrez Collett-Solberg: received travel grants and speaker fees from Pfizer, Merck-Serono, Novo Nordisk, Sandoz, and BioMarin.