To analyze the performance of the cystic fibrosis (CF) newborn screening (NBS) program over its first five years in a Brazilian northeastern state.
MethodA population-based study using a screening algorithm based on immunoreactive trypsinogen (IRT)/IRT. Data were retrieved from the state referral screening center registry. The program performance was evaluated using descriptive indicators such as the results of an active search, coverage, newborn's age at the time of blood sampling, the time between sample collection and its arrival at the laboratory, and the child's age at diagnosis of disease.
ResultsThe public CF screening program covered 82.6% of the 1,017,576 births that occurred, with an accumulated five-year incidence of 1:20,767 live births. The median (25th-75th) age at diagnosis was 3.5 (2.3–7.3) months. The sampling before 7 days of life for the first IRT (IRT1) increased between 2013 and 2017 from 42.2 to 48.3%. Around 5% of IRT1 samples and 30% of the second samples were collected after 30 days of life. In the first and second stages of screening, 23.6% and 19.9% of the infants, respectively, were lost to follow-up. In both stages of screening, the samples were retained at the health units for a median (25th–75th) of 9.0 (7.0–13.0) days.
ConclusionsThe coverage by the CF-NBS program was satisfactory as compared to other Brazilian state rates and the percentage of IRT1 samples collected within the first week of life increased progressively. However, time of samples retention at the health units, inappropriate sampling, inherent methodological problems, and loss of follow-up need to improve.
Newborn screening (NBS) accelerates the diagnosis of different metabolic, hematologic, infectious, and genetic diseases, enabling adequate timely treatment to be implemented and the disease course to be changed.1 Nevertheless, NBS programs, either addressing promotion, prevention or cure, are essentially complex and may involve federal, state and municipal agencies, thus characterizing them as governmental and public utility services.2
While the first NBS programs for phenylketonuria were initiated in the United States in the 1960s, the first phenylketonuria screening program in Brazil was implemented in São Paulo only in 1976.3,4 With the goal of including all live births in the country, the National NBS Program was created in 2001 and gradually introduced into the various Brazilian states. Concurrently, other clinical conditions were progressively incorporated into the program in the following order: congenital hypothyroidism, sickle cell disease and hemoglobinopathies, cystic fibrosis (CF), and more recently, congenital adrenal hyperplasia.1
CF is an autosomal-recessive genetic disorder resulting from the presence of two pathogenic variants of the CF transmembrane regulator (CFTR) gene that codifies the CFTR protein. Diagnosis of CF may be based on clinical findings suggestive of the disease and/or family history; however, NBS accelerates diagnosis. Confirmation requires a sweat test (ST), the gold standard for diagnosis, and/or identification of two pathogenic variants of the CFTR gene.5 Newborn CF screening proved to be beneficial and effective as children were found to be diagnosed earlier with this method, resulting in better long-term prognosis, fewer hospital admissions, better nutritional status, and delayed onset of more severe complications.6–9
The first attempts at CF-NBS date from the 1970s, with semi-quantitative measurements of the albumin content of meconium.10 In the 1980s, Crossley et al. detected elevated immunoreactive trypsinogen (IRT) levels in the blood of newborns with CF.10 CF-NBS has since then been implemented in various European, North American, and Latin American countries, while technical changes have been progressively incorporated to improve the sensitivity and specificity of the assays.11,12
The northeastern state of Bahia comprises 417 municipalities, with a total area of approximately 570.000 km2 and a population of around 15 million inhabitants. Here, CF screening was established in 2013. Each municipality collects blood samples from newborns onto filter paper for analysis at the laboratory of the Association of Friends and Parents of Individuals with Special Needs (APAE) in Salvador, the state capital. There are currently 2700 NBS collection units in the state; however, APAE is the only newborn screening referral service (NBSRC). Assessment of data on sampling, screening, and diagnostic confirmation is of key importance for process improvement and consolidating successful indicators. Since implantation, the epidemiological aspects and the performance of this program had yet to be evaluated. This study was designed to specifically evaluate the epidemiological data and the performance of the CF screening program in Bahia.
MethodsStudy design and periodThis was a population-based, observational study using individualized, descriptive incidence data, collected retrospectively for the 2013–2017 period.
Population, site and eligibility criteriaAll newborns included in the Brazilian National Health Service NBS program in Bahia were eligible for inclusion.
Procedures for newborn CF screening in Bahia stateThe adopted screening algorithm by the Newborn Screening referral center is used in most Brazilian states and is similar to that recommended by the Brazilian CF Study Group and Brazilian Health Ministry with IRT being measured at two different moments.1 A sampling of dried blood spots on filter paper is obtained at affiliated healthcare units. The NBSRC in Bahia uses a fixed cut-off point of 70 ng/mL for both measurements. The first IRT measurement (IRT1) should be performed at 3–5 days of life. A second measurement (IRT2) should be performed at 10–21 days of life whenever IRT1 is positive (≥70 ng/mL). If IRT2 is also positive (≥70 ng/mL), the infant should be referred for clinical evaluation and an ST at a CF care center. Samples for IRT1 or IRT2 taken from infants > 30 days old are considered inadequate and unreliable.
Sample processingBlood samples are collected into filter paper at primary healthcare units or maternity hospitals. Samples are then sent to the NBSRC, preferably in vehicles supplied by the municipalities. Upon receipt of the samples, an immunofluorometric assay is performed using AutoDELFIA Neonatal IRT-Kit (PerkinElmer, Finland) on an AutoDELFIA Instrument (Waltham, USA).
Sweat testAt the CF care center, infants undergo an ST using quantitative pilocarpine iontophoresis to measure chloride in sweat. Chloride measurements < 30 mEq/L are considered normal, while levels ≥ 60 mEq/L are considered positive. To confirm the diagnosis, two positive tests are required. Values of 30–59 mEq/L are considered inconclusive, and the ST must then be repeated at another moment, and/or additional genetic investigation should be performed.5,13
Study variables and endpointsThe variables of interest were the number of liveborn infants in the state each year as recorded in the Department of Informatics of the Unified Health System (DATASUS),14 newborn's birth date, date of blood sampling, date of sample arrival at the reference laboratory, date of result availability/delivery and the date of diagnostic confirmation of CF. Other variables analyzed were: IRT1 and IRT2 measurements, the number of invalid samples, the number of infants referred to the CF treatment center, and the number of infants with CF. Data were retrieved from the SMART Health System database at the reference laboratory and from patient records at the CF care center where the NS data for the state and data on the follow-up of these infants are registered regularly.
To evaluate performance NBS indicators, as proposed by Webster4 and Mendes et al.15 (supplementary material), the following variables were obtained: age in days at blood sampling; time interval in days between sample collection and sample arrival at the laboratory; time difference in days between sample arrival at the laboratory and result availability/delivery; and total screening time, i.e. the difference between sampling date and the end of the active search in cases of loss to follow-up, or death, or diagnosis of CF (either confirmed or ruled out).
Blood sampling was considered delayed when IRT1 was collected after 15 days of life and inadequate when IRT1 or IRT2 were collected after 30 days of life.5,13 Samples were also considered inadequate for CF when they could not be processed due to technical issues with sampling, storage, or transportation.
Data analysisSPSS, version 21, and Excel for Mac, version 16.48 (2020) were used to tabulate and analyze the data collected. The incidence of cases screened positive was calculated based on the percentage ratio between the total number of infants who screened positive and the total number of newborns screened. The incidence of confirmed cases of CF was obtained from the percentage ratio between the total of confirmed cases and the total number of newborns screened.
The characteristics of the sample were expressed as means and medians and interquartile range (IQR), variance, standard deviation, and coefficient of variation for quantitative indicators, and as absolute and relative frequency for categorical variables.
Ethical issuesThe study was conducted in compliance with the principles of ethics in research established in the Declaration of Helsinki and according to National Health Council Resolution 196/96. The institute's internal review board approved the protocol under reference 3.319.191.
ResultsBetween 2013 and 2017, 1,017,957 live births were recorded in the state of Bahia and 877,479 infants (86.2%) underwent NBS. In relation to CF screening specifically, IRT1 was measured in 840,976 infants (82.6%). Of those testing negative at IRT1, samples had been taken after 30 days of life in 38,986 cases (4.6%). Overall, 4,595 infants (0.5%) had an abnormal IRT1 measurement; however, in 57 of these cases (1.2%) samples were taken after 30 days of life (Table 1). The incidence of inadequate samples for CF ranged from 0 to 1.4% during the study period, The incidence of inadequate samples for CF ranged from 0 to 1.4% during the study period; however, the database did not allow either to identify which children responded or not to the recall or to distinguish between those who were recalled due to inadequate samples or abnormal screening tests.
Data on newborn screening in a northeastern Brazilian state, 2013–2017.
IRT1, first measurement of immunoreactive trypsinogen; IRT2, second measurement of immunoreactive trypsinogen; NBS, newborn screening; CF, cystic fibrosis.
IRT2 was measured in 3510/4595 (76.4%) children with abnormal IRT1 levels; however, 991 (28.3%) were > 30 days old at sampling, and it is unknown whether they were referred for CF treatment. Elevated IRT2 was found in 201/3510 infants (5.7%) and all were referred for an ST (Table 1).
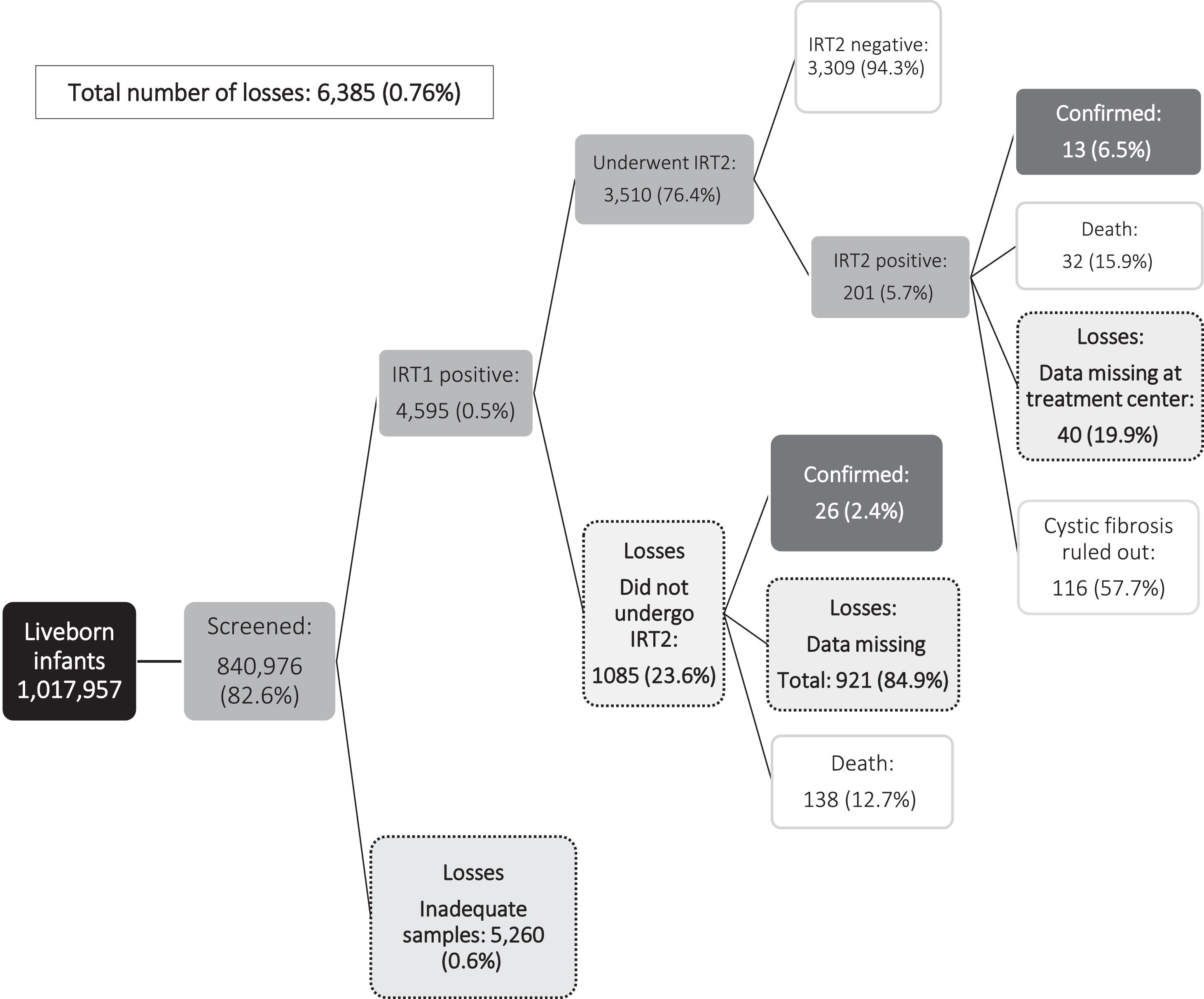
Fig. 1 shows the principal endpoint (diagnosis of CF) and the cases lost to follow-up during newborn CF screening. Over the study period, CF was confirmed in 49 children: 12 (24.5%) had increased IRT1 and IRT2 levels prior to confirmation of diagnosis, and 37/49 (75,5%) underwent IRT1 alone before CF diagnosis confirmation. In the same period was observed that 10/49 (20,4%) infants were diagnosed despite normal IRT1 levels (false negatives). One child died before completing NBS. That child underwent one ST, which was positive (Figure 1). The median age at diagnosis was 3.5 (IQR 2.3–7.3) months.
The incidence of the disease per year in Bahia ranged from 1:29,148 to 1:17,221, with an accumulated five-year incidence of 1:20,767 live births (Table 1).
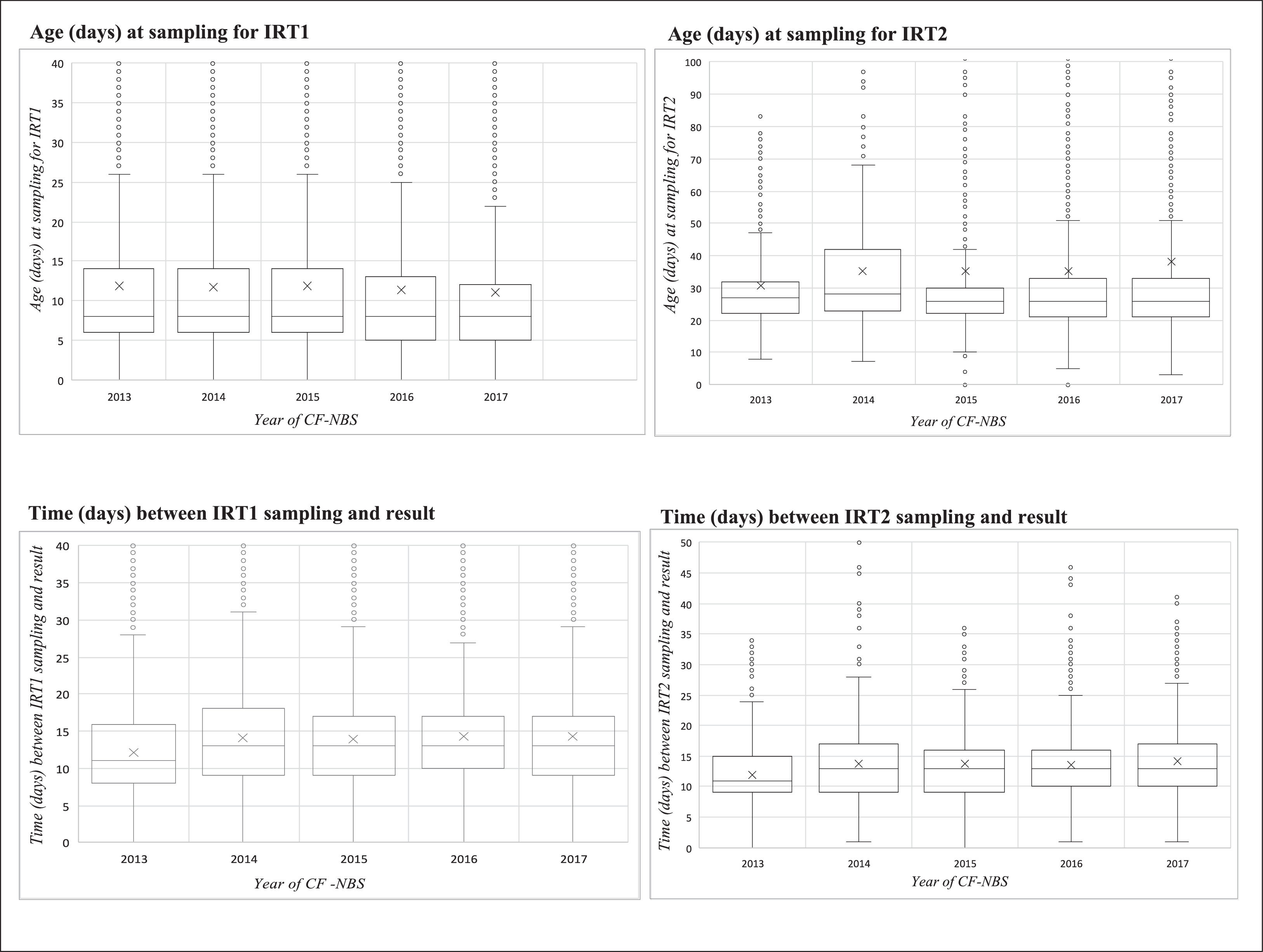
The median age of the newborns was 8 days (IQR 6–13) at first sampling and 27 days (IQR 22–33) at second sampling (Table 2). The median total screening time was 44 (IQR 33–62) days. In 2013 and 2015, median total screening times were shorter (42 days). Only 233 (23.3%) infants were submitted to IRT2 before reaching 20 days of age.
Data on the stages involved in the 1st and 2nd immunoreactive trypsinogen measurements.
IRT1, first measurement of immunoreactive trypsinogen; IRT2, second measurement of immunoreactive trypsinogen; IQR, interquartile range.
Fig. 2 shows the range in the median age of collection (days) of IRT1 and IRT2 and the time between sampling and delivery of screening test results for each study year.
Boxplot of age (days) and time (days) variables in relation to the sampling of the first and second immunoreactive trypsinogen (IRT) over the study period (2013 to 2017). IRT1, first measurement of immunoreactive trypsinogen; IRT2, second measurement of immunoreactive trypsinogen; NBS, newborn screening; CF, cystic fibrosis.
This study highlights the complexity of NBS in a populous state such as Bahia which consists of many municipalities. The coverage of CF-NBS was 82.6% for the 2013–2017 period, with the rate improving progressively over the period. The percentage of sampling for IRT1 before 7 days of life also increased from 42.2% in 2013 to 48.3% in 2017. Nevertheless, program shortcomings were detected, indicating a need to improve NBS. During the study period, samples were obtained at > 30 days of life, hence were unreliable, in 5% and 28% of infants at IRT1 and IRT2, respectively.1 The percentage of infants with elevated IRT1 levels who failed to undergo IRT2 was also high (23.6%), while 40 infants (19.9%) with elevated IRT2 levels could not be located and were consequently not referred to the treatment center for an ST. Over the evaluation period, Bahia NBS diagnosed CF in 39 infants; however, only 13 (33.3%) completed all the steps in the screening process, highlighting the difficulties involved in achieving complete CF-NBS.
In Europe, NBS has reached general coverage rates > 90%, with similar levels being found between countries.11 Sadigurschi et al. reported poor newborn CF screening coverage in Brazilian states with a low economic development level, with a mean coverage of 12.3% for the northeast and 10.9% for the north compared to 100% for the southern states and the Federal District.16 These data highlight the unequal coverage in Brazil, with poorer coverage in areas with fewer financial and social resources. Nevertheless, although the period of analysis was different, the present study showed that NBS coverage exceeds that previously reported for northeastern Brazil.
In this study, the median age of infants at sampling for IRT1 in Bahia was lower than in other Brazilian states.17,18 Despite the complexity involved in providing newborn CF screening in a large, populous state, the percentage of samples collected before 7 days of life exceeded the rates reported for Rio de Janeiro (38.3%) and Piauí (36.8%).18,19 The median time interval between collection and the sample being received at the laboratory was like that reported for Rio de Janeiro (8 days) and Piauí (5 days).18,19 Compared to the present study, however, the number of infants undergoing IRT2 following a successful active search was higher (93.4%) in São Paulo, highlighting the importance of improving follow-up in Bahia.17 Nonetheless, the median of 7 days between delivery of IRT1 results and sampling for IRT2 was satisfactory and smaller than that reported in a study in Rio Grande do Sul where the median time until attendance for IRT2 was 18 days.20 In the present study, the median age of the infants at referral to the treatment center (44 days) was less than in other states such as Piauí (56 days) and Rio de Janeiro (48 days). These data emphasize the inequality of the programs in the different states and the need for national public policies to be implemented to minimize these differences.21
The fact that this study was able to identify shortcomings in the organization of NBS in Bahia that accounted for delays in providing complete screening was a strong point that may help improve the system. Conversely, limitations include the study's retrospective nature, missing information related to the number of children who were called up to repeat the NBS of CF, the unknown outcome of infants whose IRT1 and IRT2 samples were collected after 30 days of life, absence of visits to the healthcare units where samples were collected, rendering analysis of this step impossible, and the difficulties involved in obtaining information from the CF treatment referral center. On the other hand, the NBSRC was found to be well organized and equipped, with a complete database that allowed retrospective collection of data, which proved vital in understanding the progress made in the first five years of CF-NBS.
The assessment of public health and social programs typically deals with two opposite expectations: one, skeptical, which denies their value, and the other, overly optimistic.22 Assessment contributes toward increasing rationality in decision-making by identifying problems, defining alternative solutions, foreseeing their consequences, and optimizing the use of available resources.23 Therefore, interpreting the results of this study serves to improve the newborn CF screening process in Bahia.
The Brazilian Health Ministery considers that NBS coverage is satisfactory whenever it reaches 80% or more of the target population.1 This coverage level was attained here; nevertheless, 100% coverage remains an important goal. Performing sampling in maternity hospitals and establishing a link between NBS and a social program are potential strategies to help increase coverage and sampling before 7 days of life.11,24 These two actions should be proposed in Bahia.
Although the screening coverage was satisfactory as compared to other Brazilian state rates, the results of this study show that its effectiveness was not, given that NBS CF involves two steps and only 54.8% (2519/4595) of the screened children were submitted to IRT2 in the recommended time. Thus, the effectiveness of the screening program for cystic fibrosis in Bahia was below the recommended level, also evidenced by the fact that under 1/4 of the diagnosed cases went through all the screening stages. The incidence of 1:20,767 is much lower than that found in other Brazilian regions and in other countries.11,16,25 This lower incidence can reflect both ethnic characteristics of the population and the ineffective coverage of the program, which can underestimate the real incidence. Moreover, the short time of the assessment (five years) is not enough to determine this measure precisely.
Analysis of the time spent at each step pinpoints the degree of organization and synchronization throughout the process. This study shows that at some stages the amount of time spent is satisfactory; however, due to program flaws, the median age at which infants are referred to the treatment center is 6.2 weeks. Until a diagnosis is confirmed or ruled out, these infants will undergo one, two or more STs and probably genetic profiling, adding at least another 2–3 weeks to this time and increasing the median age to at least 8.2 weeks, beyond the ideal time frame.1 Therefore, improvements are still required in Bahia to ensure that CF is diagnosed before 2 months of age of life, which has not yet been guaranteed by the NBS CF, given that the median age at diagnosis was 3.5 months. Throughout the years, the exchange of information between CF screening and CF care centers has been improved. However, there are still priority upgrades in communication services to be implemented, such as an integration of service databases, and of the main hospitals that aid these infants.
Some steps in CF-NBS merit consideration, particularly the prolonged time the samples are held at collection units. The reasons for the delay in sending these samples to the laboratory could not be determined by this study. Since Bahia is very large, with many different municipalities, road transportation may not be the best means of ensuring that the samples arrive at the laboratory in a timely fashion. Agreements between the municipal, state, and federal governments, involving the post office, aimed uniquely at transporting this high-priority biological material, may reduce this delay. Nevertheless, some studies have argued that even when the post office is involved in transportation, problems may yet persist, suggesting that it is not just the transportation method that needs to be reviewed but the entire underlying mechanism, from planning to execution.1,19 Problems with the supply of filter paper and inadequate storage of samples, factors, not analyzed in this study, may also contribute to failures in the screening process.26,27
This study highlights the large number of IRT1 and IRT2 samples collected from infants over 30 days old, a technical error with important secondary consequences. These samples should not have been taken and, moreover, parents and families should not have been given the results. Another issue to be addressed is incomplete CF-NBS resulting in a large number of infants being referred to the CF treatment center in the state capital city. This increases the costs in general, represents a burden to society, and is psychologically demanding for families. Continued training may improve awareness among primary healthcare professionals regarding the timely collection and fast shipping of samples. The future inclusion of a panel of variants or investigation of the F508del variant at first testing whenever IRT1 measurements are high could be suggested as a strategy to avoid delays in diagnosis and accelerate treatment; however, the ethnic/racial peculiarities of the target population render this strategy a complex task.
CF-NBS coverage was shown to be satisfactory as compared to other Brazilian state rates, but the effectiveness of the coverage is not as good as it should be. The percentage of IRT1 samples collected within the first week of life increases progressively. However, the percentage of samples taken > 30 days of life was high. Delays were identified at various stages and the number of infants with elevated IRT1 and/or IRT2 levels who were lost to follow-up was high, revealing difficulties in performing complete NBS, resulting in errors and delayed diagnosis, which made the median age of CF diagnosis high. The screening program in this state clearly needs to be discussed and improved, these data also highlight the importance of periodically evaluating public health actions in Brazil.
FundingFundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) - Edital 003/2017 do Programa de Pesquisa para o SUS: Gestão Compartilhada em Saúde - PPSUS/Ba, termo de outorga n° SUS0033/2018.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001
Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB), Associação de Pais e Amigos dos Excepcionais Salvador (APAE-SSA).
Study conducted at the Universidade Federal da Bahia (UFBA), Salvador, BA, Brazil.