To compare body growth, weight, and fecal moisture in recently weaned rats fed exclusively on infant soy formula and soy-based beverage.
MethodsThree similar groups were formed (n=10/group) consisting of weanling Wistar rats, maintained in metabolic cages. One group was fed soy protein-based beverage, another with soy-based infant formula, and another with cow's milk infant formula (control group). Water and diet were offered ad libitum. Body weight and length were measured. Stool was collected for three consecutive days.
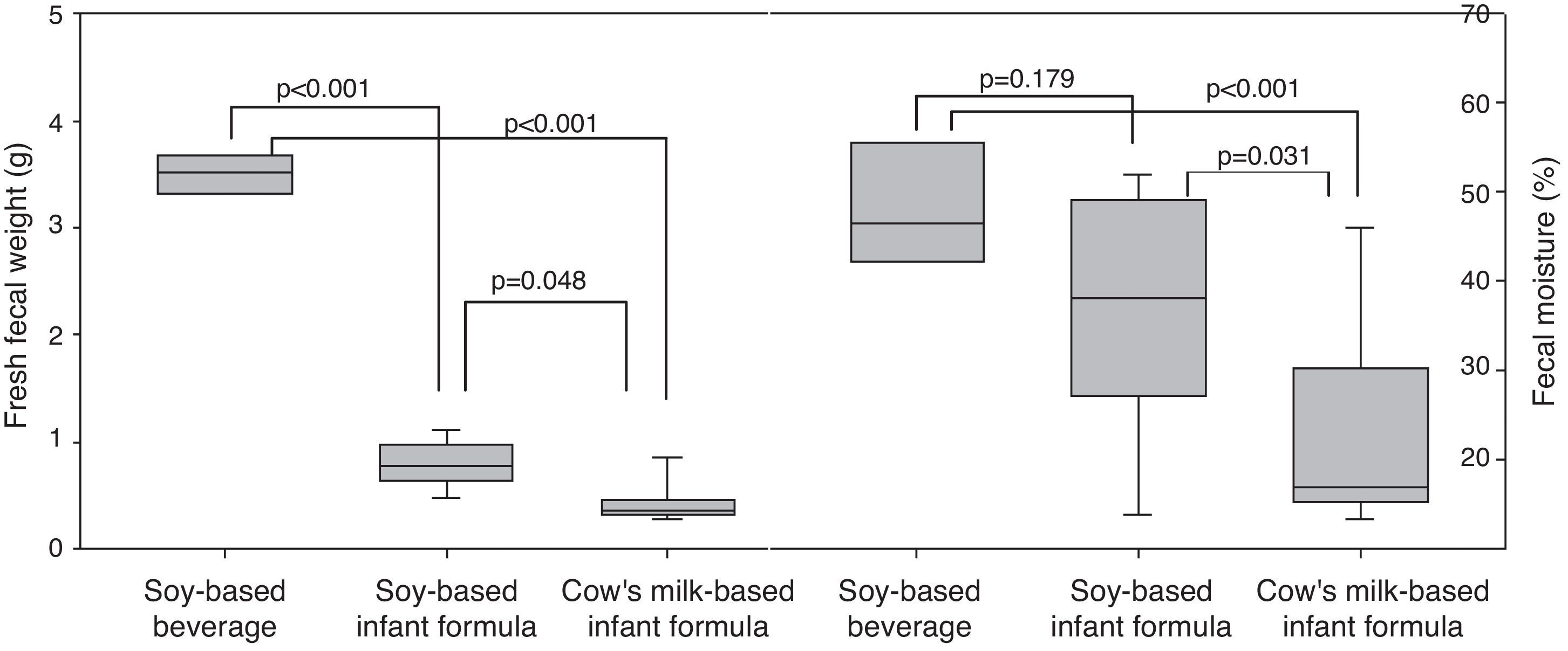
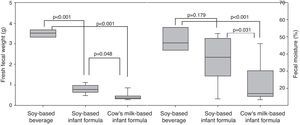
ResultsWeight and length were lower (p=0.001; p=0.001) in the groups receiving soy protein-based beverage (73.16±5.74g; 23.94±1.04cm) and soy-based formula (71.11±5.84g; 24.74±0.60cm) in relation to the group receiving cow's milk formula (84.88±9.75g; 26.01±0.91cm). Fresh fecal weight was greater (p<0.001) in the soy-based beverage (3.44±0.48g) than in the soy-based formula (0.79±0.20g) and cow's milk-based formula (0.42±0.17g). Fecal moisture was higher (p<0.001) in the group receiving soy protein-based beverage (47.28±9.02%) and soy-based formula (37.21±13.20%) than in the group receiving cow's milk formula (22.71±10.86%).
ConclusionThe growth of rats fed soy protein-based beverage and soy-based formula was lower than those fed cow's milk-based formula. The soy protein-based beverage resulted in significant increase in fecal weight and moisture.
Comparar o crescimento corporal, o peso e a umidade das fezes de ratos recém-desmamados alimentados exclusivamente com fórmula infantil de soja e com bebida de extrato de soja.
MétodosConstituíram-se três grupos similares (n=10/grupo) de ratos machos Wistar recém-desmamados, mantidos em gaiolas metabólicas. Um grupo foi alimentado com bebida de extrato de soja, outro com fórmula infantil de soja e o outro com fórmula infantil de leite de vaca (grupo controle). Água e dieta foram oferecidas ad libitum. Foram mensurados o peso e o comprimento corporal. Fezes foram coletadas durante 3 dias consecutivos.
ResultadosPeso e comprimento foram menores (p=0,001; p=0,001) nos grupos com bebida de extrato de soja (73,16±5,74g; 23,94±1,04cm) e fórmula infantil de soja (71,11±5,84g; 24,74±0,60cm) em relação ao grupo de fórmula infantil de leite de vaca (84,88±9,75g; 26,01±0,91cm). O peso fresco fecal foi maior (p<0,001) na bebida de extrato de soja (3,44±0,48g) do que com as fórmulas infantis de soja (0,79±0,20g) e de leite de vaca (0,42±0,17g). A umidade fecal foi maior (p<0,001) na bebida de extrato de soja (47,28±9,02%) e fórmula infantil de soja (37,21±13,20%) do que na fórmula infantil de leite de vaca (22,71±10,86%).
ConclusãoO crescimento de ratos alimentados com bebida de soja e fórmula infantil de soja foi menor do que os alimentados com fórmula com proteína do leite de vaca. A bebida à base de extrato de soja proporcionou aumento expressivo do peso e da umidade fecal.
Soy formulas are currently indicated for the treatment of infants in the second semester of life with suspected IgE-mediated allergy to cow's milk.1,2 However, some studies on pharmacoeconomics evaluating the impact of food allergy on the health systems of many countries3–6 demonstrated that soy formulas are also used, in practice, in patients with non-IgE-mediated allergy to cow's milk. Although inadequate, from the nutritional viewpoint, to feed infants, a study performed in Brazil7 showed that a significant number of health professionals considered that soy-based beverages could be used in the alternative diet of infants allergic to cow's milk.
Soy-based formulas have been used in infant nutrition either due to their relatively low cost or their acceptance by infants.8 Despite the very limited indications, they are used by a large number of infants around the world,9,10 representing one of the most often-used alternatives to substitute cow's milk-based infant formula, frequently introduced at a very early age or in the neonatal period. The composition of soy proteins is very complex, and differs from cow's milk proteins used in infant formula.11
The market also offers other soy-based beverages. These products should not be called formula, but rather soy-based beverages, as although some are fortified, they do not meet the legal standards for child nutrition related to protein quality or levels of minerals and their proportions.12
Initially, their consumption in the West was restricted mainly to people with lactose intolerance as a substitute for cow's milk, in addition to vegetarians and those with food restrictions.13,14 Currently, this product is very well accepted and widely consumed, indicating that consumers have been receptive and have incorporated it into their eating habits.15
It is noteworthy that the characteristics of soy formulas differ from those of soy-based beverages, commonly and erroneously marketed as “soy milk,” as the latter do not meet the nutritional needs of infants.16
Considering the nutritional inadequacy of soy-based beverages in relation to soy infant formula and that the present laboratory developed an experimental model in rats17 that allows measuring not only food intake but also growth, as well as the elimination of stool and urine, this project was planned. The objective of the study was to compare body growth, weight, and feces moisture of weanling rats exclusively fed soy-based formula and soy-based beverage. As reference, an additional group fed standard formula with cow's milk protein was used as control.
This experimental model was used in order to provide knowledge and possible benefits for humans; however, it does not accurately reflect what occurs in human beings. Considering the objective of this project, it should be noted that it could not be performed in human infants, as it is unacceptable to feed soy-based beverage to this age range. In turn, the interpretation of results must take into account the differences between the species; however, certain common characteristics justify the use of experimental models to increase the understanding of matters of interest to human health.
MethodsA total of 30 male Wistar rats, aged 21 days (the first day of weaning) were used in the study. Throughout the study period (ten consecutive days), they received deionized water through the MilliQ Plus system (Millipore Indústria e Comércio Ltda., Barueri, SP, Brazil) and liquid diet (infant formula or soy-protein based beverage) ad libitum. All animals were maintained in individual metabolic cages (Nalgene Metabolic Cages 650-0100, Tecniplast, Buguggiate, Italy) under a light cycle of 12hours and a temperature of 23±1°C. Each cage was fitted with two drinking fountains previously treated with nitric acid and rinsed in deionized water.
The study protocol was approved by the Research Ethics Committee of Universidade Federal de São Paulo – Escola Paulista de Medicina (CEP 0659/10).
Three groups were formed with ten animals each, which started receiving one of the following diets: 1) soy-based beverage; 2) soy-based infant formula; 3) cow's milk-based infant formula. The volumes consumed were measured and the feeding systems were cleaned at each change of meal (150mL/day, divided into three times per day). Group 3 represented the control group and received formula based on lactose-free cow's milk protein.
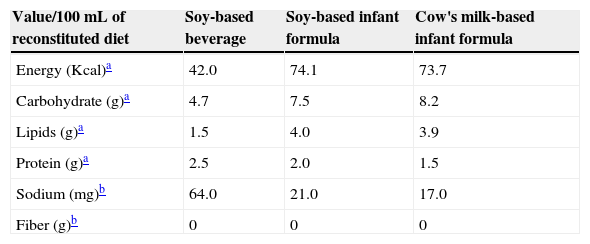
All diets were reconstituted as recommended by the manufacturer and their nutritional composition is described in Table 1. Regarding the nutritional content, the values declared by the manufacturers on the labels were considered. Sodium and fiber content shown in the table were obtained by chemical analysis conducted in the Laboratory of Food Science and Food Microbiology of Universidade Federal de São Paulo – Escola Paulista de Medicina, São Paulo, SP, Brazil, using standardized techniques.
Nutritional composition of the diets offered during the experiment according to the information on each product label.
The experiment was performed in two phases, each with 15 animals. On the first day of the study, three similar groups were formed and the animals were weighed and measured. Weight measurement was performed using a digital CG-Libror L-600 electronic scale (CG Scientific Instruments Ltda., São Paulo, SP, Brazil), with a maximum capacity of 600g and 0.1g sensitivity. The length was expressed in centimeters, considering the measurements of body and tail. These same procedures were repeated on the fifth and tenth days (31 days of life of the animals).
Dietary intake was determined by the volume ingested during the ten days of the experiment. Evaluation of the daily food intake allowed the calculation of feeding conversion efficiency, which was expressed in two ways: weight gain in grams per mL of food intake and weight gain in grams per kilocalorie of intake.
On the fifth day of the experiment, stool collection for fecal balance was performed. For this purpose, 0.1g of pink carmine dye was added to each animal's diet, and during three consecutive days, the stool eliminated from the time of occurrence of this change in color (reddish) was collected. After 72hours of the addition of pink carmine, Evans blue dye was added to the diet (Inlab; São Luís, MA, Brazil, water-soluble). The collection was stopped when the bluish stool started to be eliminated.
The stool collected during the three days were weighed on an analytical electronic scale (Mettler Toledo - AB204-S model), with a sensitivity of 0.0001, and stored in a freezer (-20°C). Subsequently, the stool samples were dried in an oven at 105°C and, after 22hours, they were weighed at 30-minute intervals until two consecutive weighings were obtained with a difference of less than 1.0mg. The stool moisture content was calculated using the formula [(fresh fecal weight - dry fecal weight/fresh fecal weight) x 100].18
At 31 days of life (tenth day of the experiment), the animals were euthanized, after being previously anesthetized with ketamine and xylazine, by exsanguination of the vena cava.
Results were expressed as mean±standard deviation when the numerical variables were normally distributed. Analysis of variance (ANOVA) was used to compare the groups. When a statistically significant difference was observed, the analysis was complemented by Tukey's test. The Jandel-Sigma Stat® software program, release 3.5 (Systat Software Inc., San Jose, California, USA) was used to perform the statistical tests and charts, with the level of rejection of the null hypothesis set at 5%.
ResultsBefore initiating the experimental diets, there was no statistically significant difference between the groups in terms of weight and length, demonstrating the similarity between them (data not shown). The similarity remained after exclusion of four animals that did not complete the entire study period. Two animals were from the group fed soy-based beverage, one from the group fed soy-based formula, and the other from the group fed cow's milk-based formula.
At the end of the experiment (tenth day) the groups fed soy-based beverage (73.16±5.74g) and soy-based formula (71.11±5.84g) had lower weight than the group fed cow's milk-based formula (84.88±9.75g), with a statistically significant difference (p=0.001). Body length at the end of the intervention was lower in the groups fed soy-based beverage (24.74±0.60cm) and soy-based formula (22.63±0.52cm) in comparison with the group fed cow's milk-based formula (26.01±0.91cm, p=0.001).
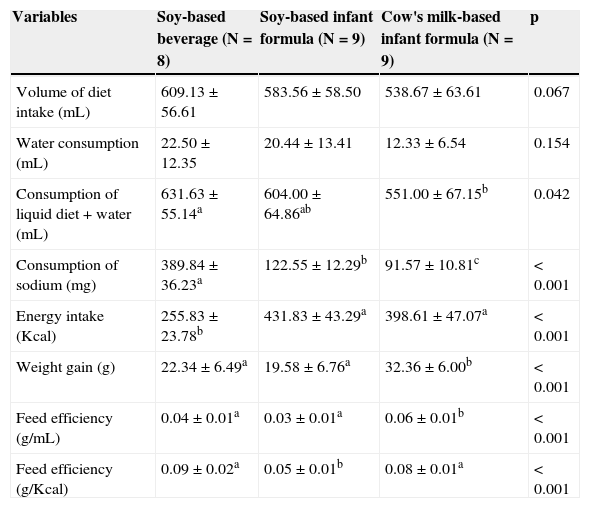
Table 2 shows that, regarding the volume consumed, there was no statistically significant difference among the three groups (p=0.067). Sodium intake was higher in the group fed soy-based beverage. The animals fed soy-based formula consumed more sodium than the group fed cow's milk-based formula. Energy intake was lower in the group fed soy-based beverage.
Food intake, weight gain, and feed efficiency during the ten days of the experiment.
| Variables | Soy-based beverage (N=8) | Soy-based infant formula (N=9) | Cow's milk-based infant formula (N=9) | p |
|---|---|---|---|---|
| Volume of diet intake (mL) | 609.13±56.61 | 583.56±58.50 | 538.67±63.61 | 0.067 |
| Water consumption (mL) | 22.50±12.35 | 20.44±13.41 | 12.33±6.54 | 0.154 |
| Consumption of liquid diet + water (mL) | 631.63±55.14a | 604.00±64.86ab | 551.00±67.15b | 0.042 |
| Consumption of sodium (mg) | 389.84±36.23a | 122.55±12.29b | 91.57±10.81c | < 0.001 |
| Energy intake (Kcal) | 255.83±23.78b | 431.83±43.29a | 398.61±47.07a | < 0.001 |
| Weight gain (g) | 22.34±6.49a | 19.58±6.76a | 32.36±6.00b | < 0.001 |
| Feed efficiency (g/mL) | 0.04±0.01a | 0.03±0.01a | 0.06±0.01b | < 0.001 |
| Feed efficiency (g/Kcal) | 0.09±0.02a | 0.05±0.01b | 0.08±0.01a | < 0.001 |
Values expressed as mean and standard deviation, analysis of variance complemented by Tukey's test.
a,b,c Different letters in the same line represent statistically significant differences in the comparison between groups (p < 0.05).
At the end of the experiment, the animals from the groups fed soy-based beverage and soy-based formula had significantly lower weight gain when compared to animals fed cow's milk-based formula. The feed efficiency (g/kcal) of soy formula was lower than the other two groups. Feed efficiency (g/mL) was highest in the cow's milk-based formula group.
Fig. 1 shows fecal weight and fecal moisture. The fecal weight of animals fed soy-based beverage (3.44±0.48g) and soy-based formula (0.79±0.20g) were higher than that in the group fed cow's milk-based formula (0.42±0.17g). The fecal weight with soy-based beverage was higher than with soy-based formula (p=<0.001). Regarding fecal moisture, the groups fed soy-based beverage (47.28±9.02%) and soy-based formula (37.21±13.20%) had higher moisture content in the stool than the group fed cow's milk-based formula (22.71±10.86%), with a statistically significant difference (p<0.001; Fig. 1).
DiscussionThe present study showed that animals fed soy protein grew less than those fed cow's milk-based formula. High sodium content was also found in the soy-based beverage. Animals fed soy protein also had higher fecal weight and moisture, especially those fed soy-based beverage.
The experimental model used allows for the evaluation of the effects of a single liquid food, similarly to what occurs in infant feeding before the introduction of complementary foods. In this study, the control group was fed lactose-free cow's milk-based formula, considering that previous studies showed that weanling rats fed formula with lactose had a greater number of loose stools, suggesting lactose intolerance.17,19 Thus, a product that did not contain lactose was used as reference, as with the soy-based beverage and soy-based formula.
This is the first study that compared a soy-based beverage to soy-based formula and cow's milk-based formula for a continuous period that was sufficient to allow for the identification of differences in weight and length. It should be also noted that, throughout the experiment, the animals from the three groups did not receive food other than the liquid diet.
The difference in the voluntary food intake of the rats may be attributed to the different composition of the diets, probably the characteristic taste of soy extract and soy protein or the smell of the milk, thereby making it more acceptable.
Attention should be paid to the significant difference in weight gain and length, with lower values in the groups receiving the soy-based beverage and soy-based formula. This result could be explained by the nutritional composition of the soy-based beverage, which has lower energy content when compared to the other two diets used. Other differences regarding the composition of the soy-based formula such as carbohydrates (lower content), protein (higher content), and amino acids (e.g., relatively low content of methionine, lysine, and proline, and higher content of arginine, cysteine, glycine, and asparagine) when compared to cow's milk-based formula could also have contributed to this finding. Soy-based formula contains anti-nutritional factors that could affect nutrient availability.11
A surprising result was that the soy-based beverage, although it has lower energy density when compared to soy-based formula, resulted in similar growth, which could be explained by the higher feed efficiency in terms of grams of weight gain per kilocalorie consumed. This study found no clear explanation in the literature for this result, considering the composition of the two foods. It is worth mentioning that although differences were observed in growth among the three groups, the animals in this study showed weight gain and body length similar to that shown by rats of the same age fed a conventional diet, demonstrating that the experimental model did not affect growth in the animals fed a liquid diet.20
The higher sodium intake (p<0.001) by the rats that received the soy-based beverage should be attributed exclusively to the higher sodium content in this group's diet. It should be emphasized that the sodium content found in soy-based beverages was higher than that specified in the product labeling. With regard to the formula, the sodium content was close to the expected value. It should be noted that the sodium content in the diet can contribute to the development of eating habits with a preference for salty foods, and also is associated with higher blood pressure levels in childhood and adulthood.21,22 This study demonstrated that, although there was no statistically significant difference, the group fed soy-based beverage consumed almost twice the amount of water when compared to the group fed cow's milk-based formula. Similar results were obtained with the soy-based formula. This fact may be related to the higher sodium content in the soy-based beverage and soy-based formula.
It must be recalled that the soy-based formula was developed to meet the nutritional needs of the full-term newborn, and several studies in humans have shown that it promotes growth and development similar to those of infants fed cow's milk-based formula, but it shows no advantage over cow's milk-based nutritional formulas and contains high levels of phytate, aluminum, and phytoestrogens (isoflavones), which can result in undesirable effects. Among the indications for soy-based formula use are persistent lactose intolerance, galactosemia, and ethical considerations (e.g., vegetarian diet).8–10 It is noteworthy that soy-based formulas do not have a role in the prevention of allergic diseases and should not be used in children with food allergy during the first six months of life.8
In turn, based on their composition and the results observed in this study, including the sodium content, soy-based beverages should not be used in infant feeding. However, according to a study7 evaluating the knowledge of pediatricians and nutritionists on a diet excluding cow's milk and dairy products, with emphasis on issues related to child nutrition, it was shown that soy-based beverages or juices were considered by many professionals as possible substitutes for cow's milk protein.
The market offers a number of such low-cost products, compared to soy protein-based formulas, which could erroneously encourage their use in the diet of infants allergic to cow's milk proteins, especially when there is no access to adequate alternative formulas. In this context, it should be noted that most of these products are not fortified and/or specifically formulated for the age group of infants, which may cause insufficient intake of nutrients, especially calcium.23,24
Another interesting result was the higher fecal weight and moisture observed in animals fed soy-based beverage. As previously observed, it was found that fecal weight and moisture depend on the fiber content in feed.25 In this study, the three groups were fed diets that, according to information from the manufacturer and the results obtained in laboratory tests, do not contain dietary fiber. Thus, it was not possible to identify what component in the soy extract would be responsible for higher fecal weight and moisture. No explanation was found in the literature for this finding. Considering the potential use of soy extract in the prevention and treatment of constipation, this aspect should be investigated in further studies.
Finally, it should be emphasized that there are no publications similar to this study in the literature, which prevents direct comparison of results. Another limitation refers to the difficulty in extrapolating the results obtained to humans. However, the importance of experimental models with animals, despite their limitations, cannot be disregarded in situations where experiments with humans are impermissible from an ethical standpoint.
In summary, the present study observed less body growth (weight and length) in mice fed soy protein-based beverages and soy protein-based infant formula when compared to cow's milk protein infant formula without lactose, results that corroborate the recommendation not to use soy protein-based beverages in infant feeding. There was also a significant increase in fecal weight and moisture in rats fed soy protein-based beverages, when compared to those fed soy protein-based formula and cow's milk-based formula.
FundingThis study received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES. CAPES had no role in the study design, data analysis, or drafting of this manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Silva MdL, Speridião PdG, Marciano R, Amâncio OM, de Morais TB, de Morais MB. Effects of soy beverage and soy-based formula on growth, weight, and fecal moisture: experimental study in rats. J Pediatr (Rio J). 2015;91:306–12.
Study conducted at Research Laboratory of the Department of Pediatrics, Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.