The objective of this meta-analysis was to study the diagnostic value of lung ultrasound (LUS) for transient tachypnea of the newborn (TTN).
MethodsEmbase, Cochrane Library, PubMed, Web of Science, and Google Scholar were searched, and the last search date was October 31, 2020. Studies on the diagnostic accuracy of pulmonary ultrasound for transient tachypnea were included. The quality assessment of the included study was assessed using the Diagnostic Accuracy Studies-2 tool. A meta-analysis was performed using Meta-Disc 1.4. A random-effects model was used and subgroup analysis was carried out to identify possible sources of heterogeneity.
ResultsA total of 378 articles were retrieved and nine studies with 3239 patients were included in the present meta-analysis. The overall quality of the included studies was moderate to high. The result of threshold analysis shows that there was no threshold effect. However, there was a significant heterogeneity caused by non-threshold effects in the included studies. A random-effects model was used. The pooled sensitivity, specificity, PLR and NLR were 0.55 (95% CI: 0.51-0.58), 0.98 (95% CI: 0.98-0.99), 58.30 (95% CI: 14.05-241.88) and 0.28 (95% CI: 0.18-0.43). The pooled DOR and AUC were 689.12 (95% CI: 68.71 to 6911.79) and 0.994. The results of subgroup analysis showed that the LUS diagnostic criteria and gold standard might be responsible for heterogeneity. Choosing "DLP combined with B line" as the diagnostic standard of LUS and choosing CXR as the gold standard could significantly improve the diagnostic performance of LUS.
ConclusionLUS is a promising method to diagnose TTN. Only DLP is not enough to diagnose TTN, while DLP combined with B-line has good diagnostic performance.
Transient tachypnea of the newborn (TTN) is a self-limited respiratory disease caused by delayed fluid clearance from the fetal lungs.1 It is the most common cause of dyspnea in newborns, with an incidence of 4-5.7% in full-term newborns and 10% in premature infants.2 Although symptoms of TTN are generally transient and usually improve within 24 to 48 h, the dyspnea it causes can be severe. Clinically, the diagnosis of TTN is mainly used to differentiate other causes of dyspnea, such as respiratory distress syndrome (RDS), pneumonia, and meconium aspiration syndrome (MAS). Generally, the diagnosis of TTN is mainly based on the medical history, clinical manifestations and chest X-ray (CXR) examination. In recent years, the application of lung ultrasound (LUS) in the diagnosis and differentiation of pulmonary diseases in neonates, children, and adults has been rapidly increasing.3-5 Ultrasound examination of the lung is different from other organs in that it combines the interpretation of real anatomical images with the interpretation of artifacts generated by ultrasound beam at the air/fluid interface.6 In 2007, Copetti et al.7 first used LUS to diagnose TTN and proposed a "double lung point (DLP)" to describe the sharp limit of echogenicity between the lower lung fields with a hyperechoic, thin pleural line and compact B-line, and the normal or near-normal upper lung areas. However, the value of DLP in diagnosing TTN varies greatly in published studies2,7 (sensitivity range 100–45.6% and specificity range 100–94.8%). In addition, the ultrasonic image of TTN is also variable, such as the “prevalence of A-lines” in the initial stage of the disease and the “white lung” in severe cases. These differences may cause confusion for physicians and hinder the application of LUS in diagnosing and differentiating TTN. The objective of this systematic review and meta-analysis was to study the diagnostic value of LUS for TTN.
MethodsSearch strategyThis meta-analysis followed PRISMA guidelines. The authors searched five databases, including Embase, Cochrane Library, PubMed, Web of Science, and Google Scholar, and the last search date was October 31, 2020. Studies published in English languages were included. The search strategy using the following text words: ‘Transient Tachypnoea’ or ‘wet lung’ or ‘TTN’, and ‘ultrasound’ OR ‘Ultrasonography’ OR ‘ultrasonographies’ OR ‘ultrasonic’ OR ‘ultra sound’ OR ‘ultra-shell’ OR ‘sonography’ OR ‘LUS’ OR ‘sonographies’ OR ‘monofixation’ OR ‘ultrasonographies’ OR ‘echography’ OR ‘echographies’ OR ‘sonogram’ OR ‘echogram’ OR ‘echoscopy’ (When retrieving PubMed, the authors used MeSH terms for these words).
Inclusion and exclusion criteriaThe inclusion criteria were as follows: (1) diagnostic accuracy studies, cohort, and case-control studies were included; (2) studies that evaluated the diagnostic accuracy of LUS for TTN; (3) TTN was clearly diagnosed; (3) the study subjects were newborns, including term and preterm infants; (4) the extracted data can be used to calculate true-positive, false-positive, false-negative, and true-negative values;
The exclusion criteria were as follows: (1) basic experiments, case reports, reviews, conference abstracts, and letters to journal editors were excluded; (2) studies published in non-English languages; (3) not enough data to form 2∗2 tables.
Data extraction and methodological quality evaluationThe authors (YQ and CP) independently perform the search as described above and screen the titles and abstracts to select relevant studies. Selected articles were evaluated by two independent authors (YQ and HW) according to the inclusion and exclusion criteria. The authors (YQ and XL) independently extracted data, including first author, year of publication, study design, sample size, inclusion criteria, exclusion criteria, diagnostic criteria, and key results from the included studies. If more than one LUS diagnoses exist in a study, the result of diagnosis associated with DLP was used. The results of the conflict were resolved through discussion and consensus. The methodological quality of the included study was assessed independently by the two authors (YQ and HW) using the diagnostic Accuracy Studies-2 (QUADAS-2), and difference was resolved by discussion and consensus. As an evidence-based quality assessment tool, QUADAS-28 consisted of four domains: patient selection, index test, reference standard, and flow and timing. The quality assessment of the included study was performed using RevMan 5.3 (Cochrane Collaboration).
Statistical analysisSpearman correlation coefficient was measured to assess the potential threshold effect. If p > 0.05, heterogeneity was considered to be caused by the non-threshold effect. The p-value of Cochran-Q statistic and I-square statistic were used to assess the heterogeneity. If p > 0.05 or I2 ≤ 50%, a fixed-effect model was used to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR)with 95% confidence intervals. If p < 0.05 or I2 >50%, a random-effects model was used. The value of diagnostic tests was assessed by constructing a summary receiver operator characteristic (SROC) and calculating the area under the curve (AUC). All statistical analysis was performed using Meta-Disc 1.4 (Cochrane Colloquium, Barcelona, Spain).
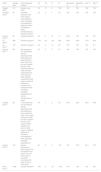
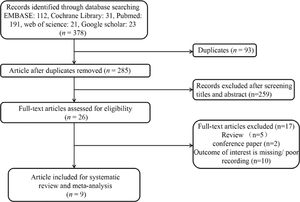
ResultsCharacteristics of the included studiesThe flow diagram of the study selection process is shown in Fig. 1. A total of 378 articles (EMBASE: 112, Cochrane Library: 31, PubMed: 191, web of science: 21, Google scholar: 23) were retrieved, and nine studies2,7,9-15 with 3239 patients were included in the present meta-analysis. The characteristics of included studies were shown in Table 1. Among the nine included studies, 2 were case-control studies, and 7 were cohort studies. Only one study included premature, while others included both premature and full-term infants. Two studies used CXR diagnosis as the gold standard, and seven studies combined CXR diagnosis and clinical history as the gold standard. There were two main LUS diagnostic criteria used in the included studies, including DLP and DLP combined with B-line. The accuracy of included studies of LUS in diagnosing TTN is shown in Table 2.
Characteristics of included studies.
| Study | Sample size (n) | Study type | Gestational age | Inclusion criteria | Exclusion criteria | LUS operator | LUS equipment, time | LUS diagnostic criteria | Blinding of test result interpretation | TTN diagnostic criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Copetti 20077 | 137 | Case-control study | premature | TTN patients and non-TTN patients | None | A pediatrician and a cardiologist skilled in lung and heart sonography | A high-resolution 10-MHz linear probe (Megas CVX Esaote Medical Systems, Florence, Italy)and a sector 5- to 7.5-MHz probe, first hour of life | Double Lung Point | Yes | CXR diagnosis |
| Grimaldi 20199 | 52 | Prospectivecohort | newborns | Newborns who needed a CXR because of respiratory conditions occurring at birth or during the first 24 h of life, and could perform a TUS less than 3 h before or after the CXR. | Any treatment or event susceptible of changing the chest imaging between TUS and CXR, insufficient quality of the CXR or TUS, and the absence of parents’ authorization for their child's participation | Six senior neonatologists trained for at least 2 weeks by an experienced senior radiologist. | A Philips1 HD100 device and one linear 5- to 12-MHz transducer, less than 3 h before or after the CXR | Interstitial syndrome with either diffuse noncompact B-lines or gradient of echogenicity between inferior and superior areas corresponding to double lung point | Yes | Presenting with mild or moderate respiratory distress starting immediately after birth, no significant cyanosis, clinical improvement within 24–72 h, mild hyperinflation on CXR with perihilar interstitial syndrome, sometimes with pleural effusion or fluid in the fissures. |
| Ibrahim 201810 | 65 | Prospectivecohort | newborns | Near and full-term neonates | Neonates presented with chest deformity, multiple congenital anomalies or gestational age less than 35 weeks | One single expert | A high-resolution linear transducer with a frequency of 7–12 MHz (Philips HD7) , within the first 24 h of admission | Double lung point | Yes | Clinical signs of respiratory distress, persistence of tachypnea for at least 12 h, chest X-ray (CXR) consistent with TTN and absence of any other cause of RD. |
| Liu 20162 | 886 | Retrospective cohort | newborns | Newborn who underwent lung ultrasonography | Patients without lung diseases | One doctor | GE Voluson E6,E8 and Logiq C9 ultrasound equipment was used. The frequency of the linear array probe was 10-14 MHz.At admission | Double lung point | Yes | Typical clinical symptoms, chest x-ray findings and exclusion and vigilance other reasons for respiratory distress. |
| Liu 201411 | 120 | Case control study | newborns | Newborns with TTN and newborns with RDS /no lung disease | None | An expert | GE Volusioni or Volusion E8 (GE Medical Systems, Milwaukee, USA) ultrasound instruments and a linear array probe with a frequency of 9.0–12.0 MHz | Double lung point | unknow | Based on medical history, clinical manifestations, arterial blood gas analysis, and CXR examination. |
| Rachuri 201712 | 94 | Prospectivecohort | newborns | Neonates who underwent x-ray chest and ultrasound (PoC-USG) within 4 h of admission to NICU and the age was less than 24 h after birth. | Neonates born with major congenital malformations or hydrops | The research associate | Philips machine using a linear probe of frequency 10–12 MHz. Ultrasound chest and chest x-ray (CXR) were done within 4 h after admission and within a maximum gap of not more than 4 h between them. | Normal pleural line and pleural sliding;Associated with the presence of predominant B-lines in the inferior pulmonary fields and less compact B-lines in the superior fields (double lung point)in both lungs, or bilateral presence of numerous noncompact B-lines indicating interstitial engorgement or Normal echogenicity of lungs | Yes | Combination of radiological and clinical criteria;Radiological features of prominent peri-hilar vascular markings, edema of the inter-lobar septae, fluid in the fissures, and hyperinflation. Respiratory distress onset at birth and progressively decreasing with time. |
| Vergine 201413 | 59 | Prospectivecohort | newborns | Neonates with respiratory distress that started within the first 24 h after birth | Patients with a diagnosis of a major congenital malformation, structural heart disease, or chromosomal diseases/syndromes | A trained neonatologist and external referee | LUS was done with Vivid-i (GE Medical Systems, Milan, Italy) using a high-resolution 10–12 MHz linear probe, with a dedicated preset. Within 1 h after admission | A normal pleural line and pleural sliding, associated with the presence of very compact B-lines in the inferior pulmonary fields and less compact B-lines in the superior fields (double lung point) in both lungs, or bilateral presence of numerous noncompact B-lines indicating interstitial engorgement. | Yes | TTN was diagnosed when the oxygen requirements and respiratory support were mild or moderate, the clinical condition improved within the first 72–96 h after birth, and CXR (if done) appearance was consistent. |
| Corsini 201914 | 134 | Prospectivecohort | newborns | Infants ≥23 weeks of gestational age and had respiratory distress requiring CXR in the first 24 h of life. | Lack of parental consent or necessity of cardiopulmonary resuscitation. | Neonatologist trained in ultrasound | A Philips CX50 ultrasound machine (Philips, Eindhoven, The Netherlands) using a high-frequency (10–12 MHz) linear transducer. | Normal, thickened, or blurry pleural line, and double lung point or numerous noncompact B-lines | Yes | CXR diagnosis |
| Chen 201715 | 1692 | Prospectivecohort | newborns | Infants with pulmonary disease | Examination time of cases> 48 h after admission | A senior neonatal physician proficient inLUS | A high-frequency linear 10- to 14-MHz probes (GEVoluson E6 or E8 and Logiq C9 ultrasound equipment. Within 48 h after admission | double lung point | unknow | Diagnoses were based on medical history, clinical manifestation, laboratory examination, and signs on chest radiography (CR) and/or computed tomography (CT). |
The accuracy of included studies of LUS in diagnosing TTN.
| Study | Sample Size | LUS Diagnostic Criteria | Tp | Fp | Tn | Fn | Sensitivity % | Specificity % | Ppv % | Npv % |
|---|---|---|---|---|---|---|---|---|---|---|
| Copetti 20077 | 137 | Double Lung Point | 32 | 0 | 0 | 105 | 100 | 100 | 100 | 100 |
| Grimaldi 20199 | 52 | Interstitial syndrome with either diffuse noncompact B-lines or gradient of echogenicity between inferior and superior areas corresponding to double lung point | 22 | 0 | 0 | 30 | 100 | 100 | 100 | 100 |
| Ibrahim 201810 | 65 | Double lung point | 33 | 0 | 15 | 17 | 68.8 | 100 | 100 | 53.1 |
| Liu 20162 | 886 | Double lung point | 104 | 34 | 124 | 624 | 45.6 | 94.8 | 75.4 | 83.4 |
| Liu 201411 | 120 | Double lung point | 46 | 0 | 14 | 60 | 76.7 | 100 | 100 | 81.1 |
| Rachuri 201712 | 94 | Normal pleural line and pleural sliding; Associated with the presence of predominant B-lines, which can be very compact B-lines in the inferior pulmonary fields and less compact B-lines in the superior fields (double lung point)in both lungs, or bilateral presence of numerous non-compact B-lines indicating interstitial engorgement or normal echogenicity of lungs | 33 | 0 | 0 | 61 | 100 | 100 | 100 | 100 |
| Vergine 201413 | 59 | A normal pleural line and pleural sliding, associated with the presence of very compact B-lines in the inferior pulmonary fields and less compact B-lines in the superior fields (double lung point) in both lungs, or bilateral presence of numerous noncompact B-lines indicating interstitial engorgement. | 28 | 1 | 2 | 28 | 93.3 | 95.5 | 96.5 | 93.4 |
| Corsini 201914 | 134 | Normal, thickened, or blurry pleural line, and double lung point (in one or both lungs) or numerous noncompact B-lines (in one or both lungs) | 32 | 2 | 0 | 100 | 100 | 97.8 | 94.1 | 100 |
| Chen 201715 | 1692 | Double lung point | 109 | 0 | 211 | 1372 | 34.1 | 100 | 100 | 86.7 |
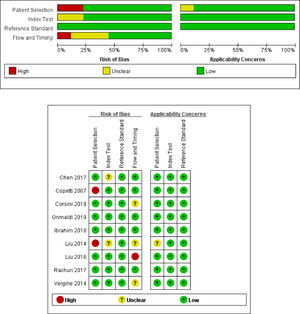
The result of methodological quality assessment (bias risk and applicability) is shown in Fig. 2. Except for 2 case-control studies,7,11 the other 7 were cohort studies, and patients were continuously included. All studies have predefined criteria for LUS to diagnose TTN. The gold standard for TTN was defined as CXR or CXR combined with clinical history in all studies. Seven studies interpreted the LUS results without knowing the results of the gold standard, and two studies11,15 did not have detailed information on the use of blinding methods. Seven studies provided the time of LUS diagnosis, and two studies did not elaborate.11,14 The overall quality of the included studies was moderate to high.
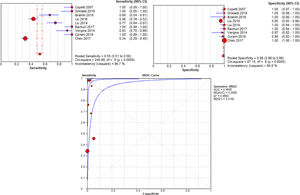
Primary outcomeThe authors performed a meta-analysis of the included studies, and the diagnostic performance was shown in Fig. 3. The result of threshold analysis shows that there was no threshold effect (Spearman correlation coefficient = -0.201, p = 0.604). However, there was a significant heterogeneity caused by non-threshold effects in the included studies. The I2 value of sensitivity, specificity, PLR, and NLR were 96.7%, 90.8%, 83.4%, and 92.3%. As significant heterogeneity was observed, a random-effects model was used. The pooled sensitivity, specificity, PLR and NLR were 0.55 (95% CI: 0.51-0.58), 0.98 (95% CI: 0.98-0.99), 58.30 (95% CI: 14.05-241.88) and 0.28 (95% CI: 0.18-0.43). The pooled DOR and AUC were 689.12 (95% CI: 68.71 to 6911.79) and 0.994.
Subgroup analysisThe subgroup analysis was used to identify possible sources of heterogeneity (Table 3). The results of subgroup analysis showed that the LUS diagnostic criteria and gold standard might be responsible for heterogeneity. In addition, the type and subject of the study had less influence on the diagnostic performance of LUS, while choosing "DLP combined with B line" as the diagnostic standard of LUS and choosing CXR as the gold standard could significantly improve the diagnostic performance of LUS.
Subgroup analysis of diagnostic effect.
Ultrasound is increasingly being used in the NICUs. However, the use of LUS in NICUs is more delayed, and the knowledge acquired is still far from that of adult intensive care units.16 Besides, there is a lack of evidence-based international recommendations for an emergency, and critical care settings, and the diagnostic value of LUS for a neonatal pulmonary disease is still controversial.17 In this meta-analysis, the authors evaluated the performance of LUS in diagnosing TTN.
The included studies used two criteria to evaluate the diagnostic performance of LUS for TTN. Most studies used DLP, followed by DLP in combination with B lines. Although there are differences between the criteria, the principles are similar. The results of a meta-analysis of all included studies showed that the sensitivity and specificity were 55% and 98%. Zhang et al. reported that DLP might also be present in pneumothorax patients.18 However, DLP is a rare sign of pneumothorax.19 The present study shows that the specificity of DLP in diagnosing TTN is high, but the sensitivity is not satisfactory.
The results of subgroup analysis showed that this low sensitivity was mainly due to the use of only DLP as diagnostic criteria. DLP used to be a specific and sensitive sign to diagnose TTN7. However, it has been challenged by some studies. Chen et al.15 reported that double lung points were observed in 109 of 320 TTN patients. Raimondi et al.6 found DLP may disappear during the course of TTN. Liu et al.2 reported in a large sample study that the sensitivity of DLP to diagnose TTN was only 45.6%. In the present study, the pooled sensitivity and specificity for DLP were 47% and 98%. Therefore, although DLP is a sign of TTN, it is insufficient for the diagnosis. The combination of DPL and B-line greatly improved the diagnostic value, with a pooled sensitivity of 98% and a pooled specificity of 99%. This combination fits well with the pathophysiology of TTN since TTN is mainly an ab extrinsic edema and B-line is the main manifestation of pulmonary edema.20,21 In addition, line B is also a manifestation of interstitial engorgement, which may be caused by edem.22 Therefore, the B-line could exist much longer in the course of TTN than DLP, which makes up for the disadvantage of the low sensitivity of DLP. The meta-analysis shows that LUS is a promising diagnostic method for TTN. The combination of DLP and B-line may be a better diagnostic criterion than only DLP. It is worth noting that this combination is also slightly different in the included studies, and prospective multicenter studies are still needed to determine the best diagnosis.
In addition, the subgroup analysis also showed that the selection of the gold standard had a significant influence on the diagnostic performance of LUS. The studies included used two gold criteria to diagnose TTN, including CXR diagnosis and CXR combined with clinical history. The pooled sensitivity and specificity for CXR diagnosis were 100% and 99%. This suggests that the diagnosis of LUS may be highly consistent with that of CXR. Similar results have been reported in previous studies. Based on three years of clinical experience, Gao et al.23 reported that LUS could completely replace CXR in the diagnosis and differentiation of pulmonary diseases in NICU. Liu24 also believed that LUS is more suitable than CXR for use in NICU. Since the advantages of no radiation, non-invasion, and simplicity of LUS,25,26 the present study's results suggest that LUS may be a reasonable alternative to CXR in diagnosing neonatal TTN.
There were two meta-analyses evaluating the performance of LUS in diagnosing TTN, and the high accuracy of LUS in diagnosing TTN was proved.27,28 The present meta-analysis further summarized the existing problems of LUS in TTN diagnosis and compared the diagnostic performance of two commonly used LUS diagnostic criteria, providing a direction for future research. Furthermore, QUADAS-2 was used to assess the quality of included studies, making diagnostic bias and suitability scores more transparent. Since fewer included studies may lower the value of the funnel plot, no funnel plot was drawn to assess selection bias. There are some limitations in the present study: (1) Although DLP combined with B-line has a good diagnostic performance, diagnostic criteria vary slightly from studies, and more prospective large sample studies are still needed to determine the optimal diagnostic criteria for LUS; (2) The LUS profile varies in technique and interpretation, and also depends on the probe used. (3) Six of the nine included studies used only one LUS operator, which may have increased the bias of the results.
In conclusion, LUS is a promising method to diagnose TTN. Only DLP is not enough to diagnose TTN, while DLP combined with B-line has good diagnostic performance. More prospective large sample studies are still needed to determine the optimal criteria for LUS to diagnose TTN.
Availability of data and materialsThis is a meta-analysis and all data are included in the manuscript.