Periventricular-intraventricular hemorrhage is the most common type of intracranial bleeding in newborns, especially in the first 3 days after birth. Severe periventricular-intraventricular hemorrhage is considered a progression from mild periventricular-intraventricular hemorrhage and is often closely associated with severe neurological sequelae. However, no specific indicators are available to predict the progression from mild to severe periventricular-intraventricular in early admission. This study aims to establish an early diagnostic prediction model for severe PIVH.
MethodThis study was a retrospective cohort study with data collected from the MIMIC-III (v1.4) database. Laboratory and clinical data collected within the first 24 h of NICU admission have been used as variables for both univariate and multivariate logistic regression analyses to construct a nomogram-based early prediction model for severe periventricular-intraventricular hemorrhage and subsequently validated.
ResultsA predictive model was established and represented by a nomogram, it comprised three variables: output, lowest platelet count and use of vasoactive drugs within 24 h of NICU admission. The model's predictive performance showed by the calculated area under the curve was 0.792, indicating good discriminatory power. The calibration plot demonstrated good calibration between observed and predicted outcomes, and the Hosmer-Lemeshow test showed high consistency (p = 0.990). Internal validation showed the calculated area under a curve of 0.788.
ConclusionsThis severe PIVH predictive model, established by three easily obtainable indicators within the NICU, demonstrated good predictive ability. It offered a more user-friendly and convenient option for neonatologists.
Periventricular-intraventricular hemorrhage (PIVH) is the most common type of neonatal intracranial hemorrhage, especially in preterm newborns, and it usually occurs at a gestational age of less than 32 weeks.1,2 The incidence of PIVH increases with the reduction of gestational age and birth weight.3 Over the past several years, with the advent of antenatal steroid administration and improvements in neonatal care, the increase in low birthweight newborns has kept the total incidence rate of intraventricular hemorrhage remains high.4 PIVH is a progressive disease that can be classified into grades I to IV based on the location and progression of the bleeding.5 Grades I and II are considered mild PIVH, and grades III and IV are considered severe PIVH. Studies have shown that the degree of neurological damage increases with the progression of PIVH, and therefore severe PIVH is regarded as an independent risk factor for poor neurological prognosis.6,7 Mild PIVH and severe PIVH have different prognoses, patients who progress to severe PIVH are more likely to have neurological sequelae, including hydrocephalus, cerebral palsy, periventricular leukomalacia, and cognitive deficits.8,9 These severe sequelae create enormous social and financial burdens.
PIVH usually occurs within 72 h after birth and lacks apparent clinical symptoms.10 Cranial ultrasonography (CUS) and Magnetic Resonance Imaging (MRI) are reliable tools for diagnosis and grading of PIVH.11,12 However, even with an early and definitive diagnosis, there is no clear method to predict whether a patient with mild PIVH will progress to severe PIVH in the future. As far as we know, there are few open predictive models for PIVH from mild to severe grade. Considering the time of PIVH onset and the simplicity of data collection, clinical data and laboratory indicators within the first 24 h of NICU are essential references for predicting PIVH development. This will enable early and personalized intervention and treatment for affected neonates, thereby reducing adverse long-term neurodevelopmental outcomes.
Currently, the potential for clinical application of diagnostic prediction models is continuously expanding, and this holds true for the prediction of severe PIVH in neonates. A nomogram is a novel type of visual model, which assigns a specific score to each variable and finally calculates the disease risk probability after accumulating the total score of all variables. It converts complex regression equations into visual graphs, making the results of the diagnostic prediction model more readable and useful, and is widely used to predict disease risk.
The present study aims to establish and validate a model presented by nomogram for the early identification of severe PIVH using conveniently measured factors within 24 h of NICU admission, thereby neonatologists in clinical practice can use it to assess whether mild PIVH might progress to severe PIVH at an early stage and help make treatment decisions.
MethodsDatabase introductionThe present study leverages data obtained from the MIMIC-III (v1.4) database(https://physionet.org/content/mimiciii/1.4/), an extensive, single-center and publicly accessible database managed by the MIT Computational Physiology Laboratory. The database encompassed a comprehensive collection of clinical information pertaining to patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) between 2001 and 2012, comprising a cohort of 7870 NICU individuals. In this database, detailed records regarding each patient's diagnoses, laboratory examinations, vital signs, and medication regimens were documented. This study aimed to analyze these indicators collected from the MIMIC-III to build a clinical predictive model for severe PIVH.
Population selection criteriaHospital admission information for patients was included according to the following criteria (1) patients who were diagnosed and graded with PIVH by head ultrasound according to International Classification of Diseases, 9th Revision (ICD - 9) code 772.1X: patients whose clinical and laboratory data were complete. Further, patients who meet the following criteria will be excluded from this study: patients with an age greater than 28 days upon admission to NICU; patients who were admitted to the NICU for the second time or more; patients with a duration of less than 48 h in the NICU.
Data extractionWithin 24 h of admission to the NICU, the following variables were extracted to avoid interference from subsequent treatments. In cases where a variable was measured multiple times within this 24-hour period, the maximum or minimum value was retained based on the characteristics of the variable and its impact on the patient's condition. The extracted variables included Demographics (Gender, Age, Ethnicity, Gestational Age, Weight), Vital Signs (Heart Rate, Mean Arterial Pressure, Temperature, Output), Interventions (Mode of Delivery, Mechanical Ventilation, Use of Vasoactive Drugs (Epinephrine, Dopamine, Dobutamine)), Early Hypoxia (Intrauterine Distress, Neonatal Asphyxia, Epileptic Seizure, Respiratory Pauses), Laboratory Indicators (Red Blood Cells, Hemoglobin, Hematocrit, Mean Corpuscular Volume, White Blood Cells, Platelets, PH, PaCO2, K+, Na+, Cl−).
Statistical analysisThe statistical analysis was performed by the SPSS version 25.0 (SPSS, Chicago, IL, United States) and R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Variables were analyzed by univariate logistic regression analysis, continuous variables were compared by T-test with normal distribution or Wilcoxon rank-sum test with non-normal distribution, and categorical variables were compared by Chi-square test or Fisher's test. Correspondingly, the results were presented as mean±standard deviation, medians with the 25th and 75th percentiles, cases, and percentages. Subsequently, a p-value < 0.05 was considered statistically significant, leading to the inclusion of the variables in a multivariate logistic regression analysis. Finally, the selected predictors were assigned scores based on the coefficients of multivariate logistic regression analysis, and the “rms” package of R software (https://CRAN.R-project.org/package=rms) was used to integrate all factors and draw a nomogram model.
ResultsBaseline data and clinical featuresThe baseline data compared with mild PIVH and severe PIVH neonatal patients was presented in Table 1. A total of 181 PIVH patients were included in this study. There were 73 (40.33%) females and 108 (59.67%) males among them. 94 (51.93%) whites were included, and the median age of all patients was 0.56 (0.35–0.82) days. The progression rate from mild PIVH to severe PIVH was 15.47%. Of particular interest were the basic demographic characteristics in the population, where severe PIVH patients had lower (p < 0.05) gestational age and birth weight compared to a mild PIVH group, there might be a linear relationship between these two variables. Additionally, concerning vital signs within the first 24 h of NICU admission, severe PIVH patients exhibited significantly lower (p < 0.05) minimum temperature, minimum mean arterial pressure, and urine output, as well as a higher rate (p < 0.01) vasoactive drug usage.
Baseline characteristics between mild PIVH and severe PIVH patients.
GA, gestational age; Tmin, minimum temperature within 24 h; HRmin, minimum heart rate within 24 h; MBPmin, minimum mean arterial pressure within 24 h.
Output, minimum PLT and the use of vasoactive drugs were independent risk factors for the progression from mild to severe PIVH.
Table 2 shows the univariate and multivariate analysis of variables by logistic regression analysis associated with severe PIVH in patients. Significant variables (p < 0.05) identified through univariate analysis were selected for further multivariable stepwise logistic regression analysis and results were considered statistically significant with p < 0.05 also. Final results demonstrated that output (0.98; 95% CI, 0.97–1.00; p = 0.021), minimum platelets (PLT) (0.99; 95% CI, 0.99–1.00; p = 0.015) and the use of vasoactive drugs (4.09; 95% CI, 1.65–10.12; p = 0.002) within the initial 24-hour period following admission to the NICU were independent risk factors for the incidence rate of severe PIVH.
Univariate and multivariate analysis of variables by logistic regression analysis.
GA, gestational age; Tmin, minimum temperature within 24 h; HRmin, minimum heart rate within 24 h; HRV, heart rate variability within 24 h; MBPmin, minimum mean arterial pressure within 24 h; MBPV, mean arterial pressure variability within 24 h; RBCmin, minimum red blood cell within 24 h; HBmin, minimum hemoglobin within 24 h; HCTmin, minimum hematocrit within 24 h; MCVmin, minimum mean corpuscular hemoglobin within 24 h; WBCmax, maximum white blood cell within 24 h; PLTmin, minimum platelets within 24 h; PHmin, minimum pH value within 24 h; PCO2max, maximum PCO2 value within 24 h; PCO2V, PCO2 value variability within 24 h.
In order to predict severe PIVH in all patients with PIVH, a nomogram was constructed by incorporating output, minimum PLT, and the use of vasoactive drugs. As shown in Figure 1(a), the nomogram not only consisted of the aforementioned three predictive factors and their corresponding scores but also included the total score and the corresponding risk of developing severe PIVH.
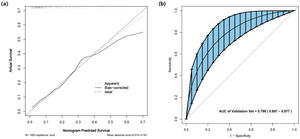
Firstly, the discrimination capacity of the nomogram was assessed by using the Receiver Operating Characteristic (ROC) curve with the Area Under the Curve (AUC) and the AUC value was 0.792 of this model, indicating a high accuracy compared with the other three independent risk factors shown in Figure 1(b). Secondly, the calibration plot was employed to assess the alignment between the actual probability and predicted probability of severe PIVH with 1000 bootstrap resampling, which showed good fitness with a p-value of 0.990 for the Hosmer-Lemeshow test and revealed a high level of concordance (Figure 2(a)). Finally, internal validation by bootstrap analysis based on 1000 Bootstrap samples was performed and results were similar with an AUC of 0.788 (Figure 2(b)).
In addition, to explore the predictive abilities of pairwise combinations of these three predictive factors compared with this model, three other combinations have been tested separately, and the results were that the AUC value of vasoactive drugs and PLT was 0.766 (Supplementary A), the AUC value of vasoactive drugs and output was 0.769 (Supplementary B), the AUC value of output and PLT was 0.758 (Supplementary C). Although all of them showed good predictive capacities, they were still inferior to the model.
DiscussionAlthough the pathogenesis of PIVH is multifactorial, it is likely that the intrinsic fragility of the germinal matrix (GM) capillary network and cerebral hemodynamic instability play a role.13,14 GM is most abundant in the fetal brain at 24 to 34 weeks of gestation, then it gradually decreases and almost disappears by 36 to 37 gestational weeks.14 The substance of GM is an immature capillary network consisting of only one layer of endothelial cells, unsupported by connective or muscular tissue, which is structurally fragile and less resistant to the impact of blood flow.15 In general, cerebral autoregulation is the ability of the blood vessels in the brain to maintain constant cerebral blood flow (CBF) despite fluctuations in blood pressure.16 Fluctuations in peripheral arterial blood pressure can be caused by intrauterine distress, hypoxia, seizures and apnoea in the perinatal period, as well as by vaginal delivery during labor, and by clinical stimuli such as the use of vasoactive drugs and mechanical ventilation. When peripheral arterial blood pressure rapidly changes, it can cause abrupt changes in cerebral hemodynamics and impaired regulation of CBF, as well as rupture and hemorrhage of the GM in the event of pressure-passive cerebral blood flow formation.17
Hypoxia is associated with an increase in carbon dioxide partial pressure, and cerebral blood vessels are sensitive to carbon dioxide, which will lead to cerebral vasodilatation, increase cerebral perfusion and cerebrovascular pressure, and cause vascular rupture and hemorrhage. Furthermore, GM is rich in mitochondria, its metabolism is vigorous, it is extremely sensitive to hypoxia, and prone to necrosis and brain hemorrhage.16 Mechanical ventilation may damage the brain by causing a localized inflammatory response and hemodynamic instability, which is a risk factor for PIVH. Studies in the literature show that cesarean section (CS) birth reduces the risk of PIVH, especially in extremely premature neonates, as well as studies that argue that the mode of delivery is not associated with the risk of PIVH.18,19 During spontaneous delivery, the birth canal squeezes the fetal head significantly, leading to the deformation of intracranial blood vessels under pressure, and changes in CBF autoregulation and hemodynamics, causing rupture of capillaries and small veins. Another study showed that vaginal delivery and emergency CS are independent risks for PIVH in premature neonates.20 The reduction in the incidence of PIVH in premature neonates by CS may be related to the need for mothers planning CS to receive prenatal medications, and these treatments significantly improved preterm outcomes.21 However, this present study showed no significant differences in hypoxia, mechanical ventilation, and mode of delivery, possibly because of the small sample size or some potential indirect association that needs to be further analyzed in future studies.
Vasoactive drugs may cause rapid changes to systemic blood pressure, thereby resulting in rapid fluctuations in CBF. In the present study, both univariate and multivariate regression analyses showed a significant effect of vasoactive drugs in predicting severe PIVH. Previous studies have shown that using vasoactive drugs in premature neonates can affect cerebral circulatory perfusion, thus contributing to the development of PIVH due to damage to and rupture of immature cerebral vessels.22 Another study confirmed an increased risk of severe PIVH development and higher mortality rates among neonates treated for hypotension.23 Moreover, brain injury was higher among newborns who received early inotropes compared to those not treated with inotropes.24 This might suggest that refraining from treatment of hypotension for premature neonates is safer than treatment with vasoactive drugs. Currently, permissive hypotension should be considered in premature neonates. A numerical blood pressure value below gestational age should not be used as the sole indicator for treating early-period hypotension in neonates. A comprehensive evaluation should be conducted by continuously monitoring blood pressure and considering tissue perfusion indicators, such as urine output, capillary refill time, lactate levels, and base excess.
The kidney plays an integral role in blood pressure regulation as well as cerebral perfusion and blood flow. The regulation of renal microcirculation probably best illustrates the crucial role of neonatal vasoactive balance and the risk of drug-induced disturbances.25 Urine output indirectly reflects the hemodynamic status of the organism and directly reflects the renal function and renal perfusion. A study indicated that newborns with severe PIVH exhibited significantly reduced urine output on the first day after birth compared to the control group, and this difference was statistically significant,26 which is consistent with the present study's findings. Reduced urine output may be associated with poor circulation in newborns, particularly in premature neonates. This circulatory impairment can further exacerbate hemodynamic abnormalities, affecting blood pressure and cerebral perfusion, placing additional strain on the delicate GM in premature neonates, leading to microvascular rupture and brain hemorrhage. Additionally, decreased urine output partially reflects renal dysfunction, which is also commonly observed among premature neonates. A multicenter retrospective study showed premature neonates with acute kidney injury have a 1.6-fold increased risk of PIVH compared to premature neonates without acute kidney injury. Of course, more comprehensive studies are needed to clarify the potential relationship between renal injury and PIVH.27
Another risk factor for severe PIVH identified in the present investigation is the lowest platelet count. A systematic review suggested that platelet counts < 100 * 109/L were associated with an increased risk of PIVH in premature neonates.28 In another retrospective cohort study,29 a lower platelet count or thrombocytopenia was significantly associated with increased risks of PIVH and mortality. An inherent fragility of the GM vasculature sets the ground for hemorrhage.14 If there are associated platelet or coagulation disorders, the homeostasis processes may be compromised, accentuating the hemorrhage. The functionally immature liver of premature neonates is unable to effectively carboxylate and activate vitamin K-dependent coagulation factors, thus impeding their coagulation activity. Additionally, premature neonates have lower platelet counts at birth and immature platelet function, which may compromise their ability to aggregate and adhere to the vessel wall. These factors contribute to the imperfect coagulation and hemostatic function in neonates, further increasing the risk of PIVH or exacerbating the progression of the condition. Therefore, it is important to be vigilant when a newborn presents with a low platelet count.
In summary, based on the vulnerability of the GM, the use of vasoactive drugs increases the risk of fluctuating cerebral blood flow in neonates. Meanwhile, decreased urine output indicates a certain degree of impairment in systemic blood perfusion, and reduced platelet count further indicates the presence or exacerbation of bleeding.
To the best of our knowledge, this is the first model that collected data within 24 h of NICU admission to analyze and predict the occurrence and development of severe PIVH. All the indicators included in the study analysis are common and easily obtainable in clinical practice, and the three independent factors also have received widespread attention in clinical work. The nomogram visually presents this prediction model, making the clinical use of this model more convenient and quicker. The internal validation analysis demonstrates great accuracy and discrimination, which means that this model is reliable.
However, this study also has several potential limitations. First, the authors acknowledge that the present study was only a retrospective analysis, which might lead to selection bias, and the omission of a small portion of data was unavoidable. Then, due to the limitations of MIMIC-III itself, and the differences in medical conditions or concepts in different eras, some laboratory data that the authors thought meaningful have not been collected or tested, such as coagulation function indicators that helped to judge the occurrence and development of PIVH, including APTT, PT, INR, etc., were not found in this database. In addition, the number of patients included in this study was relatively small, and only internal validation was performed in this study, but more external validation based on a larger sample size and multicenter could make the present results more recognized. Finally, because of the limitations of the MIMIC-III database, follow-up data were not available for these patients.
ConclusionOutput, minimum PLT, and the use of vasoactive drugs within 24 h of NICU admission are independent risk factors for the progression of severe PIVH. A severe PIVH predictive model, constructed by these three indicators demonstrated good predictive ability and is friendly for neonatologists to use.
Ethics approval and informed consentThe study was authorized by the institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center and obtained a waiver of informed consent. The authors were granted a certificate after application and completion of the course and test. All procedures of this study were conducted in accordance with the Declaration of Helsinki.
Availability of data and materialsData can be downloaded from the MIMIC-III database, https://mimic.physionet.org/iii/.
CRediT authorship contribution statementZhiyue Deng: Conceptualization, Data curation, Methodology, Software, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Jiaxin Tang: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing, Supervision. Chengzhi Fang: Conceptualization, Project administration, Supervision, Funding acquisition. Bing-Hong Zhang: Conceptualization, Project administration, Supervision, Funding acquisition.