Evaluate the effect of combinations of green banana biomass and laxatives in children and adolescents with chronic constipation.
MethodsThis was a randomized study of 80 children and adolescents with functional constipation according to the Rome IV Criteria, who were divided into five groups: (1) green banana biomass alone; (2) green banana biomass plus PEG 3350 with electrolytes; (3) green banana biomass plus sodium picosulfate; (4) PEG 3350 with electrolytes alone; and (5) sodium picosulfate alone. Primary outcome measure was the reduction of the proportion of patients with Bristol Stool Form Scale ratings 1 or 2. Secondary outcome measures were: increase of the proportion of >3 bowel movements/week and reduction of the proportion of fecal incontinence, straining on defecation, painful defecation, blood in stool, abdominal pain, and decreased laxative doses.
ResultsOn consumption of green banana biomass alone, a statistically significant reduction was observed in the proportion of children with Bristol Stool Form Scale rating 1 or 2, straining on defecation, painful defecation, and abdominal pain. Conversely, no reduction was observed in fecal incontinence episodes/week, blood in stool, and no increase was observed in the proportion of children with >3 bowel movements/week. The percentage of children who required decreased laxative dose was high when green banana biomass was associated with sodium picosulfate (87%), and PEG 3350 with electrolytes (63%). Green banana biomass alone and associated with laxatives was well tolerated, and no adverse effects were reported.
ConclusionGreen banana biomass is advantageous as an adjunct therapy on functional constipation, mainly for reducing doses of laxatives.
Avaliar o efeito das combinações da biomassa de banana verde e laxantes em crianças e adolescentes com constipação crônica.
MétodosEstudo randomizado de 80 crianças e adolescentes com constipação funcional de acordo com os Critérios de Roma IV divididos em cinco grupos: 1) Somente biomassa de banana verde; 2) Biomassa de banana verde mais PEG 3350 com eletrólitos; 3) Biomassa de banana verde mais picossulfato de sódio; 4) PEG 3350 somente com eletrólitos e 5) somente picossulfato de sódio. O desfecho primário foi a redução da proporção de pacientes com as classificações 1 ou 2da Escala de Bristol para Consistência de Fezes. Os desfechos secundários foram: aumento da produção de > 3 evacuações/semana e redução da proporção de incontinência fecal, esforço na defecação, defecação dolorosa, sangue nas fezes, dor abdominal e redução nas dose de laxantes.
ResultadosNo consumo somente de biomassa de banana verde há uma redução estatisticamente significativa na proporção de crianças com classificação 1 ou 2da Escala de Bristol para Consistência de Fezes, esforço na defecação, defecação dolorosa e dor abdominal. Por outro lado, não houve redução nos episódios fecais/semana de incontinência, sangue nas fezes e nenhum aumento na proporção de crianças com > 3 evacuações/semana. O percentual de crianças que tiveram sua dose de laxante reduzida foi alto quando a biomassa de banana verde foi associada a picossulfato de sódio (87%) e PEG 3350 com eletrólitos (63%). A biomassa de banana verde sozinha e associada a laxantes foi bem tolerada e não houve efeitos adversos relatados.
ConclusãoA biomassa de banana verde é vantajosa como uma terapia adjuvante na constipação funcional, principalmente na redução das doses de laxantes.
Functional constipation is a common problem worldwide and is among the most common complaints that bring a child to a pediatric gastroenterology clinic.1 A systematic review reported a mean and median prevalence in children of 14% and 12%, respectively.2 The treatment consists of disimpaction, maintenance with laxatives, and behavioral interventions. As low consumption of dietary fiber has been associated with an increased risk factor for functional constipation, a balanced diet with increased dietary fiber ingestion must be implemented.3
Dietary fiber from vegetables and fruits are very valued for constipation treatment. Green banana is known for its dietary fiber content, as well as for a solid concentration of amylase-resistant starch that is not digested or absorbed in the intestine, stimulating the colonic production of short-chain fatty acids and being useful in treating constipation. Indeed, resistant starch has attracted interest due to its positive effects in the human colon and implications for health.4,5 Accordingly, resistant starch could be considered a functional fiber by its indigestible nature and beneficial physiological effects.6
Banana is mainly produced in tropical and subtropical developing countries. Brazil is the second biggest producer worldwide, which makes banana an easily available and affordable fruit, making part of the Brazilian diet.7 The dietary fiber and the high quantity of resistant starch in green banana (approximately 74% of its composition) appear to contribute as a potential therapeutic agent for functional constipation treatment.8–11 Given the beneficial intestinal effects of dietary fiber in functional constipation treatment and the fact that green banana is a major source of resistant starch, the knowledge of therapeutic properties of resistant starch from green banana should be explored for human dietary applications. This study aimed to evaluate the effects of green banana biomass with different combinations of laxatives in the management of children and adolescents with functional constipation.
MethodsStudy design and participantsThis was a prospective, interventional, randomized clinical study of consecutively recruited cases of children and adolescents, referred from the Brazilian Unified Health System to the pediatric gastroenterology and pediatric surgery tertiary outpatient clinic of the Botucatu Medical School for the treatment of functional constipation. Direct contact was used to recruit participants, who received detailed explanations of the study. All children older than 12 years and all parents signed the informed consent form approved by the local ethical committee (Protocol 4349/2012). In order to obtain a homogeneous group, all patients eligible for the study met the inclusion criteria: toilet-trained children and adolescents, aged 5–15 years; functional constipation according to Rome IV Criteria12; rating types 1 or 2 of stool consistency assessed using the seven-point Bristol Stool Form Scale,13,14 and with no use of drugs except laxatives. Exclusion criteria were: genetic, neurological, behavioral, or cognitive problems.
Interventions and outcomesThe protocol was carried out at enrolment of initial one-week baseline period to establish current bowel habits and after eight weeks of treatment. During the study, all parents and patients were given information on functional constipation and Bristol Stool Form Scale was given by personal instructions and handouts. Children were instructed to try to defecate on the toilet at the same time of the day for 10–15min.
Socio-demographic, clinical, and laboratory data were collected on the day of the first visit using a standardized research protocol. Body weight and height were measured, and growth was determined using Z-scores according to WHO growth standards.
During the baseline period (before the start of supplementation with green banana biomass), and during the treatment period (eight consecutive weeks), each patient was monitored throughout a standardized week diary (stool frequency, stool consistency according to the Bristol Stool Form Scale, frequency of fecal incontinence episodes, painful defecation, abdominal pain, excretion of blood, laxative use and their doses, and adverse effects such as pain, nausea, vomiting, diarrhea, and flatus).
After the first seven days, patients returned to the clinic with the completed questionnaire and were randomized into one of five intervention groups: (1) green banana biomass alone; (2) green banana biomass plus PEG 3350 with electrolytes (GBB+PEGE); (3) green banana biomass plus sodium picosulfate (GBB+SP); (4) PEG 3350 with electrolytes (PEGE) alone; and (5) sodium picosulfate (SP) alone. Before the start of the study, all children were submitted to rectal disimpaction. Laxatives were prescribed according to the clinical guideline reported.1 The patients’ follow-up and clinical evaluation, as well as the assessment of the frequency of adverse effects and compliance with the study protocol, were carried out at routine clinic appointments every two weeks, and via weekly phone calls.
Definitions of clinical outcome measuresThe primary outcome measure was the reduction of the proportion of children and adolescents with stool consistency ratings 1 or 2 using Bristol Stool Form Scale after eight weeks of treatment schedule when compared with the baseline. Secondary outcome measures were the increase the proportion of children with >3 bowel movements/week, reduction in the proportion of fecal episodes/week of incontinence, and reduction in straining defecation and laxative use.
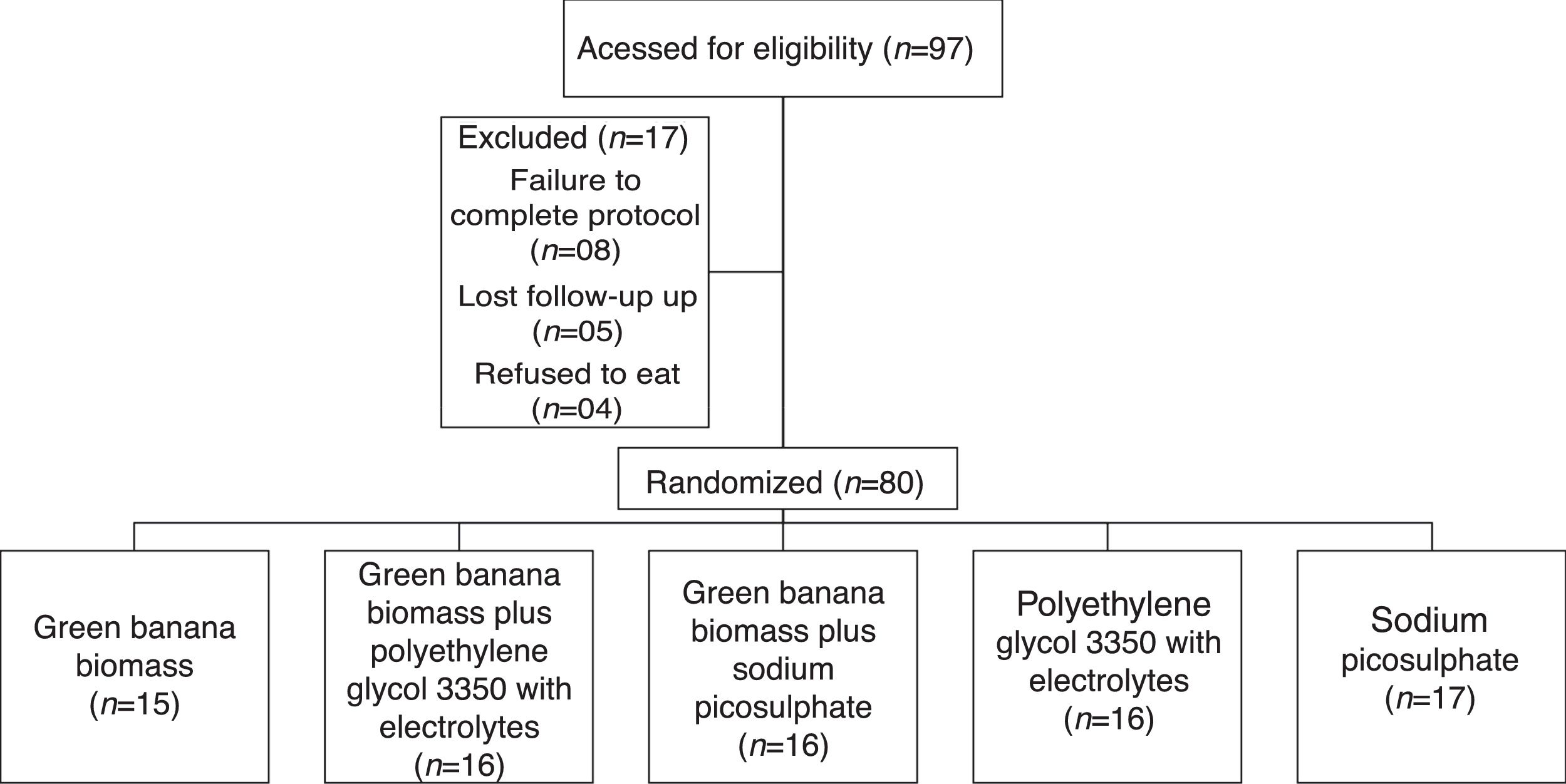
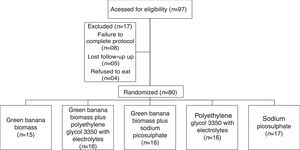
Sample size and randomizationThe sample size was estimated as 80 patients, with 16 subjects in each group, based on a reduction to 30% in the proportion of the primary outcome measure after the intervention, with a two-sided significance of 0.05 and a power of 0.8. These subjects were randomly assigned into five treatment groups by a mathematical algorithm generated using StatMate version 1.0 (GaphPad Software, Inc, San Diego, CA, USA), Random allocation sequence was generated by the second author (NCM) and all dietetic interventions by the first author (VMGC; Fig. 1).
Preparation, composition, and consumption of study dietGreen banana biomass was produced by a local grocery store, provided weekly, and free of charge. Commercial unripe green dwarf banana (Musa spp. AAA) was purchased from the local market. The biomass was prepared from the unpeeled banana. Fruits were washed and cooked in a pressure cooker with boiling water for 20min. A total of 50mL of water were added for each 200g of green banana. After that, the biomass was homogenized and stored at 12°C in a refrigerator. The green banana biomass looked identical as a thick, white, homogenous mass, and had no substantial variation in color, taste, and smell. A pilot study was previously conducted to assess children's acceptance to adding green banana biomass into juice, yogurt, milk, and soups with one, two, or three tablespoons per preparation. Good acceptance and initial positive results were achieved with two tablespoons. Because no studies have yet analyzed the optimal dosage of resistant starch, the authors adopted the dose of two tablespoons/day of green banana biomass (30g). During weeks two to nine, parents and children were advised (verbally and by a leaflet created by a research dietitian) to consume their usual diet and fluid intake. Adherence to green banana biomass intake was stimulated through the following measures: reinforcement of the importance of ingestion in the bi-weekly returns in clinical consultations; reinforcement of the importance of ingestion, in the weekly returns for the withdrawal of the biomass of the green banana; telephone contact, made by the author of the study, to reinforce the importance of ingestion; and mixing of green banana biomass in the child's favorite foods, considering the absence of odor and flavor of green banana biomass.
Random samples in green banana biomass were analyzed for resistant starch content at Centro de Raízes e Amidos Tropicais of Botucatu Agronomy School, in accordance with Goñi et al.15 (certificate number 035/2014), presenting a mean value of 7.8±0.2% of resistant starch and 4.4±0.5% of dietary fiber except for resistant starch.
Statistical methodsBaseline data were expressed as median and interquartile range (25–75), and mean and standard deviation, depending on the normality of the distribution for continuous variables using the Shapiro–Wilk normality test, and counts (n) and percentages (%) for qualitative variables. The difference in proportions of various variables between baseline and after eight weeks was analyzed with Fisher's exact test. All statistical tests were two-sided, and values of p<0.05 were considered statistically significant. Data management and analysis were performed using Graph Pad Prism version 5.0, 2005 (Graph Pad Software Inc. – San Diego, CA, USA).
ResultsThe diagram presents participant flow of 97 consecutive children and adolescents enrolled in the study. Therefore, 80 (82%) children and adolescent with functional constipation were randomly allocated into five groups, and all outcomes data of these children who completed the eight-week treatment period were collected for the final analysis.
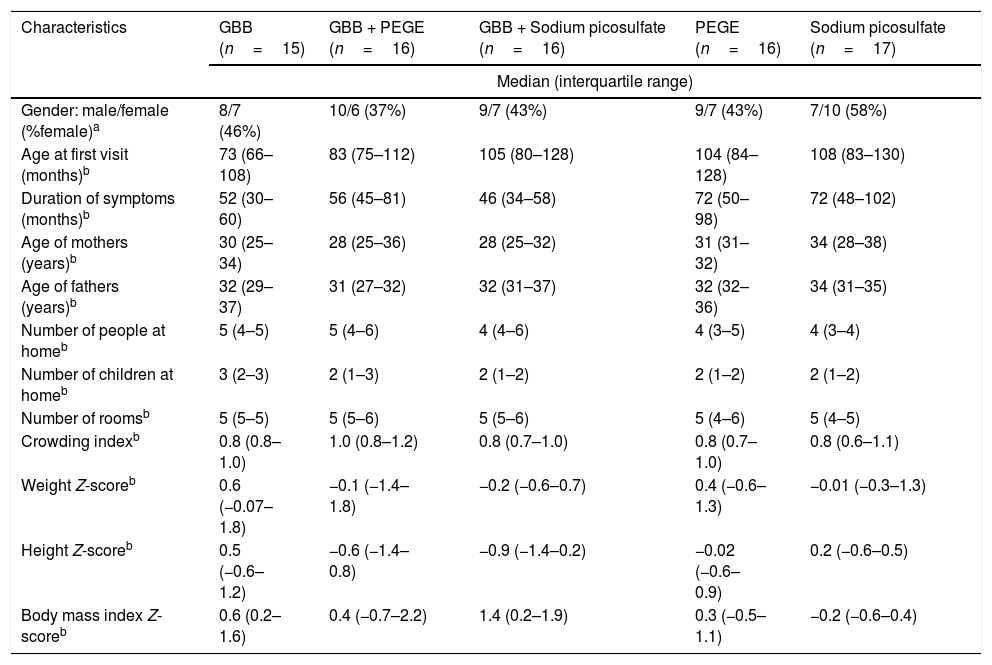
Baseline demographical, anthropometric, and symptoms characteristics of the five groups before trial are summarized in Table 1. No statistical differences were observed in all variables between groups. Long periods of constipation were observed in all children and adolescent.
Characteristics at baseline of all children with functional constipation included in the present study.
| Characteristics | GBB (n=15) | GBB + PEGE (n=16) | GBB + Sodium picosulfate (n=16) | PEGE (n=16) | Sodium picosulfate (n=17) |
|---|---|---|---|---|---|
| Median (interquartile range) | |||||
| Gender: male/female (%female)a | 8/7 (46%) | 10/6 (37%) | 9/7 (43%) | 9/7 (43%) | 7/10 (58%) |
| Age at first visit (months)b | 73 (66–108) | 83 (75–112) | 105 (80–128) | 104 (84–128) | 108 (83–130) |
| Duration of symptoms (months)b | 52 (30–60) | 56 (45–81) | 46 (34–58) | 72 (50–98) | 72 (48–102) |
| Age of mothers (years)b | 30 (25–34) | 28 (25–36) | 28 (25–32) | 31 (31–32) | 34 (28–38) |
| Age of fathers (years)b | 32 (29–37) | 31 (27–32) | 32 (31–37) | 32 (32–36) | 34 (31–35) |
| Number of people at homeb | 5 (4–5) | 5 (4–6) | 4 (4–6) | 4 (3–5) | 4 (3–4) |
| Number of children at homeb | 3 (2–3) | 2 (1–3) | 2 (1–2) | 2 (1–2) | 2 (1–2) |
| Number of roomsb | 5 (5–5) | 5 (5–6) | 5 (5–6) | 5 (4–6) | 5 (4–5) |
| Crowding indexb | 0.8 (0.8–1.0) | 1.0 (0.8–1.2) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 0.8 (0.6–1.1) |
| Weight Z-scoreb | 0.6 (−0.07–1.8) | −0.1 (−1.4–1.8) | −0.2 (−0.6–0.7) | 0.4 (−0.6–1.3) | −0.01 (−0.3–1.3) |
| Height Z-scoreb | 0.5 (−0.6–1.2) | −0.6 (−1.4–0.8) | −0.9 (−1.4–0.2) | −0.02 (−0.6–0.9) | 0.2 (−0.6–0.5) |
| Body mass index Z-scoreb | 0.6 (0.2–1.6) | 0.4 (−0.7–2.2) | 1.4 (0.2–1.9) | 0.3 (−0.5–1.1) | −0.2 (−0.6–0.4) |
GBB, green banana mass; PEGE, polyethylene glycol 3350 with electrolytes.
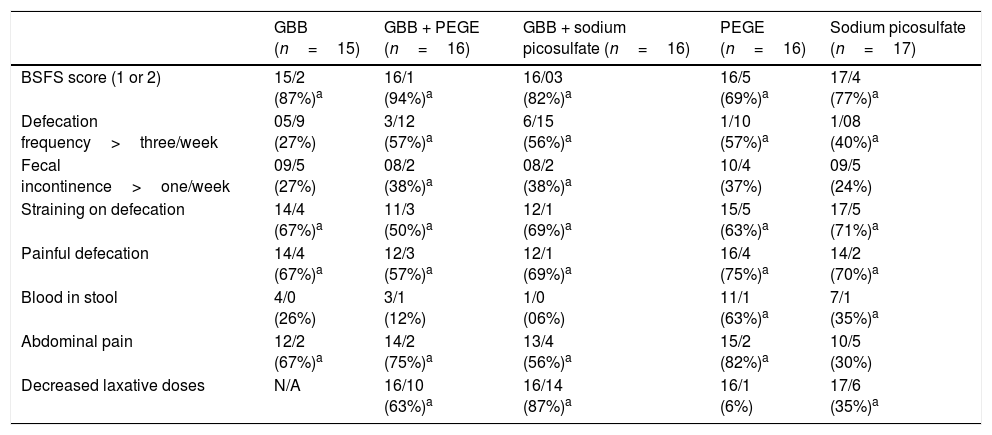
Table 2 summarizes the number and proportion (%) of children before and after intervention for all five groups. At the end of the intervention period, there was a statistically significant reduction in the proportion of children with Bristol Stool Form Scale ratings 1 or 2, straining on defecation, and painful defecation in all five groups. A statistically significant increased proportion of children and adolescent with >3 bowel movements/week was observed in four groups, except in the green banana biomass alone group. The proportion of children with abdominal pain was reduced in all groups, except in SP. There were a statistically significant reduced proportion of children with the frequency of fecal incontinence only in GBB + PEGE and GBB + SP groups. The proportion of children with blood in stool was reduced in PEGE and SP groups. During the eight-week treatment period, the proportion of children that required decreased laxative doses was: 87% in the GBB+SP group; 63% in the GBB+PEGE group; 35% in the SP group; 6% in the PEGE group. Green banana biomass alone and associated with laxatives was well tolerated, and adverse effects were not reported.
Number of children before and after eight weeks of treatment schedule for different variables and proportion of reduction or increase.
| GBB (n=15) | GBB + PEGE (n=16) | GBB + sodium picosulfate (n=16) | PEGE (n=16) | Sodium picosulfate (n=17) | |
|---|---|---|---|---|---|
| BSFS score (1 or 2) | 15/2 (87%)a | 16/1 (94%)a | 16/03 (82%)a | 16/5 (69%)a | 17/4 (77%)a |
| Defecation frequency>three/week | 05/9 (27%) | 3/12 (57%)a | 6/15 (56%)a | 1/10 (57%)a | 1/08 (40%)a |
| Fecal incontinence>one/week | 09/5 (27%) | 08/2 (38%)a | 08/2 (38%)a | 10/4 (37%) | 09/5 (24%) |
| Straining on defecation | 14/4 (67%)a | 11/3 (50%)a | 12/1 (69%)a | 15/5 (63%)a | 17/5 (71%)a |
| Painful defecation | 14/4 (67%)a | 12/3 (57%)a | 12/1 (69%)a | 16/4 (75%)a | 14/2 (70%)a |
| Blood in stool | 4/0 (26%) | 3/1 (12%) | 1/0 (06%) | 11/1 (63%)a | 7/1 (35%)a |
| Abdominal pain | 12/2 (67%)a | 14/2 (75%)a | 13/4 (56%)a | 15/2 (82%)a | 10/5 (30%) |
| Decreased laxative doses | N/A | 16/10 (63%)a | 16/14 (87%)a | 16/1 (6%) | 17/6 (35%)a |
GBB, green banana biomass; PEGE, polyethylene glycol 3350 with electrolytes; BSFS, Bristol Stool Form Scale.
The present interventional study was designed to evaluate the effects of the use of green banana biomass with different combinations of laxatives in the management of children and adolescents with functional constipation. To the best of the authors’ knowledge, no studies on childhood constipation treatment had been performed with green banana biomass as the source of dietary fiber and resistant starch. The primary results demonstrated that the consumption of green banana biomass alone for eight weeks (30g/day) reduced the proportion of children with Bristol Stool Form Scale ratings 1 or 2, straining, and painful defecation, but there was no increased percentage of children with >3 bowel movements/week.
A limitation of this study is that it was conducted in a tertiary single-center, where there are higher chances of children with more severe symptoms of constipation, and the study did not plan a long-term follow-up. The strengths of this study include the degree of homogeneity between the groups in baseline and high adherence to the treatment schedule, and the use of alterations in the Bristol Stool Form Scale as the primary outcome; the latter allowed a more objective treatment analysis in the five groups studied. All patients and mothers/caregivers were instructed to observe the Bristol score through direct education by the authors and through handouts provided during the first visit. The Bristol Score is recommended for use in children with constipation before Rome IV criteria can be applied.16–18 Therefore, categorizing stool into seven criteria according to stool consistency is useful for evaluating the effectiveness of the intervention on functional constipation.
Constipation treatment in childhood is a challenging problem, and the primary objectives are to re-establish a regular defecation pattern without fecal incontinence. The role of fiber in the treatment of chronic constipation is a controversial issue still under debate. A study in Hong Kong showed that dietary fiber intake in children with constipation was lower when compared to non-constipated children, corresponding to 40% of reference dietary fiber intake. Similar data were reported in Greece and Pakistan, where functional constipation was negatively correlated with fiber intake.19,20 An intervention with increased fiber intake in children with constipation did not result in the reduction in laxative use or increased stool frequency.21 In turn, a clinical study found that administration of dietary fiber supplements in addition to laxatives may be beneficial in symptomatic children who were already on laxatives.22 The results of the present study demonstrated that polyethylene glycol 3350 with electrolytes and sodium picosulfate, when associated with green banana biomass, reduced the required laxative doses in a higher proportion of children when compared with the group in which the same laxatives are used alone. Therefore, the two most recommended laxatives in children presented a real association with green banana biomass.
Conversely, dietary fiber is widely recommended as therapy for constipation and has been approved for the treatment of constipation since at least the 16th century. Fibers may affect gastrointestinal transit time and bowel movements by increasing the water content and the bacterial proliferation that produces softer and more frequent stools.22,23 Green banana (Musa spp. AAA) is a major source of resistant starch with several physiological effects consistent with those of dietary fibers. The results of the present study showed that green banana biomass has therapeutic properties and should be explored for human nutritional applications. Further research adopting with extensive follow-up on childhood constipation is necessary. Studies to elucidate the role of resistant starch fermentation and intestinal microbiota to explain the positive results of green banana biomass in the treatment of constipation are also required.
In conclusion, the results of the present study indicated that adding green banana biomass to the routine treatment of constipation with laxatives in children and adolescents can significantly increase symptom improvement, and the use of this combination for eight weeks allows a reduction in laxative dose. The reductions in the doses of laxatives are a very interesting result, considering side effects and price. As it is easily found in most parts in Brazil, these results demonstrate the high potential for its use in the treatment of constipation, presenting low cost, simple preparation, and high reproducibility.
FundingThis study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Cassettari VM, Machado NC, Lourenção PL, Carvalho MA, Ortolan EV. Combinations of laxatives and green banana biomass on the treatment of functional constipation in children and adolescents: a randomized study. J Pediatr (Rio J). 2019;95:27–33.