Evidence of oxidative stress was reported in individuals with Down syndrome. There is a growing interest in the contribution of the immune system in Down syndrome. The aim of this study is to evaluate the coenzyme Q10 and selected pro-inflammatory markers such as interleukin 6 and tumor necrosis factor α in children with Down syndrome.
MethodsEighty-six children (5–8 years of age) were enrolled in this case-control study from two public institutions. At the time of sampling, the patients and controls suffered from no acute or chronic illnesses and received no therapies or supplements. The levels of interleukin 6, tumor necrosis factor α, coenzyme Q10, fasting blood glucose, and intelligence quotient were measured.
ResultsForty-three young Down syndrome children and forty-three controls were included over a period of eight months (January–August 2014). Compared with the control group, the Down syndrome patients showed significant increase in interleukin 6 and tumor necrosis factor α (p=0.002), while coenzyme Q10 was significantly decreased (p=0.002). Also, body mass index and fasting blood glucose were significantly increased in patients. There was a significantly positive correlation between coenzyme Q10 and intelligence quotient levels, as well as between interleukin 6 and tumor necrosis factor α.
ConclusionInterleukin 6 and tumor necrosis factor α levels in young children with Down syndrome may be used as biomarkers reflecting the neurodegenerative process in them. Coenzyme Q10 might have a role as a good supplement in young children with Down syndrome to ameliorate the neurological symptoms.
Foram relatadas evidências de estresse oxidativo em indivíduos com a síndrome de Down. Há um interesse cada vez maior na contribuição do sistema imunológico na síndrome de Down. O objetivo deste estudo é avaliar a coenzima Q10 e marcadores pró-inflamatórios selecionados, como interleucina 6 e fator de necrose tumoral α, em crianças com a síndrome de Down.
Métodos86 crianças (5-8 anos de idade) de duas instituições públicas foram inscritas neste estudo de caso-controle. No momento da amostragem, os pacientes e os controles não sofriam de nenhuma doença aguda ou crônica e não recebiam nenhuma terapia ou suplementos. Foram medidos os níveis de interleucina 6, fator de necrose tumoral α, coenzima Q10, glicemia de jejum e quociente de inteligência.
Resultados43 crianças com síndrome de Down e 43 controles foram incluídos em um período de 8 meses (janeiro-agosto 2014). Em comparação ao grupo de controle, os pacientes com síndrome de Down mostraram aumento significativo na interleucina 6 e no fator de necrose tumoral α (p=0,002), ao passo que a coenzima Q10 apresentou significativa redução (p=0,002). Além disso, o índice de massa corporal e a glicemia de jejum eram significativamente maiores nos pacientes. Houve uma correlação significativamente positiva entre os níveis de coenzima Q10 e do quociente de inteligência, bem como entre a interleucina 6 e o fator de necrose tumoral α.
ConclusãoOs níveis de interleucina 6 e fator de necrose tumoral α em crianças mais novas com síndrome de Down podem ser utilizados como biomarcadores, refletindo o processo neurodegenerativo neles. A coenzima Q10 pode ter um papel como bom suplemento em crianças com síndrome de Down para melhorar os sintomas neurológicos.
Trisomy 21 is the most frequent chromosomal abnormality, which characteristically has significant cognitive disability and neurologic deficiencies. It affects 1/700 to 1/1000 live births.1 Excess inhibition in the brain of patients with an extra chromosome 21 could be responsible for cognitive deficits noticed throughout their lives.2 Oxidative stress is known to have a substantial role in the pathology because of genetic and epigenetic factors, which suggests that oxidative imbalance contributes to the clinical manifestations in Down syndrome (DS).3 In Down syndrome the oxidative damage has a major role in the neurodegenerative processes.4 Coenzyme Q10 (CoQ10) works as a reactive oxygen species (ROS) scavenger. Possibly, in addition, it stimulates oxidative damage repair enzymes and has a role in the regulation of gene expression. It also might work as a modulator of DNA repair mechanisms.5,6 The effect of CoQ10 has been studied in some neurological disorders where mitochondrial dysfunction was detected.7 This could explain the biochemical process by which exogenous CoQ10 improves the bioenergetic impairment in some mitochondrial myopathies and in cardiomyopathy.8,9 Coenzyme Q10 has been administered in patients affected by DS, attempting to counteract the oxidative imbalance present due to its secondary deficiency, with promising results.10,11 Individuals having DS are more prone to infections and autoimmune disorders. Ineffective immune responses in DS lead to recurrent viral/bacterial infections and contribute to the development of various pathophysiological symptoms, including cognitive impairment.12
The dysfunction of the immune system in DS has been attributed to decreased number of B-lymphocytes, T-cell subset modifications, as well as changes in the levels of anti- and pro-inflammatory cytokines. Tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) have been implicated as key components of immune and also inflammatory processes.13 An improved and better understanding of the relationship between these different elements may help in the discovery of new approaches to ameliorate the progression of dementia in trisomy 21 patients.
The aim of the study was to evaluate the level of some pro-inflammatory markers (IL-6 and TNFα) and CoQ10 in 5–8 year-old children with DS.
MethodsThis prospective study included all Down syndrome patients presenting to the Clinical Genetics Clinic, National Research Centre, and outpatient clinic of New Children's Hospital, who met the inclusion criteria over a period of eight months. Both the centre and hospital are general governmental establishments serving middle- and low-income patients.
The patients’ inclusion criteriaCytogenetically proven Down syndrome patients, having no congenital anomalies or chronic diseases, aged 5–8 years old, who consented to participate in this investigation, were enrolled in the study. The age group 5–8 years was selected, according to the frequency of young DS cases fulfilling the inclusion criteria. At the time of blood sampling, they had no acute illnesses and were not on any type of medications or supplements for at least one month. The study period was determined by availability of kits. The control group consisted of healthy children of similar age and sex fulfilling the inclusion criteria.
IQ assessment and determination of plasma CoQ10 and cytokines levelsThis study was blinded. All samples were coded and numbered by the investigator responsible for collection of clinical data and only she had access to the full data on the subjects. Intelligence quotient (IQ) assessments were done using the 4th edition of Wechsler Intelligence Scale for Children (WISC-IV).14 Levels of CoQ10 were assayed by a dedicated high-performance liquid chromatography (HPLC) system with electrochemical detector (ECD) produced by Shiseido Co. Ltd. (Tokyo, Japan). CoQ10 levels in plasma were measured as μmol/L. Cytokine quantification was conducted by ELISA using commercial kits for human TNFα and IL-6 (eBio-Science, CA, USA), according to the manufacturer's instructions. The presence and concentration of cytokines were identified by the intensity of the color measured by spectrometry in a micro ELISA reader. Plasma levels of human TNFα and IL-6 were expressed in pg/mL. An Olympus AU400 automatic analyzer (Olympus Corporation, Tokyo, Japan) was used to measure fasting blood glucose with commercial kits (Roche Diagnostics, IN, USA).
The recruitment and experimental protocols of the study were conducted in compliance with the Declaration of Helsinki (as revised in Edinburgh, 2000) and approved by local ethical committee. All legal guardians of participants consented to the study.
Statistical analysisAll data are expressed as mean±standard deviation. Correlation analyses were performed by calculating the Pearson correlation coefficient (r) to assess the relationships between the studied parameters. Statistical analysis was performed using SPSS Statistics 20.0 for windows (IBM SPSS Statistics for Windows, Version 20.0, NY, USA). The results were considered statistically significant at the 0.05 significance level.
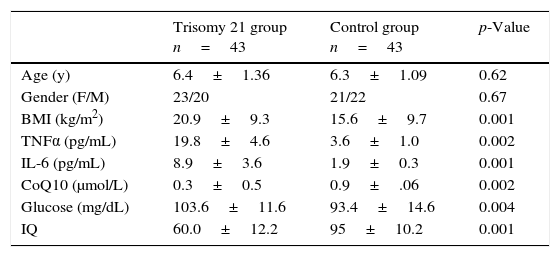
ResultsForty-three Down syndrome patients and 43 controls were enrolled in a prospective study from January 2014 to August 2014. Table 1 summarizes clinical data, BMI, and investigations on the studied individuals.
Clinical data and investigations of studied DS children and controls.
| Trisomy 21 group n=43 | Control group n=43 | p-Value | |
|---|---|---|---|
| Age (y) | 6.4±1.36 | 6.3±1.09 | 0.62 |
| Gender (F/M) | 23/20 | 21/22 | 0.67 |
| BMI (kg/m2) | 20.9±9.3 | 15.6±9.7 | 0.001 |
| TNFα (pg/mL) | 19.8±4.6 | 3.6±1.0 | 0.002 |
| IL-6 (pg/mL) | 8.9±3.6 | 1.9±0.3 | 0.001 |
| CoQ10 (μmol/L) | 0.3±0.5 | 0.9±.06 | 0.002 |
| Glucose (mg/dL) | 103.6±11.6 | 93.4±14.6 | 0.004 |
| IQ | 60.0±12.2 | 95±10.2 | 0.001 |
BMI, body mass index; CoQ10, coenzyme Q10; DS, Down syndrome; IL-6, interleukin 6; IQ, intelligence quotient; TNFα, tumor necrosis factor α.
DS patients had significantly higher body mass index (BMI) than controls (p=0.001). Also, TNFα and IL-6 levels were significantly higher in DS patients compared to controls, with p-values of 0.002 and 0.001, respectively. The CoQ10 level was significantly lower DS patients (p=0.002). The redox status of CoQ10 (% of oxidized/total CoQ10) was found to be in the range of 28–35% in the plasma of DS children compared to 8–12% in controls. The level of fasting blood glucose was significantly higher in DS patients (p=0.004), while the IQ score significantly lower (p=0.001) than controls. The mean IQ score of DS patients was in the range of mild mental disability. Table 2 summarizes the results of Pearson correlation coefficients r between CoQ10, pro-inflammatory cytokines, and BMI. There was a significant positive correlation between CoQ10 levels and IQ scores, as well as between IL-6 and TNFα levels.
Correlation matrix of CoQ10, pro-inflammatory cytokines, and various parameters in DS children.
| CoQ10 | TNF α | IL-6 | IQ | BMI | Glucose | |
|---|---|---|---|---|---|---|
| CoQ10 | 1 | −0.2 | −0.2 | 0.4a | 0.01 | −0.3 |
| TNF α | 1 | 0.9b | −0.2 | −0.2 | 0.03 | |
| IL-6 | 1 | −0.04 | −0.2 | 0.04 | ||
| IQ | 1 | 0.2 | −0.2 | |||
| BMI | 1 | −0.09 | ||||
| Glucose | 1 |
BMI, body mass index; CoQ10, coenzyme Q10; DS, Down syndrome; IL-6, interleukin 6; IQ, intelligence quotient; TNFα, tumor necrosis factor α.
It has been postulated that a triplicated chromosome 21 causes a 50% increase in the expression of trisomic genes as a primary dosage effect, which translates directly into biochemical aberrations.13,15 A number of studies have shown that trisomy 21 related increase of oxidative stress might be involved in different aspects of DS phenotypes.4,16
In this study the inflammatory markers and oxidative stress mechanisms in children with Down syndromes are evaluated and compared to controls. In the present study, all patients showed significant decrease in plasma CoQ10, a potent endogenous antioxidant, which may be an important factor associated with oxidative imbalance in children with trisomy 21. Miles et al.6 found significant decrease of CoQ10 in DS patients. Another study by Tiano et al.17 concluded that lymphocyte and platelet CoQ10 content were significantly lower in DS patients, a fact which probably underlie oxidative imbalance at the cellular level. However, the present study included a larger number of patients, with an age range of 4 years (5–8 years of age). The CoQ10 redox mechanism is possibly related to maintaining the mitochondrial homeostasis and prevention of free radical production. However, studies comparing data on oxidative DNA damage and systemic oxidative stress parameters in CoQ10-treated DS patients concluded that CoQ10 does not simply work as an ROS scavenger. This is because of the absence of a measurable plasma antioxidant response, which could be masked by the hyperuricemia usually found in DS, together with unchanged DNA levels.7
The cytokines IL-6 and TNFα are considered major orchestrators of both immune and inflammatory responses.18 The present study's data showed significant increase in the pro-inflammatory markers IL-6 and TNFα level in serum collected from DS subjects, when compared to the control group, a similar finding to other studies.13,19
High rates of overweight and obesity among children worldwide and the range of health problems associated with them from psychosocial to adverse metabolic findings warrant development of a number of action plans and setting of global targets for the prevention of obesity in children and adolescents. New preventive strategies, highlighting the important role of physical activity and nutrition education, are necessary. Individual, family, and community variations can affect having a healthy lifestyle.20,21
High BMI is associated with a specific pattern of low-grade immune activation.22 The present study's data confirm previously described associations between DS and high BMI.23,24 The mean IQ score of the studied DS patients was 60.0±12.2. Gardiner, in 2014,25 delineated that although intellectual disability in DS can be only mild delay, the most frequently reported IQ is in the range of 40–50 (mild to moderate delay). Shukla et al.26 reported that the IQ score of the DS patients had moderate mental disability in 31% and mild mental disability 52% of their patients. The correlation of CoQ10 levels to IQ scores in DS patients signifies the CoQ10 effect on neurodevelopment that may be due to its protective role against nuclear DNA damage, in addition to its redox role.5 It is well-known that diabetes mellitus has a higher prevalence in DS than in the general population,27,28 while in the present findings the blood glucose level was within normal, which may be explainable by the young age of the patients. The importance of these results from clinical and therapeutic points of view is that it emphasizes the importance of a proper follow-up strategy for DS individuals, and aids to outline novel strategies for the treatment of patients with DS.
In conclusion, the levels of Il-6, TNFα, and CoQ10 in young DS patients might represent a key-contributing factor to the neurodegenerative process that culminates in DS. Coenzyme Q10 should be considered a good supplement in young children with trisomy 21 to ameliorate the neurological symptoms.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Zaki ME, El-Bassyouni HT, Tosson AM, Youness E, Hussein J. Coenzyme Q10 and pro-inflammatory markers in children with Down syndrome: clinical and biochemical aspects. J Pediatr (Rio J). 2017;93:100–4.