To evaluate the validity of clinical and laboratory signs to serious dengue disease in hospitalized children.
MethodsRetrospective cohort of children (<18 years) hospitalized with dengue diagnosis (2007–2008). Serious dengue disease was defined as death or use of advanced life support therapy. Accuracy measures and area under the receiver operating characteristic curve were calculated.
ResultsOf the total (n=145), 53.1% were female, 69% aged 2–11 years, and 15.9% evolved to the worse outcome. Lethargy had the best accuracy (positive likelihood ratio >19 and negative likelihood ratio <0.6). Pleural effusion and abdominal distension had higher sensitivity (82.6%). History of bleeding (epistaxis, gingival or gastrointestinal bleeding) and severe hemorrhage (pulmonary or gastrointestinal bleeding) in physical examination were more frequent in serious dengue disease (p<0.01), but with poor accuracy (positive likelihood ratio=1.89 and 3.89; negative likelihood ratio=0.53 and 0.60, respectively). Serum albumin was lower in serious dengue forms (p<0.01). Despite statistical significance (p<0.05), both groups presented thrombocytopenia. Platelets count, hematocrit, and hemoglobin parameters had area under the curve <0.5.
ConclusionsLethargy, abdominal distension, pleural effusion, and hypoalbuminemia were the best clinical and laboratorial markers of serious dengue disease in hospitalized children, while bleeding, severe hemorrhage, hemoconcentration and thrombocytopenia did not reach adequate diagnostic accuracy. In pediatric referral hospitals, the absence of hemoconcentration does not imply absence of plasma leakage, particularly in children with previous fluid replacement. These findings may contribute to the clinical management of dengue in children at referral hospitals.
Avaliar a validade dos sinais clínicos e laboratoriais para o dengue com evolução grave em crianças hospitalizadas.
MétodosCoorte retrospectivo de crianças (<18 anos) internadas com dengue (2007–2008). Evolução grave foi definida como óbito ou pelo uso de terapia de suporte avançado de vida. Foram calculadas medidas de acurácia e área sob a curva ROC.
ResultadosDo total (n=145), 53,1% casos eram do sexo feminino, 69% de 2 a 11 anos e 15,9% evoluíram para gravidade. Letargia obteve a melhor acurácia (razão de verossimilhança positiva RVP>19 e RV negativa RVN<0,6). Derrame pleural e distensão abdominal apresentaram maior sensibilidade (se=82,6%). Relato de sangramentos (epistaxe, gengivorragia ou gastrointestinal) e hemorragia grave (pulmonar ou gastrointestinal) presente no exame físico foram mais frequentes nos casos com evolução grave (p <0,01), porém com baixa acurácia (RVP=1,89 e 3,89; RVN=0,53 e 0,60, respectivamente). Os níveis de albumina sérica foram mais baixos nas formas graves (p <0,01). Ambos os grupos apresentaram trombocitopenia, apesar da diferença estatística (p <0,05). Contagem de plaquetas, hematócrito e hemoglobina apresentaram área sob a curva ROC<0,5.
ConclusõesLetargia, distensão abdominal, derrame pleural e hipoalbuminemia foram os melhores marcadores clínicos e laboratoriais de dengue com evolução grave em crianças hospitalizadas, enquanto sangramento, hemorragia grave, hemoconcentração e trombocitopenia não tiveram boa acurácia diagnóstica. Em hospitais de referência pediátricos, a ausência de hemoconcentração não implica ausência de extravasamento plasmático, particularmente quando há reposição anterior de volume. Esses resultados podem contribuir para o manejo clínico do dengue em crianças em hospitais de referência.
Dengue is an acute febrile disease caused by a flavivirus, with four known serotypes (DENV-1, DENV-2, DENV-3, and DENV-4). In 2013, the worldwide estimate of dengue infection was 390 million people, 96 million of whom were symptomatic.1 In Brazil, from 2000 to 2010, there was an increase in dengue incidence, accompanied by a proportional increase in severe cases.2 Dengue has mainly affected the adult population in Brazil, but the epidemic in 2008 showed an increased incidence in individuals under 15 years of age, with a higher proportion of severe cases in this age group.2
The four serotypes can lead to variable clinical presentations, ranging from asymptomatic to severe forms. Infants and preschoolers frequently present an undifferentiated febrile disease. Coryza, seizures, nausea, vomiting, exanthema, and petechiae are more frequent in children under 2 years of age.3 Children with dengue could evolve into severe forms faster than adults, especially those under 5 years.4 Signs of hypoperfusion like cold skin, oliguria, and slow capillary refill can appear suddenly after a few days of the febrile phase.5
In 1997, the World Health Organization (WHO) established a classification of dengue cases: dengue fever (DF) and dengue hemorrhagic fever (DHF). DHF is subdivided into four stages of severity; stages III and IV are defined as dengue shock syndrome (DSS). In 2009, the WHO proposed a new classification, dengue and serious dengue, in which dengue is subdivided according to the presence or absence of the following warning signs: abdominal pain, persistent vomiting, edema, mucosal bleeding, lethargy, irritability, hepatomegaly (>2cm), and increased hematocrit concurrent with decreased platelet count.6 This new classification aimed at simplification and changed the focus from hemorrhage to plasma leakage, the principal factor in pathogenesis of the severe forms.7
The WHO and Brazilian Ministry of Health adopted the use of warning signs for clinical case management,6,8 although their evidence as predictors of severity is not consistent, especially in children.9 Signs and symptoms most frequently associated with severity in children were: spontaneous bleeding, hepatomegaly, and signs of plasma leakage, such as ascites and pleural effusion,10,11 abdominal pain,11,12 hemoconcentration and thrombocytopenia,13–16 and elevated plasma levels of the liver enzyme serum glutamic oxaloacetic transaminase (SGOT).11,15 The majority of studies defined platelet count below 50,000 per mm3 as a sign of poor prognosis.13,14,16 Potts et al.15 found that leukocyte count and thrombocytopenia are less significant for predicting severity, with the latter more important for the diagnosis of dengue.
The use of warning signs in dengue was proposed for early detection of potentially severe cases for timely treatment, to avoid unnecessary hospitalizations, and to decrease the case-fatality of this disease.6,8 However, various clinical and epidemiological aspects have still not been completely elucidated, especially in children. In Brazil, the 2008 epidemic showed more serious dengue cases in children with the primary infection,2 contrary to studies in Asia that showed an association between secondary infection and severity.17 Although a meta-analysis of 37 international studies showed an inversely proportional association between age and DSS, the studies were highly heterogeneous, probably due to wide variability in age brackets in the study populations.17 In this study, although hemorrhage appeared as a predictor of severity, this sign was not frequent in children.13 Finally, the majority of the validation studies17 on warning signs for dengue used the WHO criterion for DHF as the reference standard,9 which incorporates many of the signs and symptoms tested as predictors, thus committing an incorporation bias toward diagnostic studies, with a tendency to overestimate accuracy measurements.18
This study aimed to evaluate the validity of clinical and laboratorial markers for serious dengue disease in a cohort of children hospitalized during the 2007–2008 epidemic in the city of Rio de Janeiro, Brazil. During this epidemic, there were more than 100,000 suspected dengue cases reported to the local health information system; 38,808 were confirmed and classified according to the WHO system of 1997. Of those, 42.3% were under 18 years of age. At that time, the serotypes circulating were DENV-2 and DENV-3.
MethodsThis was a retrospective cohort study in a pediatric referral hospital in the city of Rio de Janeiro, Brazil. The study included all children and adolescents (from 2 months to 18 years) hospitalized with dengue during the epidemic from November 2007 to May 2008.
Since it was sought to study clinical and laboratorial markers of serious dengue disease and shock was related to the outcome variable, cases who presented a clinical picture consistent with shock on the day of the hospital admission were excluded. Shock was defined as the presence of narrow pulse pressure, arterial hypotension for age associated with cyanosis, lethargy, or coma. This study also excluded cases with lack of information from the patient's medical chart or those with clinical and/or laboratory evidence of other acute febrile diseases, such as pneumonia, acute otitis media, and sinusitis. Two local research ethics committees approved this study.
Dengue diagnosis was defined according to the Brazilian Ministry of Health criteria.19 A case was considered confirmed when it met the following criteria: a positive sample in the detection of viral RNA by RT-PCR (reverse transcription polymerase chain reaction), ELISA (enzyme-linked immunosorbent assay) for IgM antibodies; IgM or IgG seroconversion in paired samples, by ELISA; or by the clinical-epidemiological criterion: clinical picture consistent with dengue, epidemiological link to confirmed dengue case, and absence of laboratory confirmation of other diagnoses consistent with the age bracket.19 Data collection in medical charts was performed by medical students or pediatricians trained for this purpose, using a standardized form for the study.
The outcome variable “Serious dengue disease” was defined as the occurrence of death or the use of amines, inotropes, colloids, mechanical ventilation, non-invasive ventilation, peritoneal dialysis or hemodialysis, not considered under the classifications prevailing at the time.19 The explanatory variables included socio-demographic data, clinical history, physical examination on the first day of hospitalization, and initial hematological and biochemical laboratory test results. Arterial hypotension was defined according to age bracket: systolic pressure (SAP) <100mmHg for children ≥12 years, SAP<95mmHg for ≥6 to <12 years, SAP<90mmHg for ≥2 to <6 years, SAP<85mmHg for ≥1 to <2 years, and SAP<70mmHg for ≥6 months to <1 year.8 In anamnesis, asthma reported in medical charts was not clinically classified; bleeding was defined by epistaxis, gingival, or gastrointestinal bleeding. In physical examination, severe hemorrhage was defined by gastrointestinal or pulmonary bleeding; pleural effusion was confirmed by chest X-ray or ultrasound. Serum albumin was evaluated as a continuous and as a dichotomous variable. For the last, this study used the cutoff point of 3.0g/dL; above this there is no indication for albumin administration. Liver injury was evaluated through aspartate aminotransferase (AST>40 UI/L) and alanine aminotransferase (ALT>56 UI/L).
The data were entered into EpiData 3.1 (Lauritsen JM. (Ed.) EpiData Data Entry, Data Management and basic Statistical Analysis System. Dinamarca) and analyzed in Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, USA). Frequencies and proportions were calculated for the categorical variables and medians with the respective interquartile intervals (IQI) for age in years, time of fever until onset of symptoms, and hematological and biochemical laboratory parameters. Fisher's exact test was calculated for categorical variables and the Mann–Whitney test was utilized for continuous variables (non-normal distribution) to assess the association between these variables and serious dengue disease. When the association was statistically significant (p<0.05), the following accuracy measures were calculated for the categorical variables with their respective 95% confidence intervals: sensitivity (Se), specificity (Sp), positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratios (PLR, NLR). Adequate values were defined as PLR>5 and NLR ≤0.20. For the continuous variables, a receiver operating characteristic (ROC) curve was constructed in order to estimate the area under the curve (AUC) with the respective 95% confidence interval.
ResultsOf the total of 164 children hospitalized with suspicion of dengue, 19 were excluded: one dengue diagnosis was ruled out by laboratory results, three already presented at the day of hospitalization a clinical condition consistent with DSS, and 15 were ruled out due to the diagnosis of other diseases or lack of information on the patient chart. The final sample included 145 patients, the majority female (53.1%), black or brown (56.6%), residing in the city of Rio de Janeiro (74.5%), and with at least one prior medical consultation (88.3%). Most of the children were 2–11 years (69%), 21.4% 12 or more years, and 9.2% less than 2 years of age. Only three patients had a previous history of dengue, of whom none were classified as serious dengue disease. Of the 145 cases, 97 were confirmed by laboratory and 48 by the clinical-epidemiological criterion, without statistical difference across serious and non-serious dengue (p=0.436).
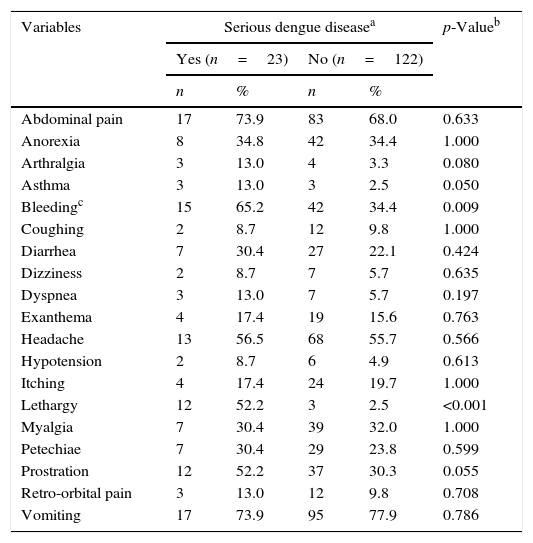
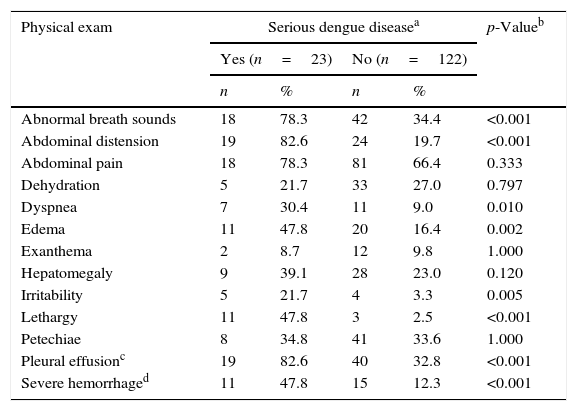
Patients who evolved to serious dengue disease represented 15.9% of the sample. Both groups were mostly represented by children aged 2–11 years (serious=78.3% and non-serious=67.2%), without statistical difference in relation to those less than 2 years and 12–18 years (p=0.167 for Fisher's exact test). Duration of fever in days was slightly lower in this subgroup of patients (serious: Md=3.0, IQI=2.0–4.5; non-serious: Md=4.0, IQI=3.0–5.0; p=0.062). History of asthma (p=0.051), report of lethargy or bleeding, and the identification of lethargy, dyspnea, irritability, bleeding, abdominal distension, edema, altered breath sounds, or pleural effusion on physical examination at hospital admission were more frequent in patients with serious outcomes (Tables 1 and 2).
Clinical history of hospitalized children with dengue.
| Variables | Serious dengue diseasea | p-Valueb | |||
|---|---|---|---|---|---|
| Yes (n=23) | No (n=122) | ||||
| n | % | n | % | ||
| Abdominal pain | 17 | 73.9 | 83 | 68.0 | 0.633 |
| Anorexia | 8 | 34.8 | 42 | 34.4 | 1.000 |
| Arthralgia | 3 | 13.0 | 4 | 3.3 | 0.080 |
| Asthma | 3 | 13.0 | 3 | 2.5 | 0.050 |
| Bleedingc | 15 | 65.2 | 42 | 34.4 | 0.009 |
| Coughing | 2 | 8.7 | 12 | 9.8 | 1.000 |
| Diarrhea | 7 | 30.4 | 27 | 22.1 | 0.424 |
| Dizziness | 2 | 8.7 | 7 | 5.7 | 0.635 |
| Dyspnea | 3 | 13.0 | 7 | 5.7 | 0.197 |
| Exanthema | 4 | 17.4 | 19 | 15.6 | 0.763 |
| Headache | 13 | 56.5 | 68 | 55.7 | 0.566 |
| Hypotension | 2 | 8.7 | 6 | 4.9 | 0.613 |
| Itching | 4 | 17.4 | 24 | 19.7 | 1.000 |
| Lethargy | 12 | 52.2 | 3 | 2.5 | <0.001 |
| Myalgia | 7 | 30.4 | 39 | 32.0 | 1.000 |
| Petechiae | 7 | 30.4 | 29 | 23.8 | 0.599 |
| Prostration | 12 | 52.2 | 37 | 30.3 | 0.055 |
| Retro-orbital pain | 3 | 13.0 | 12 | 9.8 | 0.708 |
| Vomiting | 17 | 73.9 | 95 | 77.9 | 0.786 |
First clinical exam of hospitalized children with dengue.
| Physical exam | Serious dengue diseasea | p-Valueb | |||
|---|---|---|---|---|---|
| Yes (n=23) | No (n=122) | ||||
| n | % | n | % | ||
| Abnormal breath sounds | 18 | 78.3 | 42 | 34.4 | <0.001 |
| Abdominal distension | 19 | 82.6 | 24 | 19.7 | <0.001 |
| Abdominal pain | 18 | 78.3 | 81 | 66.4 | 0.333 |
| Dehydration | 5 | 21.7 | 33 | 27.0 | 0.797 |
| Dyspnea | 7 | 30.4 | 11 | 9.0 | 0.010 |
| Edema | 11 | 47.8 | 20 | 16.4 | 0.002 |
| Exanthema | 2 | 8.7 | 12 | 9.8 | 1.000 |
| Hepatomegaly | 9 | 39.1 | 28 | 23.0 | 0.120 |
| Irritability | 5 | 21.7 | 4 | 3.3 | 0.005 |
| Lethargy | 11 | 47.8 | 3 | 2.5 | <0.001 |
| Petechiae | 8 | 34.8 | 41 | 33.6 | 1.000 |
| Pleural effusionc | 19 | 82.6 | 40 | 32.8 | <0.001 |
| Severe hemorrhaged | 11 | 47.8 | 15 | 12.3 | <0.001 |
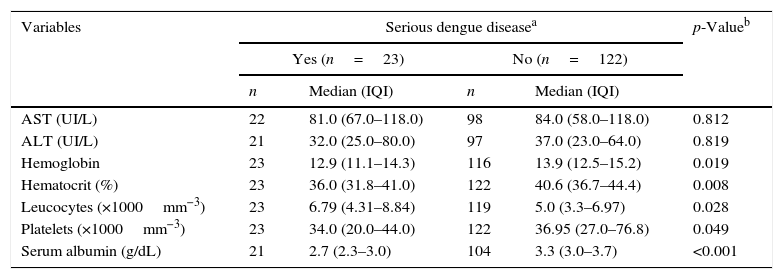
Cases that progressed toward serious dengue disease presented lower values of hematocrit, hemoglobin, platelet count, and serum albumin than other forms, while leukocyte count was higher in serious dengue. In both groups, AST levels were elevated while ALT levels were below the normal range (p>0.05; Table 3).
Median and interquartile interval (IQI) of the first laboratory exam of hospitalized children with dengue.
| Variables | Serious dengue diseasea | p-Valueb | |||
|---|---|---|---|---|---|
| Yes (n=23) | No (n=122) | ||||
| n | Median (IQI) | n | Median (IQI) | ||
| AST (UI/L) | 22 | 81.0 (67.0–118.0) | 98 | 84.0 (58.0–118.0) | 0.812 |
| ALT (UI/L) | 21 | 32.0 (25.0–80.0) | 97 | 37.0 (23.0–64.0) | 0.819 |
| Hemoglobin | 23 | 12.9 (11.1–14.3) | 116 | 13.9 (12.5–15.2) | 0.019 |
| Hematocrit (%) | 23 | 36.0 (31.8–41.0) | 122 | 40.6 (36.7–44.4) | 0.008 |
| Leucocytes (×1000mm−3) | 23 | 6.79 (4.31–8.84) | 119 | 5.0 (3.3–6.97) | 0.028 |
| Platelets (×1000mm−3) | 23 | 34.0 (20.0–44.0) | 122 | 36.95 (27.0–76.8) | 0.049 |
| Serum albumin (g/dL) | 21 | 2.7 (2.3–3.0) | 104 | 3.3 (3.0–3.7) | <0.001 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
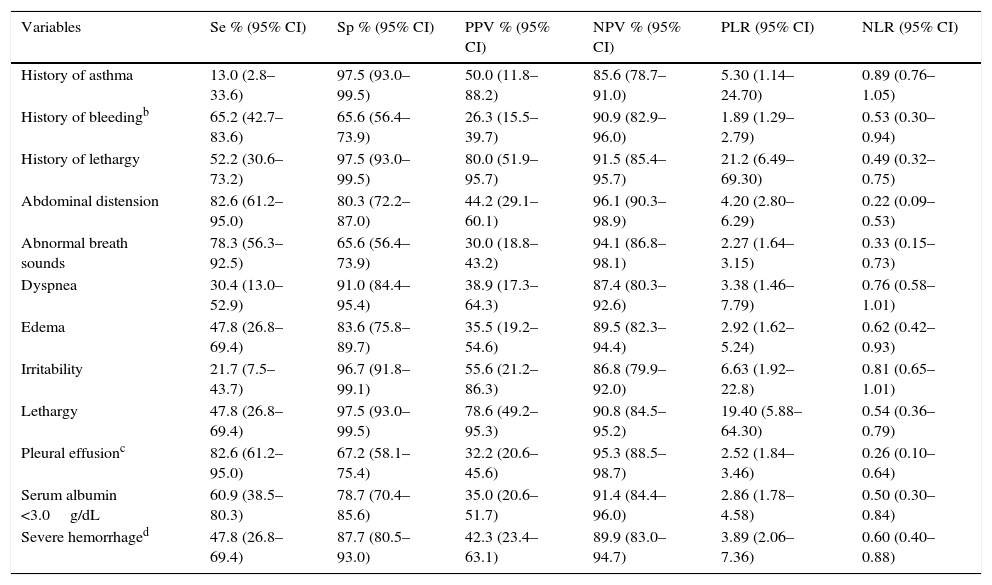
Although present in only half of the serious dengue cases, lethargy, reported in the history or identified in the initial physical examination, was the single clinical sign with the highest positive likelihood ratios (LR+=21.2 and 19.4, respectively; Table 4). Presence of irritability and history of asthma were associated with a moderate increase in the likelihood of evolving to serious dengue disease (LR+=5.3 and 6.63, respectively). Pleural effusion and abdominal distension were the signs with the highest sensitivity (82.6%) and negative predictive value (95.3% and 96.1%), besides showing the lowest LR− (0.26 and 0.22). The AUC for total leukocyte count was 0.645 (0.526–0.765). The duration of fever and other laboratory values presented AUC ≤0.5 (data not shown). Abdominal distension and pleural effusion were the signs with the highest sensitivity (82.6%) and negative predictive value (96.1% and 95.3%, respectively), besides showing the lowest negative likelihood ratios (0.22 and 0.26, respectively).
Accuracy of clinical signs and symptoms identified in the anamnesis or the first physical examination of hospitalized children with serious dengue disease.a
| Variables | Se % (95% CI) | Sp % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|---|---|---|---|---|---|
| History of asthma | 13.0 (2.8–33.6) | 97.5 (93.0–99.5) | 50.0 (11.8–88.2) | 85.6 (78.7–91.0) | 5.30 (1.14–24.70) | 0.89 (0.76–1.05) |
| History of bleedingb | 65.2 (42.7–83.6) | 65.6 (56.4–73.9) | 26.3 (15.5–39.7) | 90.9 (82.9–96.0) | 1.89 (1.29–2.79) | 0.53 (0.30–0.94) |
| History of lethargy | 52.2 (30.6–73.2) | 97.5 (93.0–99.5) | 80.0 (51.9–95.7) | 91.5 (85.4–95.7) | 21.2 (6.49–69.30) | 0.49 (0.32–0.75) |
| Abdominal distension | 82.6 (61.2–95.0) | 80.3 (72.2–87.0) | 44.2 (29.1–60.1) | 96.1 (90.3–98.9) | 4.20 (2.80–6.29) | 0.22 (0.09–0.53) |
| Abnormal breath sounds | 78.3 (56.3–92.5) | 65.6 (56.4–73.9) | 30.0 (18.8–43.2) | 94.1 (86.8–98.1) | 2.27 (1.64–3.15) | 0.33 (0.15–0.73) |
| Dyspnea | 30.4 (13.0–52.9) | 91.0 (84.4–95.4) | 38.9 (17.3–64.3) | 87.4 (80.3–92.6) | 3.38 (1.46–7.79) | 0.76 (0.58–1.01) |
| Edema | 47.8 (26.8–69.4) | 83.6 (75.8–89.7) | 35.5 (19.2–54.6) | 89.5 (82.3–94.4) | 2.92 (1.62–5.24) | 0.62 (0.42–0.93) |
| Irritability | 21.7 (7.5–43.7) | 96.7 (91.8–99.1) | 55.6 (21.2–86.3) | 86.8 (79.9–92.0) | 6.63 (1.92–22.8) | 0.81 (0.65–1.01) |
| Lethargy | 47.8 (26.8–69.4) | 97.5 (93.0–99.5) | 78.6 (49.2–95.3) | 90.8 (84.5–95.2) | 19.40 (5.88–64.30) | 0.54 (0.36–0.79) |
| Pleural effusionc | 82.6 (61.2–95.0) | 67.2 (58.1–75.4) | 32.2 (20.6–45.6) | 95.3 (88.5–98.7) | 2.52 (1.84–3.46) | 0.26 (0.10–0.64) |
| Serum albumin <3.0g/dL | 60.9 (38.5–80.3) | 78.7 (70.4–85.6) | 35.0 (20.6–51.7) | 91.4 (84.4–96.0) | 2.86 (1.78–4.58) | 0.50 (0.30–0.84) |
| Severe hemorrhaged | 47.8 (26.8–69.4) | 87.7 (80.5–93.0) | 42.3 (23.4–63.1) | 89.9 (83.0–94.7) | 3.89 (2.06–7.36) | 0.60 (0.40–0.88) |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; 95% CI, 95% confidence interval.
This study showed that lethargy, reported in the history or identified in the physical examination, was the clinical sign that best discriminates patients who progress toward serious dengue disease. Asthma and irritability showed moderate positive likelihood ratios, involving increased probability of serious dengue when present. Pleural effusion and abdominal distension showed the lowest negative likelihood ratios, meaning that patients without these signs at hospital admission were less likely to evolve to serious dengue disease. However, the clinical evolution corroborates the importance of early diagnosis, considering the rapid evolution of dengue, in which the most critical period is defervescence.20,21 Bleeding was more frequent in serious dengue cases but did not reach good accuracy.
These results are similar to those of other studies demonstrating that lethargy,17,22 irritability,17 abdominal distension, and pleural effusion10,11 were associated to severity. However, hematocrit was lower in patients with serious disease. This finding can be explained by the fact that 88.3% of the present patients were referred from other health services and may have received fluid replacement prior to hospitalization. A study conducted in the Dominican Republic found that anemia was associated with serious dengue.23
The serum albumin was lower in the group with the worse outcome, which was also found in another Brazilian study.24 This result is in accordance with the pathogenesis of dengue, in which the endothelial lesion with resulting plasma leakage is the most important marker for evolution to severe forms, especially in children.25 The high AST serum levels in the present pediatric sample was in accordance with two other studies.24,26
In the present study, although bleeding was more frequent in serious cases, both groups presented thrombocytopenia. The absence of association between thrombocytopenia and bleeding was also found in a sample of hospitalized children from Sri Lanka.26 Leukocyte count was higher in serious dengue, whose association was not consistent in two other studies.26,27
The current study has strengths and limitations. This study's strength is the evaluation of a wide set of clinical signs and symptoms as well as laboratory exams using currently recommended accuracy measures.28 In addition, the criterion adopted in this study for serious dengue disease was defined by death or medical interventions, thus minimizing the diagnostic incorporation bias. The majority of the studies11,17,29 were limited to assessing the odds ratio and adopting composite criteria consisting of one or more predictive variables. The present study's sample size was similar to other hospital-based studies in children.24,26,27 However, there were also some limitations, such as overestimation of the diagnostic accuracy of pleural effusion, the presence of which can be determinant for the use of mechanical ventilation. The study was also retrospective,28 and generalization of the results is limited to hospitalized children at school age.
In conclusion, this study showed that lethargy, abdominal distension, and pleural effusion were the signs with the greatest diagnostic accuracy; and that bleeding, severe hemorrhage, hemoconcentration, and thrombocytopenia were not associated to severity in this setting. Thus, in pediatric referral hospitals, the absence of hemoconcentration does not imply absence of plasma leakage, particularly in patients who have received previous fluid replacement. In this setting, hypoalbuminemia and pleural effusion represented better markers of serious dengue. Moreover, the results related to lethargy and pleural effusion were consistent with the warning signs listed in the revised 2009 WHO dengue classification.30
These findings can contribute to knowledge on dengue clinical epidemiology in children admitted at pediatric referral hospitals. Future prospective studies should include children in initial stages of the disease to identify earlier signs of severity, more adequate for case management in primary care.
FundingThis research was supported by Rede Dengue FIOCRUZ and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ [grant number E-26/110.964/2013 – APQ1].
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank Carlos Augusto Ferreira de Andrade for his comments and Christopher Robert Peterson for English translation.
Please cite this article as: Pone SM, Hökerberg YH, de Oliveira RV, Daumas RP, Pone TM, Pone MV, et al. Clinical and laboratory signs associated to serious dengue disease in hospitalized children. J Pediatr (Rio J). 2016;92:464–71.