To explain the high mortality of septic shock in children with cancer.
MethodsA retrospective cohort from 2016 to 2020, of children aged 0 to 18 years, and septic shock.
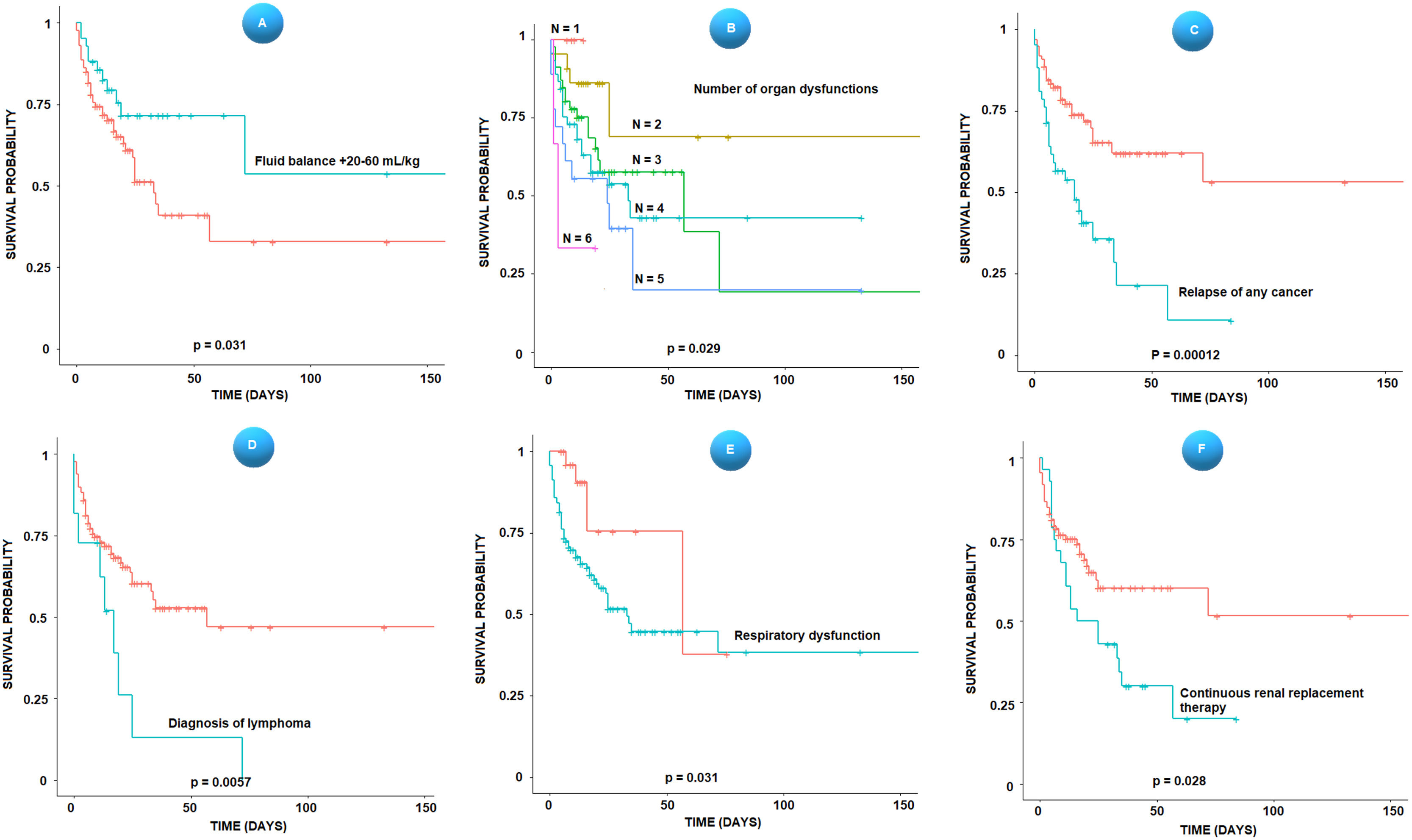
ResultsThe authors included 139 patients. Acute lymphocytic leukemia was the most frequent diagnosis (16.5%), and Gram-negative bacteria were the most frequent blood culture isolates (22.3%). There were 57 deaths in ICU (41%), 10 in the first 24 hours of shock (early death). A LASSO model with variables: neutropenia (coefficient 0.215), respiratory (0.81), hematological (1.41), and neurological (0.72) dysfunctions, age (-0.002) and solid tumor recurrence (0.34) generated AUC = 0.79 for the early death outcome. Survivors had significant differences in the PRISM-IV score (mean ± SD 10.9 ± 6.2 in the survivors, 14.1 ± 6.5 in the deceased, p = 0.004), and in the mean number of organ dysfunctions (3.2 ± 1.1 in the survivors, 3.8 ± 6.5 in the deceased, p < 0.001). A positive fluid balance in the first 24 hours of sepsis between 2% and 6% of body weight showed a reduction effect on the probability of death in ICU (hazard ratio 0.47, 95% CI 0.24-0.92, p = 0.027). The recurrence of any cancer was a predictor of in-hospital death, regardless of severity.
ConclusionsRecurrence of any cancer is an important risk of sepsis-related death. A positive fluid balance between 20 and 60 mL/kg or 2% and 6% of body weight in the first 24 hours after the onset of sepsis is related to lower mortality.
Sepsis mortality has decreased over time in cancer patients, due to improvements in the management, advances in cancer therapies, and improvements in the intensive care unit admission policies.1 Even considering this improvement, about 40% of children with cancer will need intensive care at least once during the disease period, with sepsis being one of the main reasons for hospitalization.2
Despite all knowledge about sepsis and the specific knowledge about infections in pediatric cancer patients, septic shock remains a leading cause of mortality, with a heavier burden in developing countries.3 In children with cancer requiring both mechanical ventilation and inotropic support, the mortality rates can be as high as 69%.4
Septic shock in immunosuppressed children is frequently devastating and unresponsive to therapies. The pleiotropic dysregulation of innate and adaptive immune responses and a decrease in the activities of neutrophils, monocytes, macrophages, and lymphocytes1 can be a constant characteristic of these patients. This study aimed to clinically characterize the group of cancer patients who developed septic shock and to build statistical models to try to explain the high mortality.
MethodsThe study was conducted in the oncological intensive care unit (ICU) of a pediatric cancer center in São Paulo, Brazil, and the Research Ethics Committee of the Federal University of São Paulo approved the protocol. The study design was a retrospective cohort, with data collected from May 2016 to March 2020, and included children who presented with septic shock in need of vasoactive drugs and ICU admission. Patients in palliative care, for whom intensive support such as vasoactive drugs or mechanical ventilation were suspended, were excluded. Patients whose diagnoses were not malignant neoplasms and those older than 216 months (18 years) were also excluded.
Data were collected from the beginning of the sepsis recognition. For severity assessment, the authors used the Pediatric Risk of Mortality IV (PRISM-IV),5 calculated on PICU admission, and the vasoactive-inotropic score (VIS),6 in the first 24 hours after starting the use of vasoactive drugs. To calculate the fluid balance, the authors used the information available in medical records about fluid volume administered, diuresis, and other body fluid losses, regarding the first 24 hours of the onset of sepsis.
Definitions of sepsis and organ dysfunction criteria were based on the 2005 consensus.7 The T-test was used to evaluate differences between means. Survival analysis was accomplished using Kaplan–Meier curves, comparisons with the log-rank test (Mantel-Cox), and proportional risk estimates by Cox regression. LASSO regularization (least absolute shrinkage and selection operator) was used to select variables in regression models, with an evaluation of the predictive capacity by the ROC curve. The analyses were performed with the packages survival, pROC, tidyverse, caret, glmnet, dplyr and survminer, from the R software version 4.1.1 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
ResultsThe authors analyzed data from 139 patients with septic shock. Demographic and other clinical data are shown in Table 1. A sheet with the raw data is available for other investigators, upon request. There were 57 deaths in ICU (41%), in a median time of 12 days (IQR: 4-25). All the deaths were related to sepsis complications (refractory shock and multiple organ dysfunctions).
Demographic and diagnostic data (n, %).
IQR, interquartile range 25-75; SD, standard deviation.
Table 2 shows the characteristics of shock and infection, organ dysfunctions, and vasoactive drugs. Reactivation of cytomegalovirus was observed in two patients. Screening for other viruses through viral panels was not performed for the patients in this sample.
Sites and agents of infection, vasoactive drugs, number of organ dysfunctions.
Table 3 shows the bivariate Cox regression models for the analyzed variables, targeting the outcome of “death in ICU". All patients had cardiovascular dysfunction, so this variable is considered to be constant and is not represented in the models.
Cox bivariate regression models for the analyzed variables, for the outcome “death in ICU.”
For the outcome “death in ICU,” deceased patients had significant differences in the PRISM-IV score (mean ± SD 10.9 ± 6.2 in the survivors, 14.1 ± 6.5 in the deceased, p = 0.004), and in the mean number of organ dysfunctions (3.2 ± 1.1 in the survivors, 3.8 ± 6.5 in the deceased, p < 0.001). The means of VIS were 29.5 ± 29.1 for survivors and 43.1 ± 51 for the deceased (p = 0.1).
Ten patients died within the first 24 hours of septic shock, which was considered early death. The authors built regression models with LASSO regularization to try to explain these precocious deaths. The best adjusted model was composed by the variables neutropenia < 500 cells/mm3 (coefficient: 0.21), respiratory dysfunction (0.81), hematological dysfunction (1.41), neurological dysfunction (0.72), age (-0.002), solid tumor recurrence (0.34), and an intercept of -5.3. The logit of this model generated a ROC curve for this outcome with AUC = 0.79 (95% CI 0.64-0.94). The best cutoff point of the curve showed specificity rates of 68% and sensitivity of 90%.
For the patients who survived the first 24 hours of shock (n = 129), the mean ± SD of fluid balance was 46.9 ± 49.9 mL/kg (corresponding to 4.69% ± 4.99% of body weight; these percentages were calculated as fluid balance in the first 24 hours after sepsis in liter divided by admission weight in kilograms × 100). Means were similar between patients who survived hospitalization (43.9 ± 48.8 mL/kg) and those who did not (51 ± 47.9), p = 0.58. Eighteen patients (13.6%) had a negative fluid balance, and 18 (13.6%) had a balance > 100 mL/kg (equivalent to > 10% of body weight).
In a multivariate Cox regression model, with the variables of clinical severity “PRISM IV score ” and “number of organ dysfunctions,” a positive fluid balance on the first 24 hours of sepsis between 2% and 6% of body weight showed a reduction effect on the probability of “death in ICU” (hazard ratio 0.47, 95% CI 0.24-0.92, p = 0.027), regardless of severity. Other ranges of positive or negative fluid balance showed no significant effects.
In other multivariate models with “PRISM IV score” and “number of organ dysfunctions,” the recurrence of any type of cancer was also a predictor of in-hospital death (hazard ratio 2.8 (95% IC 1.6 -5.1, p < 0.001). A figure depicting these models is in the supplementary material.
Figure 1 shows the Kaplan Meier curves for the variables: fluid balance between 20 and 60 mL/kg (2% and 6% of body weight) in the first 24 hours of sepsis, the number of organ dysfunctions, recurrence of any type of cancer, diagnosis of lymphoma, respiratory dysfunction, and continuous renal replacement therapy.
Kaplan Meier survival curves, stratified according to the fluid balance between 20 and 60 mL/kg in the first 24 hours (A), the number of organ dysfunctions (B), relapse of any type of cancer (C), diagnosis of lymphoma (D), respiratory dysfunction (E) and continuous renal replacement therapy (F). Time is represented in days after the onset of the shock.
There was also a difference in the average number of days to initiate the vasoactive drug after the sepsis recognition (2.23 ± 3.5 for the survivors vs. 5.72 ± 7.2 for the deceased, p = 0.001).
Eighteen patients required initiation of vasoactive drugs after the 10th day of sepsis diagnosis and antibiotic therapy initiation (what was called “late” shock), of whom 14 died (77.7%). Nine of them had positive blood cultures at the onset of sepsis (50%). In 4 cases (22%), a new infection was confirmed due to a different agent from the initial one (Candida krusei, pulmonary aspergillosis with positive galactomannan test, Enterococcus faecium, and Staphylococcus epidermidis). In 2 cases (11%), there was a recrudescence of the infection, with the isolation of the same agent in blood cultures (Klebsiella pneumoniae and Scedosporium prolificans). In one case there was a reactivation of cytomegalovirus. In the other cases, all the cultures were negative.
Patients who required continuous renal replacement therapy (CRRT) had, on average, a greater number of organ dysfunctions (3.79 versus 3.32, p = 0.018 by T-test). Twenty-eight patients required CRRT, and 20 died (71.4%). In a multivariate Cox regression model, the variables “recurrence of solid tumors” (Hazard ratio = 6, 95% CI 2.2 - 16.3, p < 0.001), and “PRISM IV score” (Hazard ratio = 1.1, 95% CI 1.1 – 1.2, p = 0.028) were the only independent predictors of death in these patients. The model was significant with p = 0.0012.
DiscussionThis study included only patients with septic shock admitted in the ICU, and the observed mortality (41%) corresponds approximately to five times the average rate of the ICU in the period. In 2005, Fiser et al reported a mortality rate of 64% in children with cancer, septic shock, and need for mechanical ventilation and inotropic support.8 Over the years, mortality from sepsis has decreased with the early recognition protocols and the application of measures to reduce the risk of multiple organ dysfunctions, but in the population of children with cancer, it remains unacceptably high.
One of the most interesting findings of this study is the observation that patients who had a positive fluid balance between 20 and 60 mL/kg or 2% and 6% of body weight in the first 24 hours after the onset of sepsis had a lower risk of death in the ICU. This correlation was maintained independently in a multivariate Cox regression model with variables of clinical severity and number of organ dysfunctions. Most importantly, represents a controllable situation that can be modified by the intervention of the assistant team.
Children with sepsis have a high incidence of capillary leak syndrome, vasodilatation, and decreased fluid intake, causing relative hypovolemia and a decreased preload.9 However, fluid overload can be harmful: in children with septic shock, a percentage of fluid overload above 10% of the body weight within 96 hours of shock has been correlated with higher mortality, independently of the hemodynamic profile.10 In a large observational cohort study, Barhight et al. found a dose-dependent effect of fluid overload on the odds of death: the 10-20% stratum of fluid overload had an increase of 1.8 times, and over 20% stratum had 2.6 times.11
Fluid creep (fluid administered mainly as the vehicle for drugs) and maintenance fluids can be responsible for 60% of the water administered to critically ill children and can also account for most sodium and chloride (56 and 58%).12 Controlling the amount of water and electrolytes can improve survival, and the present study suggests that would be a protective range in the fluid balance in the first 24 hours after the onset of sepsis.
Ten patients had refractory shock dying in the first 24 hours after the onset of signs of poor tissue perfusion or hypotension, within a catastrophic progression. For these patients, a statistical model that reasonably explains the early deaths included variables related to the underlying disease (neutropenia, relapse of solid tumors), age (the higher the age, the lower the risk), and acute dysfunctions such as respiratory, hematological, and neurological. Schlapbach et al. described a mortality of 36.8% in the first 24 hours in children with sepsis and septic shock, remarking that the severely deranged physiological parameters persisting during the admission may represent a description rather than a prediction of death.13
Among the patients who died, the mean time to start vasoactive drugs after the initial diagnosis of sepsis was longer than that in patients who survived. This difference was explained because shock occurred late in 18 patients, that is, ten or more days after the initial diagnosis of sepsis, and this recrudescence caused great lethality, with 14 deaths (77.7%). The phenomenon of sepsis recrudescence is poorly studied and understood. In adults, DeMerle et al. described a rehospitalization rate for new sepsis within 90 days after discharge of 30%, estimating that between 50 to 80% of cases it was not a recrudescence of the original infection, but a new infection, provided by the immune response impaired by both the original sepsis and the profound alteration in the intestinal microbiome caused by the use of antibiotics.14 The authors can suppose that, in the already immunosuppressed patients, the late shock was due to massive translocation of intestinal bacteria,15 selection of multiresistant bacteria in a new infection, inappropriate initial antimicrobial coverage, lack of infection source control, or reactivation of latent viruses or of the original infection.
The recurrence of any cancer was an important risk of death in the present study's patients and it proved to be an independent predictor in various multivariate models. The authors found a hazard ratio of 2.8 for relapse of any cancer in a model, which included severity scores; Sano et al. described a hazard ratio of 4.1 for sepsis-related death in children with cancer and relapse or refractory state of the disease.16 Along with the immunosuppression caused by the underlying disease, recurrence also carries the burden of the cumulative effect of chemotherapy: a linear association between the accumulated cycles of chemotherapy and the sepsis rate has been described, and patients who had received two or more courses of chemotherapy presented an increased incidence of a neutropenic septic shock compared with those receiving only one course.17
In the analysis of the present study's subgroup of patients who required continuous renal replacement therapy (CRRT), the authors observed higher means in the number of organ dysfunctions, and out of 28 patients, 20 died (71%). Solid tumor recurrence and PRISM-IV were the only independent predictors of death in these patients. A large observational study in the Netherlands showed that in pediatric cancer and hematopoietic stem cell transplant patients requiring continuous renal replacement therapy, the mortality was 54%, and the raw PICU mortality, 11%.18
Besides renal dysfunction, the number of organ dysfunctions was a major determinant of death in patients with septic shock in several studies. In Fiser et al., the presence of four or more organ dysfunctions resulted in survival of less than 30%.8 In the patients with four or more organ dysfunctions, the mortality was 51%. Some aggressive diseases, like acute leukemia and high-grade B-cell lymphoma, can cause multiple organ dysfunctions through tissue infiltration by tumor cells, anatomical compression, intracellular metabolite release, altered coagulation, and hemophagocytic lymphohistiocytosis.19,20
In the present study, the authors found more frequently in blood culture isolates Klebsiella pneumoniae and Escherichia coli. Recently, a predominance of Gram-negative bacteria as the cause of sepsis in cancer patients has been described, particularly E. coli and Klebsiella species.21 Gram-negative agents are more frequently isolated in pediatric cancer patients with septic shock and are also more commonly associated with mortality.22,23
There are obvious limitations in this study. The retrospective nature excluded the possibility of analyzing important variables, such as the adequacy of antibiotic administration in the first hour. As it was conducted in a single center, the observations may not be generalizable. The absence of detailed data on immunosuppression, like absolute lymphocyte counts, or concurrent immunosuppressive drug administration in addition to corticosteroids, is also a weakness. The lack of screening for viral infections also impoverishes the analysis of sepsis recrudescence and etiology.
Death within the first 24 hours of septic shock was correlated to hematological, neurological, and respiratory dysfunctions, age, neutropenia, and the status of solid tumor relapse. Sepsis recrudescence is a possible explanation for cases with late shock and high lethality. Recurrence of any cancer is an important risk of sepsis-related death. A positive fluid balance between 20 and 60 mL/kg or 2% and 6% of body weight in the first 24 hours after the onset of sepsis was related to lower mortality.
Only institutional (IOP/GRAAC).