The aim of this study was to verify the association of echocardiogram, ferritin, C-reactive protein, and leukocyte count with unfavorable outcomes in pediatric sepsis.
MethodsA prospective cohort study was carried out from March to December 2014, with pediatric critical care patients aged between 28 days and 18 years. Inclusion criteria were diagnosis of sepsis, need for mechanical ventilation for more than 48h, and vasoactive drugs. Serum levels of C-reactive protein, ferritin, and leukocyte count were collected on the first day (D0), 24h (D1), and 72h (D3) after recruitment. Patients underwent transthoracic echocardiography to determine the ejection fraction of the left ventricle on D1 and D3. The outcomes measured were length of hospital stay and in the pediatric intensive care unit, mechanical ventilation duration, free hours of VM, duration of use of inotropic agents, maximum inotropic score, and mortality.
ResultsTwenty patients completed the study. Patients with elevated ferritin levels on D0 had also fewer ventilator-free hours (p=0.046) and higher maximum inotropic score (p=0.009). Patients with cardiac dysfunction by echocardiogram on D1 had longer hospital stay (p=0.047), pediatric intensive care unit stay (p=0.020), duration of mechanical ventilation (p=0.011), maximum inotropic score (p=0.001), and fewer ventilator-free hours (p=0.020).
ConclusionCardiac dysfunction by echocardiography and serum ferritin value was significantly associated with unfavorable outcomes in pediatric patients with sepsis.
Verificar a associação do ecocardiograma, da ferritina, da Proteína C Reativa (PCR) e da contagem de leucócitos com desfechos desfavoráveis na sepse pediátrica.
MétodosEstudo de coorte prospectivo, no período de março a dezembro de 2014, com pacientes críticos pediátricos de idade entre 28 dias e 18 anos. Critérios de inclusão foram diagnóstico de sepse, necessidade de ventilação mecânica (VM) por mais de 48 horas e uso de drogas vasoativas. Avaliaram-se os níveis séricos PCR, ferritina, contagem de leucócitos, no recrutamento (D0), 24 horas (D1) e 72 horas (D3) após o recrutamento. No D1 e no D3 todos pacientes foram submetidos a ecocardiograma transtorácico para determinação da Fração de Ejeção (FE) do ventrículo esquerdo. Os desfechos avaliados foram tempo de internação hospitalar e na Unidade de Terapia Intensiva pediátrica (UTIP); duração da VM; horas livres de VM; duração do uso de inotrópicos; escore de inotrópicos máximo e mortalidade.
ResultadosVinte pacientes completaram o estudo. Ferritina elevada no D0 associou-se com menor tempo livre de ventilação (p=0,046) e maior escore de inotrópicos máximo (p=0,009). A disfunção cardíaca pelo ecocardiograma no D1 relacionou-se com maior tempo de internação hospitalar (p=0,047), de UTIP (p=0,020), VM total (p=0,011), escore de inotrópicos máximo (p=0,001) e menor tempo livre de VM (p=0,020).
ConclusãoA disfunção cardíaca pelo ecocardiograma e o valor de ferritina sérica associaram-se significativamente com desfechos desfavoráveis nos pacientes pediátricos com sepse.
Sepsis remains an important cause of morbidity and mortality in the pediatric intensive care unit (PICU) environment. Finding tools that can anticipate or monitor unfavorable evolution in sepsis can contribute to the improvement of care in these critically-ill patients.1,2
Thus, several biological markers have recently been studied as tools to evaluate disease progression in bacterial infections, sepsis, and septic shock.1–8 Among the biomarkers, the most often used in the authors’ setting are leukocyte count, C-reactive protein (CRP), and ferritin levels, the last two having limited studies in pediatrics correlating serum levels with unfavorable outcomes.1,4,6–8
In pediatric sepsis, myocardial dysfunction is one of the main causes of clinical deterioration.9 Myocardial dysfunction may be present in up to 50% of cases of severe sepsis or septic shock, causing systolic or diastolic ventricular dysfunction and contributing to shock and mortality.10 The echocardiogram is already used in the management of patients with septic shock during volumetric resuscitation and to choose the best vasoactive drug.11,12 It is speculated that evaluations obtained by echocardiographic assessment can be used as markers of sepsis evolution. Additionally, few studies have associated these measures with unfavorable outcomes in pediatric sepsis.13
The present observational study evaluated the evolution of left ventricular ejection fraction (EF) as measured by echocardiography, serum ferritin and CRP, as well as leukocyte count in critically ill patients with sepsis. Furthermore, measures of these markers were associated with unfavorable outcomes.
MethodsThis prospective cohort study was developed at the PICU of Hospital São Lucas of Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), located in Porto Alegre, state of Rio Grande do Sul, southern Brazil, from March to December 2014. This unit receives patients aged 28 days to 18 years with clinical and surgical diseases and has 12 beds for hospitalization.
All patients hospitalized during the abovementioned period who required mechanical ventilation (MV) for more than 48h using cardiovascular support (except for dopamine at a dose <5mcg/kg/min) and who had clinical diagnosis or suspicion of sepsis were included. Exclusion criteria were: congenital heart disease, presence of confirmed or suspected endocrine disease involving the somatotropic and corticotropic axes, need for hemofiltration or any other renal replacement therapy, diagnosis of congenital or acquired immunosuppression, confirmed or suspected congenital glucose metabolism alterations, severe liver impairment, preterm birth, and weight <4kg.
Sepsis was defined as the presence of two or more of the following four criteria: tachycardia, tachypnea, temperature change, leukocytosis, or leukopenia for age in the presence of confirmed or suspected infection. The organic dysfunctions were classified according to Goldstein et al.14 The presence of two or more organic dysfunctions was considered as multiple-organ dysfunction syndrome.
All patients in the study had their serum levels of CRP, ferritin, and leukocyte counts assessed at study entry (D0), 24h (D1), and 72h (D3) after recruitment. The sample was stratified according to CRP values (greater than 7.6mg/dL and 16.2mg/dL),2 ferritin (≥300ng/mL),15 and leukocyte count (<5000/μL and >15,000/μL) for association with outcomes.
On D1 and D3, all patients underwent transthoracic echocardiography to determine left ventricular ejection fraction (EF). The EF represents the ejected volume, in percentage, of the left ventricular end-diastolic volume, i.e., how much blood is ejected into the aorta at systole. The Teichholz formula was used and cardiac dysfunction was considered when the EF was <55%.16
The left ventricular shortening fraction was also measured, using the same formula, in all patients. The results obtained were overlapping; therefore, it was decided to use only the EF in the statistical analyses. The device used was the Siemens Acuson Cypress (Siemens®, Munich, Germany), with a 3MHz transducer. All assessments were performed using the same device and by the same pediatric cardiologist, with experience in the Pediatric Cardiology Service of the Hospital São Lucas of PUCRS. Each examination had three consecutive measurements to minimize the effect of respiratory variation, mainly caused by mechanical ventilation. Kappa value was calculated to evaluate the intraobserver agreement and a Kappa of 0.80 was considered acceptable.
The following outcomes were evaluated: length of hospital stay (days), length of stay in the PICU (days), time of total MV (hours), time without MV (hours), total time of inotropic use, maximum inotropic score, and mortality.
To calculate the time without MV, a maximum of 28 days of mechanical ventilation (672h) were considered, i.e., the value corresponding to the number of hours free from mechanical ventilation was calculated by subtracting the time of total mechanical ventilation (hours) from 672h. If the patient remained more than 672h with MV, a value equal to zero was considered.17 For the maximum inotropic score, the highest value, obtained on any day of the study, was calculated through a summation obtained from the formula: dose of dopamine+dobutamine+(epinephrine×100)+(noradrenaline×100)+(milrinone×10). All of them were expressed in mcg/kg/min.18 The Pediatric Index of Mortality 2 (PIM2) was calculated on the first day of the PICU, according to the routine of this service.19 A PIM2 value of 6% was chosen as the cutoff point for severity, as it is the upper limit of historical mortality in this service.
Regarding the statistical analysis, the numerical data were expressed in absolute values and percentages. Demographic data such as age, weight, gender, type of organ dysfunction, presence of infection, and origin of the patient were obtained through the electronic medical record. The Kolmogorov–Smirnov test was used to verify sample normality, with a sample being considered normal when the value was >0.05. Qualitative (categorical) variables were expressed as absolute values and percentages, and when the sample was stratified, the groups were compared using Pearson's chi-squared test or Fisher's exact test. Quantitative variables were expressed as mean and standard deviation, and those with asymmetric distribution, as median and interquartile range (IQR). When the sample was stratified, the groups were compared using Student's t-test or ANOVA for variables with normal distribution and the Mann–Whitney–Wilcoxon or Kruskal–Wallis test for variables with non-normal distribution. Values of p<0.05 were considered significant. Data analysis was performed using the IBM Statistical Package for Social Sciences (IBM SPSS Statistics 20).
This study was approved by the research ethics committee of Hospital São Lucas of PUCRS, under No. 474,050, issued on 11/27/2013. Authorization was requested to participate in the study through an informed consent from the parents or guardians of all the recruited patients.
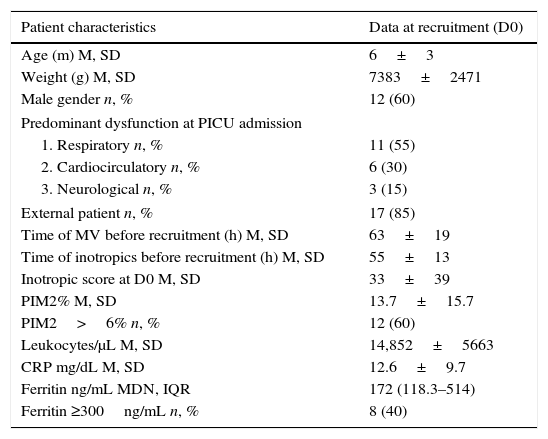
ResultsThere were 337 hospitalizations during the recruitment period and, of these, 41 patients were eligible for the study. Parental consent was not granted in four cases and one child died before the start of the examination. Of the 36 who had samples collected, eight children were excluded after they failed to complete the echocardiographic study and eight were excluded due to error, loss, or insufficient collection of some of the study material. The remaining 20 children completed the protocol. There were no baseline differences in characteristics, severity measured by PIM2, and mortality between the excluded patients and those remaining in the study. The general characteristics of the study population are described in Table 1.
Overall characteristics of the sample at recruitment.
| Patient characteristics | Data at recruitment (D0) |
|---|---|
| Age (m) M, SD | 6±3 |
| Weight (g) M, SD | 7383±2471 |
| Male gender n, % | 12 (60) |
| Predominant dysfunction at PICU admission | |
| 1. Respiratory n, % | 11 (55) |
| 2. Cardiocirculatory n, % | 6 (30) |
| 3. Neurological n, % | 3 (15) |
| External patient n, % | 17 (85) |
| Time of MV before recruitment (h) M, SD | 63±19 |
| Time of inotropics before recruitment (h) M, SD | 55±13 |
| Inotropic score at D0 M, SD | 33±39 |
| PIM2% M, SD | 13.7±15.7 |
| PIM2>6% n, % | 12 (60) |
| Leukocytes/μL M, SD | 14,852±5663 |
| CRP mg/dL M, SD | 12.6±9.7 |
| Ferritin ng/mL MDN, IQR | 172 (118.3–514) |
| Ferritin ≥300ng/mL n, % | 8 (40) |
Normality verified by the Kolmogorov–Smirnov test.
m, months; g, grams; M, mean; MDN, median; IQR, interquartile range; n, number; h, hours; %, percentage; SD, standard deviation; MV, mechanical ventilation; D0, day zero; PIM2, pediatric index of mortality 2; CRP, C-reactive protein.
At recruitment, when stratified by severity (PIM2 <6% or ≥6%), the authors did not find any differences in demographic characteristics, diagnoses at hospitalization, time of MV, and time of inotropic use and score. Among the laboratory markers of inflammatory response, leukocyte count and CRP did not differ between the groups. Only ferritin values were higher among the most severe cases (mean and standard deviation: 454.4±309.7 vs. 91.9±6ng/mL; p=0.005).
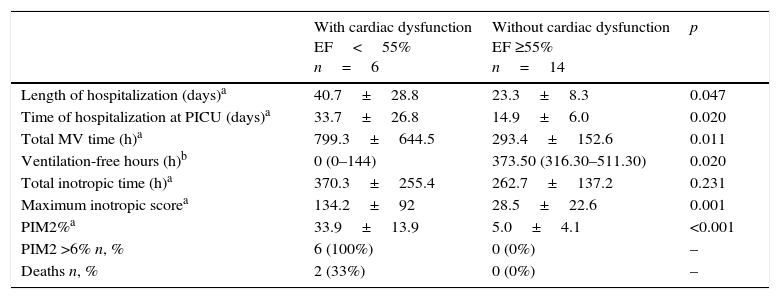
Cardiac output (EF)All patients were submitted to echocardiogram on the first and third study days after recruitment. Overall, EF values increased in a discrete and non-significant manner during the study interval. Six patients (30%) had EF<55%, characterizing cardiac dysfunction. Of these, two (10%) recovered cardiac function on D3. Patients with cardiac dysfunction on the first day had higher PIM2 at the PICU admission and had a significant association with unfavorable outcomes. Two patients from this group died (Table 2).
Cardiac dysfunction by echocardiogram on the first day (D1) post-recruitment and outcomes.
| With cardiac dysfunction EF<55% n=6 | Without cardiac dysfunction EF ≥55% n=14 | p | |
|---|---|---|---|
| Length of hospitalization (days)a | 40.7±28.8 | 23.3±8.3 | 0.047 |
| Time of hospitalization at PICU (days)a | 33.7±26.8 | 14.9±6.0 | 0.020 |
| Total MV time (h)a | 799.3±644.5 | 293.4±152.6 | 0.011 |
| Ventilation-free hours (h)b | 0 (0–144) | 373.50 (316.30–511.30) | 0.020 |
| Total inotropic time (h)a | 370.3±255.4 | 262.7±137.2 | 0.231 |
| Maximum inotropic scorea | 134.2±92 | 28.5±22.6 | 0.001 |
| PIM2%a | 33.9±13.9 | 5.0±4.1 | <0.001 |
| PIM2 >6% n, % | 6 (100%) | 0 (0%) | – |
| Deaths n, % | 2 (33%) | 0 (0%) | – |
Normality verified by the Kolmogorov–Smirnov test.
n, number; h, hours; %, percentage; MV, mechanical ventilation; PIM2, Pediatric Index of Mortality 2.
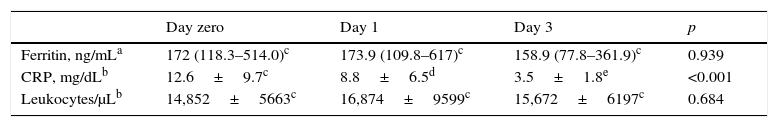
Inflammatory mediators were elevated at recruitment and showed different patterns throughout the study. Ferritin was within normal limits in most patients, with no significant decrease on D3. CRP levels were extremely high at recruitment and decreased significantly, but still showed abnormal levels on D3. Total leukocytes remained elevated throughout the study period (Table 3).
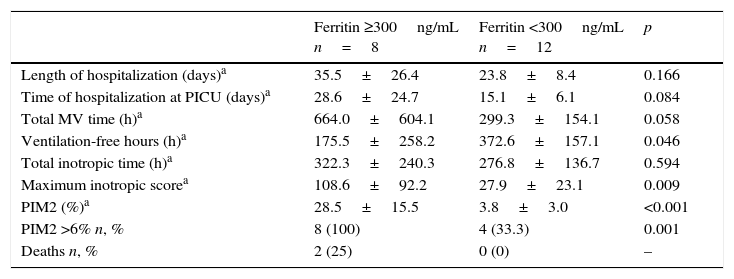
Patients with hyperferritinemia (≥300ng/mL) at recruitment (D0) had more severe disease on the first day at the PICU (higher PIM2) and had the worst outcomes. The two patients who died belonged to this group (Table 4).
Hyperferritinemia at recruitment (D0) and outcomes.
| Ferritin ≥300ng/mL n=8 | Ferritin <300ng/mL n=12 | p | |
|---|---|---|---|
| Length of hospitalization (days)a | 35.5±26.4 | 23.8±8.4 | 0.166 |
| Time of hospitalization at PICU (days)a | 28.6±24.7 | 15.1±6.1 | 0.084 |
| Total MV time (h)a | 664.0±604.1 | 299.3±154.1 | 0.058 |
| Ventilation-free hours (h)a | 175.5±258.2 | 372.6±157.1 | 0.046 |
| Total inotropic time (h)a | 322.3±240.3 | 276.8±136.7 | 0.594 |
| Maximum inotropic scorea | 108.6±92.2 | 27.9±23.1 | 0.009 |
| PIM2 (%)a | 28.5±15.5 | 3.8±3.0 | <0.001 |
| PIM2 >6% n, % | 8 (100) | 4 (33.3) | 0.001 |
| Deaths n, % | 2 (25) | 0 (0) | – |
n, number; h, hours; %, percentage; MV, mechanical ventilation; PIM2, Pediatric Index of Mortality 2.
Normality verified by the Kolmogorov–Smirnov test.
This was one of the few cohort studies that jointly analyzed sepsis biomarkers, echocardiogram measurements, evolution, and outcomes in critically ill pediatric patients. The inclusion criteria were strict; all patients were on mechanical ventilation for at least 48h and required vasoactive drug support. PIM2 was higher than 6% in 12 patients (60%), indicating high severity of the recruited individuals. The mortality rate was 10%, which is compatible with the literature.18,20 The present study demonstrated a significant association between low cardiac systolic function, represented by EF, and hyperferritinemia with unfavorable outcomes.
Cardiac dysfunction in pediatric sepsis is a widely known, but not yet fully understood condition.9 The finding of left ventricular cardiac dysfunction in septic shock (low EF) of 30% is consistent with studies recently published by Raj et al., 37%; Pulido et al., 27%; and Furian et al., 33%.9,21,22 The last two authors did not find an association between low EF and unfavorable outcomes or mortality when studying adult patients. However, Carmona et al. found increased mortality in pediatric patients with septic shock that had an EF <45% on the first day of PICU admission, a result similar to that of the present study.13 None of these studies associated cardiac dysfunction measured by EF with other clinical outcomes. A possible explanation for these findings is that cardiac dysfunction, in severe sepsis or septic shock, has a greater influence on the clinical picture of pediatric patients when compared to that of adult patients, who predominantly have vasoplegic shock.9,10 Thus, patients who are already receiving optimized inotropic therapy and still have cardiac dysfunction will tend to have a worse outcome.
Ferritin was the most prominent inflammatory marker in this study. It is an iron-storing protein, responsible for releasing it in a controlled manner. In inflammatory processes, a great production of this protein occurs, inducing a decrease in serum iron, believed to minimize the availability of iron to microorganisms. For this reason, ferritin in critically ill pediatric patients may be elevated, and it is associated with severity in some diseases.6–8 The mortality rate in patients with ferritin >3000ng/mL is 3-fold higher.8
In the present study, 40% of the patients had high levels of this biomarker on D0. When the sample was stratified into two groups using the 300ng/mL cutoff point, a significant association between hyperferritinemia and unfavorable outcomes was observed, such as fewer mechanical ventilation-free hours and higher maximum inotropic score. Additionally, the two patients who died belonged to this group. Garcia et al. had previously associated ferritin levels >500ng/mL with mortality.6 Sustained hyperferritinemia or very high values of this marker represent an intense inflammatory response scenario that should be beneficial, but seems to be an indicator of unfavorable outcomes.7 The present study used a cut-off point of 300ng/mL, since in a previous study by Laks and Garcia,15 this was the closest median value in patients with septic shock. The reduction of the cutoff point to 300ng/mL accentuates the results of this study.
Rey et al. published in their study in 2007 a stratification of CRP values in pediatric patients with systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock.2 Similarly to Rey, in the present study, CRP at recruitment was elevated as in septic shock (12.6±9.7mg/dL), and showed a significant reduction on subsequent days. On D3, patients still had abnormal values of this biomarker (3.5±1.8mg/dL). However, there was no association between the highest values of CRP found in this sample and unfavorable outcomes.
Studies that stratify CRP values in septic shock in pediatric patients are scarce. Leukocyte count was not useful as a marker of severity either, as its value remained constant. Initially, for leukocyte analysis, the authors used normal values as reference, as limit values were not found in the literature that defined prognosis for this marker. Some studies have already demonstrated the low validity of leukocyte count as a diagnostic and prognostic marker in pediatric sepsis.2,23
Study limitationsSome limitations of this study should be indicated. The first is related to EF measurement by echocardiogram, which is a professional-dependent assessment. This method was chosen, despite its limitations, because it is available in most PICU services in Brazil. The second is the use of PIM2 as an outcome, representing a prognostic index indicating mortality. Patients with cardiac dysfunction and hyperferritinemia had higher PIM2. Considering that PIM2 is used in patient populations to estimate mortality, the finding of the association of this measure of severity with biomarkers allows an interesting application of this index, which is widely used in Brazilian PICUs.
The third is the lack of other biomarkers already studied in pediatric sepsis. It was decided to study the ones that are easily obtained and most commonly used in Brazil. Finally, the number of patients was a limitation. The authors studied an expressive group of very severe patients, in whom the inclusion and exclusion criteria were strict. The sample, while producing significant differences in results, had a low statistical power.
In brief, cardiac dysfunction by echocardiogram (EF<55%) on D1 and serum ferritin values (≥300ng/mL) on DO, obtained in pediatric patients with sepsis admitted to the PICU, were significantly associated with unfavorable outcomes.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), whose funding was approved by process No. 485488/2011-6 – Research Project Support – Universal 14/2011.
Conflicts of interestDr. Pedro Celiny R. Garcia has a grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES BRASIL). The others authors declare no conflicts of interest.
To CAPES, for the supplied grants, and to the Postgraduate Program in Pediatrics and Child Health of PUCRS, for allowing the authors to carry out this study.
Please cite this article as: Tonial CT, Garcia PC, Schweitzer LC, Costa CA, Bruno F, Fiori HH, et al. Cardiac dysfunction and ferritin as early markers of severity in pediatric sepsis. J Pediatr (Rio J). 2017;93:301–7.
Study carried out at Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Postgraduate Program in Pediatrics and Child Health, Porto Alegre, RS, Brazil.