The prevalence of food allergies (FA) has increased worldwide over the last few decades. Milk, eggs, and peanuts are among the most common allergens and can cause anaphylaxis. Therefore, we aimed to identify biomarkers that could predict the persistence and/or severity of IgE-mediated allergies to milk, eggs, and peanuts via a systematic review.
MethodsThis systematic review proceeded according to a protocol registered in the International Prospective Register of Systematic Reviews. Two independent authors extracted studies of interest from PubMed, SciELO, EMBASE, Scopus, and Ebsco databases and assessed their quality using the Newcastle-Ottawa Scale.
ResultsWe selected 14 articles describing 1,398 patients. Among eight identified biomarkers, total IgE, specific IgE (sIgE), and IgG4 were the most often cited biomarkers of persistent allergies to milk, eggs, and peanuts. Skin prick tests, endpoint tests, and sIgE cutoff levels may predict positive responses to challenges with these foods. The basophil activation test is a biomarker for the severity and/or threshold of allergic reactions to milk and peanuts.
ConclusionOnly a few publications identified possible prognostic indicators of the persistence or severity of FA and outcomes of oral food challenges, indicating that more accessible biomarkers are needed to determine the likelihood of having a severe food allergic reaction.
Immunoglobulin E (IgE)-mediated food allergy (FA), an immunological response that can occur after exposure to food, has become a global public health problem.1,2 The prevalence of FA has increased over the last few decades and is considered to affect up to 45 million children and 12 million adults worldwide.2-4 Food allergies affect the quality of life of patients and their families, impact their daily activities, and can become life-threatening.2,5,6 More than 170 foods are possible causes of allergic reactions.7 Although FAs are the main cause of anaphylaxis, the risk of anaphylaxis due to food ingestion in patients with FAs has not been defined.8 The most common foods that cause allergies are milk, eggs, and peanuts; however, it is difficult to determine their prevalence due to regional variations.9,10
The effectiveness and safety of FA prevention measures remain undetermined. Preventive approaches, such as breastfeeding and the early introduction of some foods, can induce oral tolerance.11-13 Oral immunotherapy has also been used as a treatment; however, it can be associated with an increased incidence of allergic side effects, and patients do not always achieve tolerance.14,15 An allergenic food can be omitted from the diet, but accidental ingestion can lead to severe and occasionally fatal reactions.8,7,16 Current technological developments provide a deeper understanding of key immune cells and molecular mechanisms associated with FA, which might facilitate the safe treatment of different FA phenotypes and endotypes using precision medicine.17,18 The characterization of sensitive and specific biomarkers can improve diagnostic accuracy and guide decisions concerning the prevention, diagnosis, and management of FA.17
Although FA can be diagnosed using in vivo and in vitro tests, the gold standard remains the oral food challenge (OFC). Double-blind placebo-controlled food challenges (DBPFC) are expensive, time-consuming, labor-intensive, and limited to well-equipped clinics or hospitals; further, they can be potentially dangerous, because they can trigger anaphylactic reactions.19-21 Patients who are sensitized to a portion of food based only on clinical history or being positive in a screening test might not react to the same food in a DBPFC, implying that sensitization does not necessarily indicate the presence of food allergies.4,19 Therefore, diagnostic tools are needed to improve accuracy and guide decisions concerning the prevention, management, and treatment of FAs.17,22,23
This systematic review aimed to identify biomarkers that could predict the persistence and/or severity of egg, milk and peanut IgE-mediated allergy. Biomarkers could be useful to recognize patients with FAs who have become tolerant to a specific food and would not require an exclusion diet. Moreover, biomarkers could also identify patients with allergies who could have a severe reaction to an OFC and avoid exposing them to harmful events such as a severe anaphylactic reaction.
MethodsThis systematic review proceeded according to a protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42020185609) and has been reported according to the PRISMA statement.24
Data sources and search strategyWe searched the PubMed, SciELO, EMBASE, Scopus, and Ebsco databases for studies of food allergies using the terms, “biomarker” AND “food” AND “allergy” in the title and abstract. Articles were limited to those published in English in the last decade. We limited the search to this time frame to access the most recent data on biomarkers. The search results were managed using Mendeley software. Duplicate articles were excluded from the analysis. The searches were repeated before the final analysis.
Study exclusion criteriaAll titles, followed by abstracts, were screened for relevance using the following exclusion criteria: animal studies; languages other than English; studies of food allergies that did not include milk, eggs, and/or peanuts, and non-IgE-mediated food allergies. All relevant full-text articles were screened based on the following exclusion criteria: non-original articles, only congress abstracts available without a description of the study, and full-text unavailable. Systematic reviews, meta-analyses, cross-sectional studies, conference abstracts, case reports, case series, non-original articles, and animal studies were excluded.
Study eligibility criteriaWe included articles on all studies that identified a biomarker related to the persistence or severity of IgE-mediated milk, egg, and/or peanut allergy and were conducted on children and adults of any age, with IgE-mediated food allergies to milk, eggs and/or peanuts. All patients were confirmed to have allergies using skin prick tests (SPTs), serum-specific IgE (sIgE), and/or OFC. All articles were independently assessed by two authors (MM and RF).
Data extractionThe two independent authors extracted data from the selected studies. They screened titles and abstracts and excluded those that were not related to the predetermined inclusion criteria. Full texts were obtained from the selected articles. Disagreements were resolved by consultation with a third author (CP). All studies were independently assessed and extensively discussed by all authors. The authors documented the extracted data (reference, first author, type of food, biomarker, and the number of patients included) from the articles using Excel spreadsheets.
Qualitative gradingWe assessed the methodological quality evaluation and risk of bias in the included studies using the validated Newcastle-Ottawa Scale (NOS), which contains eight items within three domains: selection of study groups, comparability of groups, and ascertainment of exposure/outcomes. Scores of 0–3, 4–6, and 7–9 indicated low-, moderate-, and high-quality studies, respectively.25
Data synthesisAll descriptive analyses were performed in an Excel spreadsheet, shared by the authors (MM, RF, CP) after deciding which articles would be included in the systematic review.
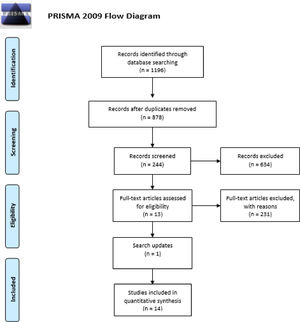
ResultsSearch outcomesThe search identified 1196 articles among which, 318 were duplicated, and 634 were excluded after screening the title and abstract. Full texts were then obtained from 244 articles. The analysis of study type excluded 63 reports that were not original, resulting in 181 articles. Analyses of the food allergies described led to the exclusion of 143 articles that were not related to peanuts, milk, or eggs. Another 25 of 38 reports were excluded because they did not meet the inclusion criteria for biomarker function. Most of them were diagnostic, and not directly related to the persistence or severity of FA.
The search was repeated before the final analysis, which led to the inclusion of an additional study.26 We finally included 14 studies (Figure 1) comprising 1398 patients, of whom 48 were allergic to two or three foods, and 1350 were allergic only to eggs (n = 256), milk (n = 370), or peanuts (n = 724).
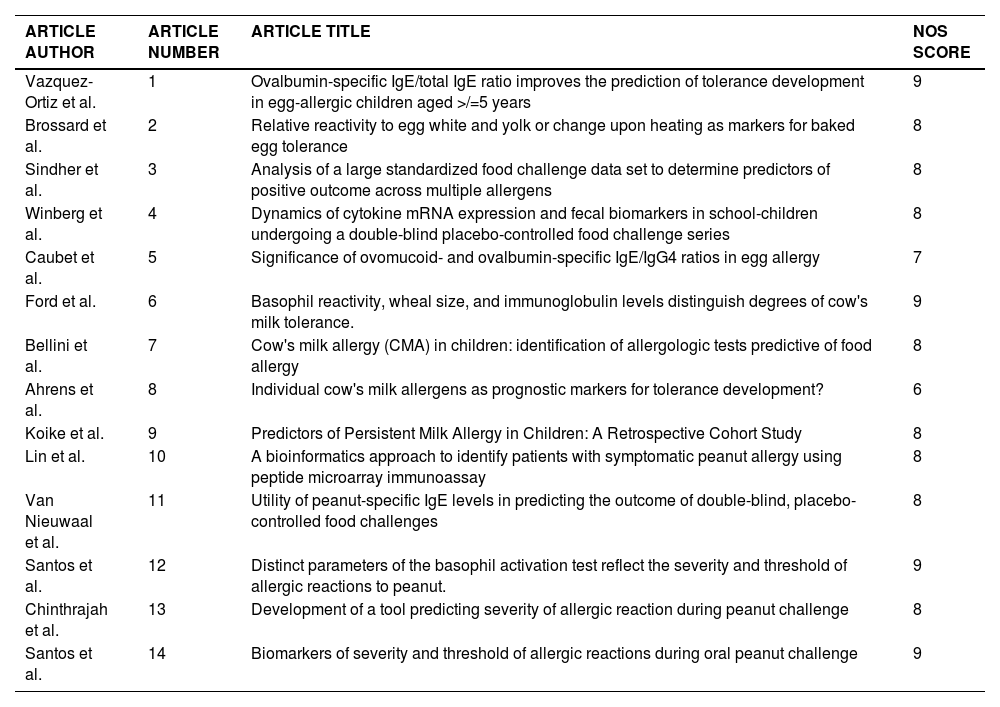
Risk of bias and quality assessmentRisk of bias and quality was assessed using the NOS, and each included study was scored on the selection of participants, comparability, and outcome standards indicated by the NOS. The quality of one article was scored as moderate, whereas the others were classified as high-quality articles (Table 1).
Article identification and Newcastle-Ottawa Scale (NOS) score.
We identified the following biomarkers in the 14 studies: positive SPT and endpoint test (EPT) outcomes, Total IgE (tIgE), sIgE, sIgG4, a positive basophil activation test (BAT) outcome, cytokines, and the fecal biomarkers calprotectin and eosinophil-derived neurotoxin (EDN). A positive SPT outcome and sIgE were biomarkers in egg, milk, and peanut allergy studies. A positive BAT outcome was described as a biomarker in both milk and peanut allergy studies. Egg allergies were tested using tIgE, sIgG4, and the ratio between them. A study on egg allergies described cytokine expression and fecal biomarkers, whereas a study on milk allergies described EPT outcomes.
Among the six peanut studies included, the major determinants of persistence and the severity of peanut allergy were positive BAT and SPT outcomes, as well as sIgE and IgG4. The BAT accurately predicted severe allergic reactions in children with peanut allergies. Higher proportions of activated basophils were associated with more severe reactions during peanut OFC. The BAT results could estimate the severity and threshold of peanut-induced allergic reactions; a high CD63 ratio led to a higher risk of life-threatening reactions in three studies, one of which included adult patients.26-28 An SPT result of ≥ 8 mm in children indicated a high risk of a reaction during OFC.26 Specific IgE cutoffs can also predict the persistence of peanut allergies. The combination of SPT and sIgE could predict the outcomes of oral food challenges, thereby indicating the persistence and severity of allergic reactions.29,30 One study applied a bioinformatics approach to access results from peptide microarray immunoassays aimed at identifying children with peanut allergies. The authors found that a combination of the peptide biomarkers Ara h 2_10, Ara h 2_16, and Ara h 2_18 could differentiate between peanut-allergic and -tolerant groups.31
Previous anaphylaxis was a risk factor for milk allergy persistence in children aged < 6 years32. SPT results were related to the persistence of allergy.30 High levels of milk sIgE were involved in the persistence of milk allergies in children who underwent an OFC.30,32,33 One study identified EPT as a more useful test than SPT and defined EPT as a marker for selecting children at high risk of developing anaphylaxis during food challenges.34 Cytokine IL-13 and IL-10 levels and fecal biomarkers were related to milk allergy persistence;35 additionally, BAT outcomes were associated with more severe clinical milk reactivity, as we found in egg and milk studies.36
A total of 230 OFCs were described in the included studies of egg allergies. The SPT and sIgE cutoffs might predict a positive food challenge and play important roles in the persistence of egg allergies.30 The sIgE;sIgG4 ratios to both ovalbumin (OVA) or ovomucoid (OVM) were higher in 107 children who were reactive to eggs than in those who could tolerate baked eggs.37 Among 95 children with egg allergies who underwent a DBPCFC, a high OVA-sIgE/total IgE ratio predicted the development of tolerance to raw eggs and was superior to sIgE or a positive SPT outcome alone.38 The SPT nullified by hard-boiling eggs or sensitization to egg fractions (low egg white [EW]: egg yolk [EY] sIgE ratio), was associated with baked egg tolerance in a group of children.39 Levels of the cytokines IL-13 and IL-10 were higher among children with FAs who were orally challenged with both egg and milk. Fecal biomarkers such as calprotectin and eosinophil-derived neurotoxin (EDN) were also associated with persistent egg allergies in the same study.35
DiscussionAssays of serum sIgE against specific allergen components or peptides in vitro are the most frequently applied means of diagnosing FAs. However, they are influenced by tIgE levels and potentially different diagnostic cutoff values among studies.40,41 The benefits of tIgE and sIgE levels have been assessed as sIgE:tIgE or sIgE:sIgG4 ratios to enhance the diagnostic and prognostic performance of sIgE. Serum sIgE and sIgE:sIgG4 ratios have assumed an important role in FA natural history and also in predicting immunotherapy response.42 In fact, nine of the 14 selected studies evaluated sIgE, tIgE, and/or sIgG4 for eggs, milk, and/or peanuts, and found that the isolated dosage and ratio between these markers were helpful to predict FA tolerance.29-33,36-39
Egg white and its four allergens (OVM, OVA, ovotransferrin, and lysozyme) are assumed to be the underlying causes of allergic reactions. The selected studies associated high levels of sIgE with egg allergy persistence. These values are important to correlate with tIgE and IgG4, since OVA-sIGE:tIgE predicted tolerance (OVA-sIgE:tIgE) and OVA and OVM-sIgE:IgG4 predicted severity, including anaphylactic reactions.37,38 Brossard et al confirmed the importance of EW over EY fractions, indicating that a high EW/EY ratio could be a marker of egg allergy persistence.39 Although these studies differed in terms of populations and Ig ratios, OVA and OVM are the most studied egg allergens with a direct association between high levels and FA persistence.
Cutoffs for SPT and sIgE have been extensively investigated, and they vary among studies. Two studies proposed that positive SPT outcomes and/or sIgE values are highly predictive of a positive egg, milk, and peanut OFC.29,30 These values can predict the dose above which an OFC outcome might be positive. Although these values could indicate allergy persistence, practical cutoffs are difficult to define, as they depend on many factors, such as patient populations, study protocols, and sample sizes.29,30
The SPT is a safe and highly sensitive method of detecting sIgE to a defined allergen. It is the most inexpensive, popular, and simplest way to evaluate IgE sensitization in vivo.40 Some articles described a high SPT value as a biomarker of FA tolerance or persistence.26,30,37,39 Although high SPT values are described in these reports as being highly predictive of a positive OFC for eggs, milk, and peanuts, the relationship between SPT and OFC outcomes remains controversial. Larger SPT wheels can be related to a positive OFC outcome, but it is not always associated with the severity of allergic reactions.26,30,37,39
The BAT is a functional assay that detects the capacity of IgE to mediate basophil activation after allergen stimulation.43 Basophils express higher levels of their activation markers, CD63 and CD203c in patients with allergies. BAT has acquired a sustained role in distinguishing allergic patients from those who are sensitized but clinically tolerant.42 Four of the selected studies considered BAT outcomes as a biomarker of severity and/or threshold of milk or peanut allergic reactions, and children underwent OFCs to evaluate their responses and degrees of reactions.26-28,36 Santos et al describe a study of 468 children.27 The BAT outcome was an important predictor of the threshold dose of allergic reactions to peanuts during OFCs and accurately identified those who were likely to develop severe or life-threatening reactions during peanut OFC. The optimal cutoff for the BAT outcomes had high sensitivity and specificity for identifying children at high risk of severe peanut allergy reactions.27 Thus, BAT is a biomarker for the severity and threshold of allergic reactions, especially to peanuts according to the studies analyzed here, and should be considered according to clinical history and other risk factors.
Interleukin-10 plays an important role in FA tolerance and is associated with the persistence of milk allergies involved in tolerance induction.44-48 Only one study, performed by Winberg et al, evaluated cytokines as FA biomarkers and showed that a combination of increased numbers of peripheral blood mononuclear cells, IL-13 and IL-10 mRNAs, and fecal calprotectin and EDN are associated with the persistence of allergies to eggs and milk in children.35
Among the 14 included studies, only one presented a high risk of bias according to the NOS (Table 1).33 However, the studies differed in terms of methodological diversity and the variety of studied biomarkers. The BAT, cytokine dosages, and fecal biomarkers are still not routinely accessible. This could explain why the most studied biomarkers were SPT and sIgE, which are the most easily accessible and inexpensive.
Considering the recent increase in FA prevalence and the severity of the reactions, identifying biomarkers of persistence and severity allows patients to avoid the possibility of a harmful event occurring during an OFC. A few studies have identified possible prognostic indicators of persistence or the severity of FA and outcomes during an OFC or a DBPFC. Among the included reports, SPT and sIgE were the most studied biomarkers of FA, and BAT was notably an important biomarker of peanut allergies. Cytokine dosage and fecal biomarkers were evaluated in only one study.
Further investigation is required to identify more accessible biomarkers that could determine the likelihood of having a severe food allergic reaction, thereby preventing the ingestion of food and unnecessary OFCs.