to assess evidence available in the literature about the use of sedation and analgesia for intratracheal intubation of newborns.
Data sourcesby means of an integrative literature review, the authors looked for evidence related to the theme from the last ten years, indexed in the Pubmed, Medline, Lilacs, Scielo, and Scopus databases, by combining the descriptors: newborn, intratracheal intubation, and analgesia. Articles in Portuguese, English, and Spanish that met the research purpose were included.
Data summaryAfter applying the eligibility criteria, ten articles on the topic were obtained, predominantly narrative reviews, retrospective studies, observational studies, and only one non-randomized clinical trial, which characterizes the literature related to the topic as having a low level of scientific evidence. There is still no consensus in the literature on which medications and indications are for use in non-elective intubations, despite the ethical recommendation.
Discussionpain and its deleterious effects should not be neglected. Neonatal Intensive Care Units should have their own protocols regarding sedation and analgesia for intubation considering the individual characteristics of each patient. There is an ethical recommendation regarding the use of sedation and analgesia for intubation since it is a known painful procedure.
In the last three decades, there has been a significant advance in the production of knowledge related to the assessment and management of neonatal pain. The finding that newborns (NB) can detect, process and react to algic1 stimulus and demonstrate permanent changes in immature brains in these patients2,3 have driven research aimed at understanding pain in this population, as well as strategies for its identification and management. In the past, invasive procedures and potentially painful, such as intratracheal intubation itself, were performed without any analgesia or sedation, which today is considered ethically unacceptable.
Intratracheal intubation is a stressful, painful, and potentially dangerous procedure that can lead to airway trauma, laryngospasm, bronchospasm, hemodynamic changes, and increased risk of intracranial hemorrhage. The practice of using premedications before intubation in adults and children, aiming to facilitate the procedure, and reduce pain, stress, and the deterioration of vital signs, is already well established. In the scope of newborns, there is no well-established practice of sedation and analgesia and few studies on the use of premedications.4
Among the main reasons for not using premedications for intratracheal intubation in the neonatal period are the short and long-term effects of these drugs, ranging from respiratory depression, hemodynamic instability, and even induction of neuroapoptosis. Therefore, on one hand, there are studies showing that the exposure to sedatives and analgesics in immature brains of this population is able to lead to important neurological changes, on the other hand, there is sufficient evidence showing the susceptibility to the pain of these patients and the negative neurological impact that these repeated experiences cause.
In this approach and aiming to contribute and add to the efforts to improve newborn care, this research aimed to assess the available evidence in the literature on the use of sedation and analgesia for intratracheal intubation of newborns.
Data collection and synthesisThis is a qualitative study, with data collected from primary and secondary sources, through a bibliographical survey of the integrative literature review type. The following steps of the integrative literature review method were followed: 1st) theme identification and selection of the hypothesis or research matter for the integrative review; 2nd) establishment of criteria for inclusion and exclusion of studies/sampling or literature search; 3rd) definition of the information to be extracted from the selected studies/categorization of the studies; 4th) evaluation of the studies included in the integrative review; 5th) interpretation of results; and 6th) presentation of the knowledge review/synthesis.5
Therefore, the guiding question of this research was: is sedation and/or analgesia used for intratracheal intubation of newborns? Then, a search for scientific articles on the subject was conducted in the period from March to October 2022.
The inclusion criteria for the studies were: articles in Portuguese, English and Spanish, published in the last ten years, in order to have more current evidence about the theme; complete texts that present in their discussions considerations on the use of sedation and/or analgesia for intubation of newborns admitted to neonatal intensive care units, indexed in the Pubmed, Lilacs, Medline, Scopus, and Scielo databases
The exclusion criteria were: articles repeatedly indexed in the databases and articles that did not meet the research objectives.
To perform the search, a combination of the following descriptors was used DeCS (Descritores em Ciência de Saúde - Health Science Descriptors) and MeSH (Medical Subject Heading): analgesia; newborn; and intubation, intratracheal. The boolean operator and other terms were used.
For the analysis and subsequent synthesis of the articles that met the inclusion criteria, a synoptic summary was used especially built for this purpose, which contemplated the following aspects: name of the research, year of publication, country, author, journal in which it was published, results/conclusion, and level of evidence.
The presentation of the results and discussion of the data obtained was descriptive, allowing the reader to evaluate the applicability and the positive impact on the quality of care of the newborn.
In this research the authors used, mainly, as a theoretical reference by Fritjof Capra, who through Systems Theory demonstrated that all problems are interconnected and interdependent, and this characteristic should be considered when thinking about an intervention.
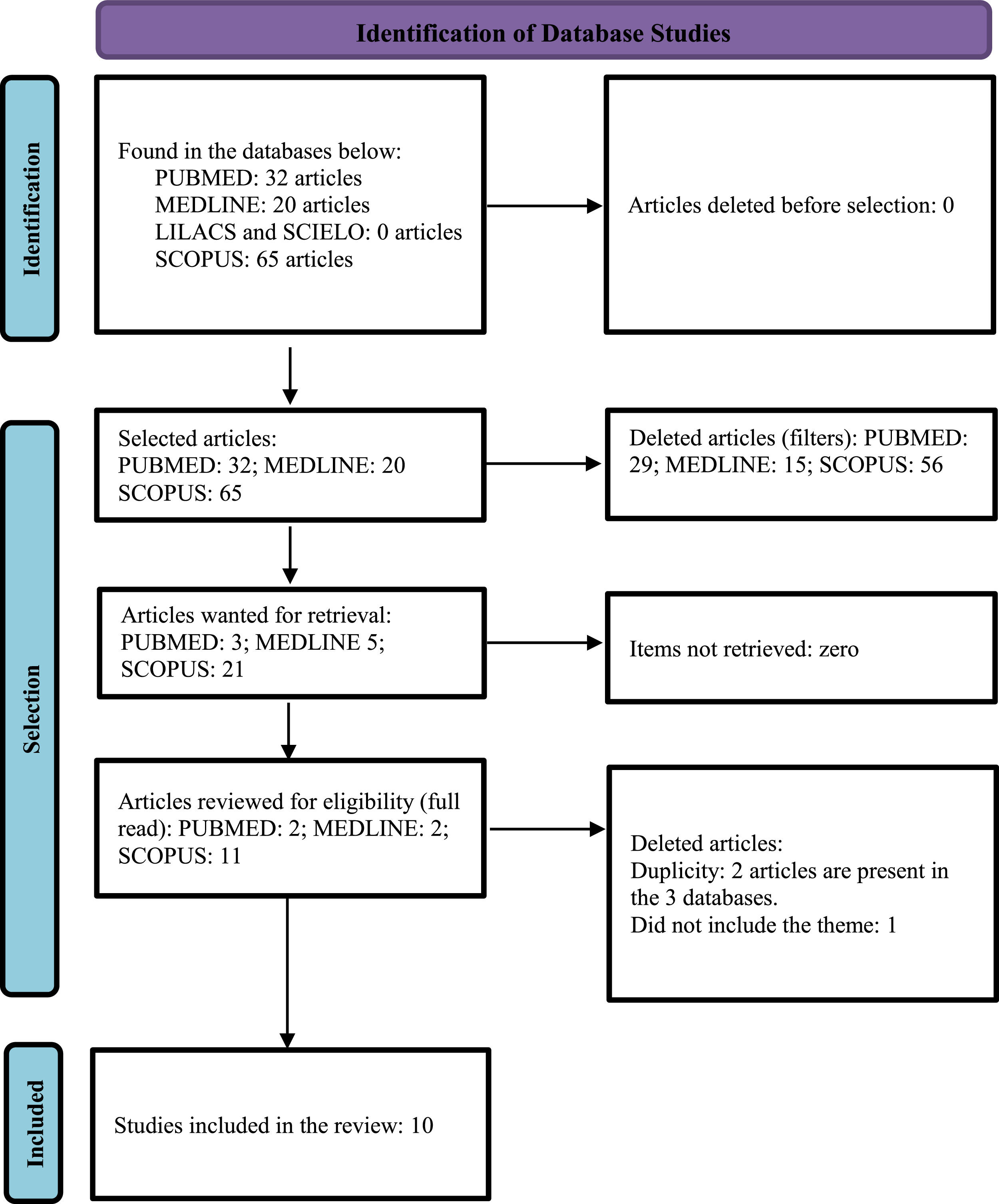
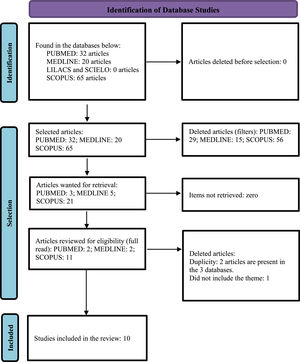
After searching for the descriptors, 32 scientific articles were initially identified in the Pubmed database, and after applying the inclusion and exclusion criteria, three articles remained, which were read in full and included two in the review. In the Lilacs and Medline database via Biblioteca Virtual em Saúde (BVS) (Virtual Public health library), 20 articles were initially identified, and after applying inclusion and exclusion criteria, two articles remained (both from Medline), these were then read in full and included in this review. Initially, 65 articles were identified in the Scopus database, and after applying inclusion and deletion criteria, eleven articles remained, which were submitted to an exploratory reading of the abstracts, ten of which were eligible for full reading, and seven were included in this review. In the Scielo database, after searching for descriptors, no article was found. The steps of this process are represented in the PRISMA flow chart (Fig. 1).6
The final sample of this review consisted of ten scientific articles. Table 1 shows a summary of the articles selected for this review.7-16
Summary table of the articles selected for the review.
| No. | Title | Year and Country | Author | Magazine | Method/Results/Conclusion | Evidence Level |
|---|---|---|---|---|---|---|
| 1. | Propofol for endotracheal intubation in neonates: a dose-finding trial | 2020; The Netherlands | Kort et al.7 | Archives of Disease in Childhood: Fetal and Neonatal Edition | A non-randomized, multicenter, prospective, dose-ranging clinical trial that had the primary objective of finding the optimal dose of propofol for elective intubation in newborns that would produce sufficient sedation without side effects at each gestational age. They used doses of 1–2.5 mg/kg IV. The ideal dose cannot be established, with the need for high doses of the medication for sedation and a high risk of hypotension, and its use is recommended with caution in this population. | II |
| 2. | Adequate analgesia and sedation should be given to neonates during non-emergency endotracheal intubation | 2020; Germany | Tippmann et al.8 | Acta Paediatrica, International Journal of Paediatrics | Narrative review on the possible reasons for not using premedication for intubation in neonates. It concludes that: the cerebral alterations induced by painful procedures, the low success rate of intubation with increased risk of intracranial hemorrhage with each new attempt, combined with the fact that there is no evidence that contraindicates premedication, make its use ethically unquestionable. It mentions the lack of sedation in the use of morphine, which is widely used for this purpose. Importance of protocols. | V |
| 3. | Premedication practices for tracheal intubation in neonates transported by French medical transport teams: a prospective observational study | 2019; France | Carbajal et al.9 | BMJ Open | A multicenter, observational, prospective study that observed for two months several neonatal transport teams in the Paris region as to whether or not sedation and analgesia were used when intubation was necessary. It found that more than 70% of intubations were performed after premedication; such complications were more frequent when more than one intubation attempt was made, and that there was no agreement on the drugs to be used (the most frequent association being sufentanil + midazolam). | III |
| 4. | Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing ventilation and endotracheal intubation | 2019; Italy | Ancora et al.10 | Acta Paediatrica, International Journal of Paediatrics | A systematic review, using the GRADE system to assess the quality of the literature on pain and stress management of newborns on mechanical ventilation or during intubation. Publications from 1986 to 2017 were used, from which strong recommendations were extracted. This would reduce pain, facilitate the procedure, and reduce side effects of the procedure. There is no consensus about the best combination of drugs and doses for this, but some indications according to gestational age, hemodynamic status, and extubation plans. It highlights the favorable pharmacokinetic profile of remifentanil and ketamine, despite few studies in NBs yet. It advises against the use of morphine as monotherapy. It also cites Drugs such as propofol, fentanyl and midazolam combined. He mentioned the possibility of using antagonist drugs to reverse sedation and analgesia after the procedure. | II |
| 5. | Intranasal midazolam and fentanyl for procedural sedation and analgesia in infants in the neonatal intensive care unit | 2019; USA | Ku et al.11 | Journal of Neonatal-Perinatal Medicine | A retrospective, observational study that analyzed the use of intranasal fentanyl and midazolam for analgesia and sedation of neonates in a given US ICU from 2009 to 2015. As a result, its use in RNT and PMT was shown to be safe and well tolerated in this small cohort of 17 patients, and was used for several procedures, including intubation. The dose used was 0.1–0.2 mg/kg midazolam and 1–2 ug/kg/dose fentanyl, and could be repeated. | III |
| 6. | Premedication for endotracheal intubation in the neonate | 2018, USA | McPherson12 | Neonatal Network | Literature review article on the use of premedication prior to neonatal endotracheal intubation with a description of the main pharmacological agents available. It is concluded that analgesia should be given in all cases, with strong consideration given to the use of a sedative, vagolytic, and muscle relaxant based on patient demographics and physician comfort. To ensure safety and optimize logistics, each neonatal unit should develop its own premedication protocol. | V |
| 7. | Respiratory care for the ventilated neonate | 2018; Portugal | Rocha et al.13 | Canadian Respiratory Journal | Review article on the care and monitoring of neonatal patients on mechanical ventilation. Cites the use of intranasal midazolam/ketamine for intubation in the delivery room and for performing MIST (minimal invasive surfactant therapy). Cites the need for more studies to compare the risks and benefits. | V |
| 8 | Ketamine and atropine decrease pain for preterm newborn tracheal intubation in the delivery room: an observational pilot study | 2013, France | Barois and Tourneux14 | Acta Paediatrica | An observational and prospective study of the feasibility and efficacy of inserting a short venous catheter for analgesia in intubating premature infants in the delivery room. Patients were screened at the discretion of the attending physician to receive ketamine (1.8 +- 0.9 mg/kg) and atropine (20 ucg/kg) 1 min before intubation, when this was indicated. As a result, these medications proved to be effective in reducing pain and preventing vagal bradycardia during intubation, being easy to use and without major side effects observed, allowing the use of surfactant in the first 30 min of life in 79.5% of cases when indicated. | II |
| 9 | Premedication for neonatal endotracheal intubation | 2013, France | Durrmeyer et al.15 | Pediatric Critical Care Medicine | An observational and prospective study, which described the frequency and nature of premedication used before intratracheal intubation in certain neonatal units in France. A premedication use rate of 56% was found, being mainly composed of opioids (67%) and midazolam (53%). It concluded that the use of premedication for intubation has increased but remains inconsistent and not systematized. It also described that the medications when used were either not recommended or not described by the American Academy of Pediatrics (AAP). | III |
| 10 | Premedication for Tracheal Intubation | 2013, Italy | Biban and Gaffuri16 | Pediatric Critical Care Medicine | Article with a narrative about possible reasons for not using premedication for intubation of neonates, among them: lack of guidelines, adverse effects of medications, lack of large studies of medications in this period. It reports the progressive increase over the years in the use of analgesia for intubation, and in the use of institutional protocols. | V |
The studies were characterized according to their level of evidence, using the JBI (Instituto Joanna Briggs).
Among the articles included, narrative literature reviews and analytical observational studies without a control group predominate, with only one systematic review of quasi-experimental studies, and two quasi-experimental studies.
Table 2 presents the main medications used for sedation and analgesia in intratracheal intubation of neonates described in the selected articles.
Medications used for intratracheal intubation.
For newborns, intubation is a common procedure in the neonatal ICU and also in the delivery rooms, but despite this, there is no well-established practice of sedation and analgesia and few studies on the use of premedications in this age group. This is confirmed by the small number of articles found on the subject in the various databases used in this research. There is evident little scientific production on the subject, and the absence of controlled and randomized clinical trials on the subject, which represent the highest degree of scientific evidence. The lack of randomization present in the clinical trials of the selected studies can generate biases and some limitations in the generalization of their conclusions.
In a recent review of evidence-based clinical guidelines, included in this study, there are strong recommendations for reducing neonatal stress and using non-pharmacological methods of pain control, combined with bolus analgesia of opioids before invasive procedures, as well as the use of premedications before intratracheal intubation. This recommendation is in line with the knowledge already built up on neonatal pain and also aims to make the procedure faster, less traumatic, and safer.
In the selected studies, there is a consensus recommending the use of premedications in non-emergency intratracheal intubations, which do not occur in emergency cases. This is reflected in the data obtained in a study conducted in France, where 100% of elective intubations were performed with analgesia and sedation versus 50% in emergencies and 90% in those considered semi-emergency. There is a trend described in the literature towards an increase in the use of analgesia, sedation, and protocols for intratracheal intubation over the years, which demonstrates a concern of the scientific community regarding the management of neonatal pain and its short and long-term effects.
Among the medications that could be used, one capable of producing analgesia and sedation quickly was sought, and that had a short half-life and no major side effects. Regarding to this, the authors have two prospective studies. One of the clinical trial dosage types, is where the author studies the use of propofol for the intubation of newborns and investigates the dose capable of producing analgesia and sedation without side effects. In this study, it was found that high doses were needed to produce good sedation and analgesia, which led to hypotension in almost 60% of the patients, making its use impractical, in view of the wide variety of responses in the different gestational ages of newborns. The other is a study on the feasibility and efficacy of the use of ketamine as an analgesic for intubation in the delivery room. Both studies are characterized by the lack of randomization of patients submitted or not to drug intervention, leaving this decision at the discretion of the attending physician, which can generate biases.
The remaining articles that make up this review are descriptive retrospective studies and reviews. There are several possible combinations of medications that are used according to the clinical situations of each patient, but they mostly consist of an analgesic, a sedative, a vagolytic, and a muscle relaxant.
As for the most used medications: the recommendation of not using midazolam as a sedative in premature infants (due to its hypotensive effect) is maintained; there is a strong tendency to use remifentanil instead of morphine for analgesia, in view of its more favorable pharmacokinetics; the use of muscle relaxants is restricted to professionals with more experience in the procedure; atropine can be used to reduce bradycardia induced by intubation; propofol is a possibility in full-term and near-term newborns, and in patients, without venous access, there is the possibility of using intranasal midazolam and fentanyl.
Despite the INSURE technique, often used in neonatal ICU, where the patient is intubated only to receive surfactant replacement and then quickly extubated, there is a need to maintain a good respiratory drive after the procedure, this has long led to resistance to the use of analgesia and sedation, due to fear of drug-induced respiratory depression. In the current literature, there is a preference in these cases for the use of remifentanil as a premedication, followed, if necessary, by reversal with naloxone, with excellent results.
Pain in the neonatal period and its deleterious effects should not be neglected, it should always be considered and treated. It is ethically unacceptable, in view of the already demonstrated ability of the NB to feel pain, to perform any procedure considered potentially painful in adults or children, without the use of analgesia. More studies on the best combinations of medications are needed, including investigations on efficacy and safety. Each neonatal ICU should develop its own premedication protocol in conjunction with its neonatal pain identification and management protocol.
To our little patients.