To review, critically analyze and synthesize knowledge from the international literature regarding the association between allergic rhinitis (AR) and sleep disorders, the impact of AR treatment on children's sleep, and lay the foundation for future research on this topic.
Source of dataA literature search using PubMed database including original and review articles, systematic reviews and meta-analyses using keywords related to AR, sleep disorders and sleep-disordered breathing.
Synthesis of dataSleep is fundamental to health, and its assessment and control of conditions that trigger or aggravate disturbances are of the uttermost importance. Allergic rhinitis (AR) is common in children and may interfere with both their quality of life and quality of sleep. It has emerged as one of the most important risk factors for habitual snoring in children and appeared to increase the risk of Obstructive Sleep Apnea (OSA), with AR severity exhibiting a significant and independent association with pediatric OSA severity. However, in some studies, those associations between AR and OSA in children are not very consistent.
ConclusionsA substantial level of controversy exists regarding the interactions between AR and OSA in children. Notwithstanding, identifying and treating AR in clinical settings is probably an important step toward improving symptoms and preventing deterioration of sleep quality in children and may improve the severity of underlying OSA. Considering the high prevalence, morbidity, economic and social implications of both AR and sleep problems, it is crucial that healthcare providers improve their understanding of the relationships between those conditions among children.
The mechanisms, signs, and symptoms of allergic conditions such as AR present with clear circadian and ultradian oscillations with a striking day-night pattern. Patients with seasonal and perennial AR complain of disturbed sleep at night, as well as symptoms of awakening in the morning.1 The earliest chronobiological characterization of AR was by Trousseau in 1865. Since then, many studies have examined the day-night variation and intensity of AR,2 with several controversial issues remaining under debate.
AR classical presentation involves sneezing, pruritus, rhinorrhea, and nasal congestion, as a conglomerate of symptoms and functional changes either predisposing to or worsening underlying sleep disorders, including sleep apnea.
OSA is a relatively prevalent condition in children, affecting 1% -5% of children aged 2-8 years, and is caused or aggravated by a variety of different conditions, including AR.3
Despite the low quality of evidence from many studies examining the associations of AR with sleep impairment, both in children and adults, it has been shown that AR patients present with higher levels of disturbance and sleep latency scores and a decreased sleep efficiency score using Polysomnography (PSG). AR was also associated with a higher risk of nocturnal sleep-related dysfunctions, including insomnia, nocturnal enuresis, restless sleep, sleep-disordered- breathing (SDB) as OSA or snoring. Additionally, AR was found to be associated with a higher risk of daytime sleep-related impairment.4
The management of children with chronic disorders such as AR has become a frequent component of the activities within any pediatric clinic and usually involves both the general pediatricians and pediatric subspecialists. Because of the high prevalence of AR and OSA, its associated morbidity, and the economic and social implications of either respiratory allergies or sleep problems, it is imperative that healthcare providers acquire a deeper understanding of the relationships between both conditions in children and their mutual interaction.
In light of the contradictory findings on the relationship between AR and sleep and its disorders, the authors aimed to review a potential pathophysiological matrix for the crosstalk between AR and sleep disorders, as well as the implications of rhinitis control measures on sleep.
Allergic rhinitis prevalence in childrenAllergic rhinitis (AR) is a widespread and common disease. In the United States, the Agency for Healthcare Research and Quality estimates the prevalence of AR in adults to be between 10% and 30%, and several studies have estimated the prevalence of AR in children to be closer to 40%.5,6 In the International Study of Asthma and Allergies in Childhood (ISAAC), the overall prevalence of rhinoconjunctivitis was 8.5% in children aged 6–7 years and up to 14.6% in 13-14-year-old children.7 AR is a major non-communicable disease that can impose a significant impact on quality of life, considering its effects during both wakefulness and sleep.8
Although the prevalence of AR is clearly heterogeneous and greatly varies with location and socioeconomic status in different countries, the apparent escalation in AR prevalence in children over the last several decades has been paralleled by a similar increase in the frequency of other allergy-related disorders, such as asthma and atopic eczema.9 AR can present at any age but will usually become manifest after 2 years of age, with incremental increases in prevalence in older children and adolescents.9
Basic mechanisms of allergyAs pathophysiological pathways of allergic disorders are well studied, it is known that after allergen sensitization, whereby antigen is presented to naïve T lymphocytes via an antigen-presenting cell (APC), T cells will undergo differentiation into Th2 cells upon stimulation by thymic stromal lymphopoietin (TSLP) and other cytokines (IL-4, IL-5, IL-13).
Th2 lymphocytes bind to B cells via major histocompatibility complex class II (MHCII) and secrete IL-4 and IL-13. These cytokines induce the B cell to undergo class switching and to produce IgE specific to the sensitizing antigen.10 Besides stimulating IgE production, Th2 cells produce a variety of cytokines (type-2 cytokines), which enhance eosinophilopoiesis (IL- 5), mast cell development (IL-9), and goblet cell hyperplasia.
Type I hypersensitivity processes involve a 2-step temporal process: the early response, reaching a maximum within 30 minutes and usually resolving within 1–3 hrs, and the late response, whereby symptoms start to increase again after 3–4 hrs and reach their peak over 6– 12 hrs. The allergen-induced responses are initiated when IgE binds to high-affinity receptors (FcεRI) located on the surface of mast cells and basophils. IgE is subsequently cross-linked by allergen, causing mast cell degranulation, and the release of preformed mediators such as histamine, neutral proteases, and chemotactic factors, along with the activation of eicosanoid pathways to produce newly formed mediators, such as cysteinyl leukotrienes (CysLTC4 and CysLTD4) and their metabolite cysLTE4, as well as prostaglandins (PG), especially PGD2. CysLTs are the mediators underlying nasal congestion and nasal mucosal swelling by increasing microvascular permeability and stimulating mucus secretion. Histamine also contributes to inflammation during the early phase response,11 whereas in the late response, these early mediators are involved in further recruitment of cells including eosinophils, basophils, monocytes, macrophages, and lymphocytes into the inflammatory milieu.12
Diurnal variation in AR symptomsAR and other allergic diseases are underpinned by frank day-night oscillatory patterns either in clinical symptoms, laboratory parameters, or response to treatment.13 In most AR patients, symptoms worsen overnight or early in the morning, thereby compromising night-time sleep and resulting in poor daytime quality of life.2,13-15 The circadian nature of allergic diseases pathophysiology is well documented, and evidence has been accumulated that the circadian clock regulates IgE/mast cell-mediated allergic reactions.13 It was demonstrated an occurrence of 24h clock based variations in histamine release, cysteinyl leukotriene production, and IL-6/IL-13 expression in mast cells following IgE-mediated activation, eventually due to circadian variation in FceRIa expression.16 Although rhythmic humoral signals such as cortisol from the adrenal gland17 and periodically recurrent activity of the autonomic nerves13 have been implicated in diurnal oscillations, the biological basis of this phenomenon remains poorly understood.13
It was demonstrated that endogenous glucocorticoids could synchronize circadian expression of the clock genes, and the core circadian clock gene Per2 may contribute to symptomatic temporal variations in AR by its possible anti-inflammatory effect on controlling rhythmic changes in levels of Th2- and Th17-associated transcription factors (eg, GATA- binding protein 3 [GATA3], and RORγ t).18 Recent reviews highlighted that oscillatory allergic reactions are generated by rhythmic expression of key molecules in the pathophysiology controlled by the circadian clock.19
The worsening of symptoms during nighttime may be related to exacerbation of inflammatory processes, usually as a manifestation of the late-phase allergic response to provoking environmental antigens imposed earlier in the day, and such processes also involve key neuroendocrine rhythmic dynamics.20 The day-night differences in symptom intensity, however, may also represent, at least to some extent, circadian rhythm differences in vulnerability to antigen exposure as diurnal variation in nasal reactivity suggests a circadian rhythm-dependent difference in the ability of specific antigens as well as non-specific chemical substances to induce pro-inflammatory activities.21
When considering perennial AR, it is suspected that greater exposure loads to mites and their associated antigens at night, when in contact with mattress and pillows, clearly account for a substantial proportion of the nocturnal symptomatic severity increases20 with effects on sleep.22
Sleep disorders and its consequencesSleep is an important pillar of a healthy life. Yet, sleep disorders are common in children and adolescents, with an estimated prevalence ranging from 11% to 47%.23 OSA, as a major sub-group of overall sleep disorders, may conservatively affect up to 4% of children.3,24 Impaired nocturnal sleep in children results in daytime fatigue and sleepiness, often associated with altered immune function, anxiety, attention deficit problems, memory deficits, behavioral issues, irritability, depression, growth retardation, hormone imbalance, hypertension, poor academic performance, increased accident rates, substance abuse, as well as increased risk for cardiovascular and metabolic dysfunction, ultimately leading to lower overall and health-related quality of life.25-27 As such, any condition as AR that affects sleep integrity and continuity and reduces its restorative properties is liable of generating symptoms that are not directly related to the disease per se but rather reflect the consequences of disrupted sleep homeostasis.
Association between AR and SDBIn a systematic review analyzing the association between AR and SDB in children, it was concluded that although the majority of the studies showed a significant association between AR and SDB, in all of these studies, the level of evidence was 3b and 4, for an overall grade of B−evidence (Oxford Evidence-Based Medicine Center). As such, the evidence for the association between rhinitis and OSA in children is not very strong, in part because most pediatric studies, until not long ago, used indirect measurements to document SDB (i.e., snoring) instead of overnight PSG to confirm the diagnosis of pediatric OSA.28 Yet, significant associations between snoring and AR were reported in preschool and primary school children29,30 and in school children.31
SDB is an important component of the morbidity associated with AR, and it is currently accepted that AR increases the risk of OSA.32,33 Although AR severity was found to have a significant and independent association with the severity of OSA in children,34 it was not confirmed in other PSG-diagnosed studies, despite the high prevalence of AR in patients with SDB.35-38 The Apnea-hypopnea index (AHI) during rapid eye movement (REM) sleep in children with moderate-severe OSA was significantly increased in subjects with rhinitis and OSA compared with those with OSA alone.35 The AHI severity (determined by total events occurring in REM + NREM) may be affected by different levels of nasal congestion occurring in REM sleep versus NREM sleep. Given that REM sleep occupies only a small proportion of the night (around 20-25%), breathing abnormalities during REM sleep may not necessarily correlate with the overall AHI severity score (which includes NREM sleep that represents 75- 80% of the night).35
Although controversial issues subsist regarding the role of nasal inflammation in primary snoring and OSA, allergic rhinitis appears to be present in 35% of children with primary snoring and in 6% with OSA. On the other hand, non-allergic rhinitis was found in 19% of children with primary snoring and in 34% with OSAS. In this sense, nasal cytology could provide interesting information that can be used to plan possible treatment strategies.39
Accordingly, rhinitis may be considered as a set of symptoms potentiating SDB, whereas in other studies, symptoms of AR may be considered an independent predictor for SDB and failure of adenotonsillectomy (AT) in children.25
Notwithstanding, a recent study that enrolled 1.578 children with habitual snoring did not find a significant association between AR and OSA,40 and no association was found between AR and OSA severity in children who were admitted for inpatient AT.41 Therefore, the presence of enlarged tonsils and adenoids as a dominant factor in the genesis and severity of OSA in children might mask the relative contributions of AR to this condition considering the dominant contribution of ATH. The authors should, however, consider that before ATH reaches a stage that results in OSA, the proliferation of such lymphoid tissues may be driven by underlying AR, such that the contribution of AR to upper airway dysfunction may be more readily apparent during the early pathophysiological stages of OSA, and progressively lose its prominence as ATH and OSA become more firmly established.
Atopic status and SDBIn one cohort of 105 young children with habitual snoring, significant relationships emerged between this condition and atopic status.42
In a retrospective study with 120 children treated for snoring, the percentage of children with abnormal serum levels of total IgE or abnormal allergen-specific IgEs was comparable in PSG- diagnosed OSA and non-OSA groups. The proportions of abnormal total airway resistance and of abnormal serum levels of total IgE were significantly higher in children with severe OSA compared with children with mild or moderate OSA. The authors suggested that allergic sensitization might contribute to the exacerbation of existing OSA in children.43
Association between AR and sleep disordersIt is worth pointing out that AR may cause daytime fatigue or sleep fragmentation without having evident effects on breathing.44 Over the last 15 years, about one-third of the studies failed to show the presence of a significant association between rhinitis and overall sleep disorders (Table 1).35,38,40,41,45-63 The very low-grade level of evidence was further corroborated in a more recent systematic review and meta-analysis evaluating the association between AR and sleep, based on observational investigations in children and adults.4 Adjusted odds ratios revealed that AR was associated with a higher risk of nocturnal dysfunction, including insomnia, nocturnal enuresis, restless sleep, SDB, OSA, snoring, daytime dysfunction, including difficulty waking up, daytime sleepiness, morning headache, and the use of sleep medications.4
Summary of published studies examining the relationship between AR and Sleep Disorders in children and adolescents over the last 15 years.
| Authors, year, reference | Age | Sample size/AR cases | Study type | Measurement-AR | Measurement sleep outcome | Outcomes |
|---|---|---|---|---|---|---|
| 2020 Xu Z et al.40 | 2-15 | 1578/244 | Cross-sectional | Face-to-face interview | PSG | OSA |
| 2018 Shen L et al .45 | <14 | 676/121 | Case- controlled | Self-reported Rhinolaryngosc opy | PSG | OSA |
| 2018 Loekmanwidjaja J et al.46 | 4–10 | 167/112 | Case- controlled | Medical records | Self-reported | Parasomnias, daytime sleepiness |
| 2018 Lai PH et al.47 | <18 | 655.529/327.928 | Cohort | Medical records | Medical records | OSA, nocturnal enuresis |
| 2018 Bilgilisoy Filiz et al.48 | 8–18 | 287/143 | Case- controlled | Medical records, clinical examination and SPT | Face-to-face interview | Sleep quality (PSQI outcomes) |
| 2017 Tsai JD et al .49 | 5–18 | 8616/4191 | Case- controlled | Medical records, clinical examination | Medical records | Nocturnal enuresis |
| 2017 Nguyen- Hoang Y et al.50 | 6-17 | 85/52 | Case- controlled | Clinical examination | Medical records | OSA in asthmatics |
| 2016 Di Francesco RC, Alvarez J38 | 3–14 | 135/57 | Case- controlled | Self-reported, Rhinoscopy and SPT | PSG | PSG outcomes (AHI) |
| 2015 Poachanukoon O, Kitcharoensakkul M51 | 6-15 | 175/65 | Case- controlled | Medical records, clinical examination and SPT | Self-reported | Sleep duration, difficult waking up, snoring, morning headache, mouth breathing, night sweating, nocturnal enuresis, OSA daytime sleepiness, restless sleep, SDB, sleep bruxism |
| 2014 Weinstock TG et al.41 | 5-9.9 | 464/110 | Cross-sectional | Physician-diagnosed | PSG | AHI severity |
| 2014 Ng DK et al.52 | 15.3 ± 1.7 | 175/65 | Cohort | Self-reported | Self-reported | Snoring |
| 2014 Huseni S et al.35 | 2-13 | 145/63 | Cross-sectional | Medical records | PSG | AHI during REM and non-REM |
| 2012 Fadzil Abdullah AA et al.53 | 6-10 | 550/22 | Case-controlled | survey booklet | survey booklet | SDB |
| 2012 So HK et al.54 | 7–10 | 6381/2661 | Cross-sectional | Self-reported | Self-reported | Night sweating |
| 2012 Ishman SL et al.55 | 0-18 | 134/21 | Case-controlled | SPT or RAST | PSQ; OSA18; PDSS | SDB, sleepiness |
| 2010 Bhattacharjee R et al.56 | 6.9±3.8 | 578/212 | Case- controlled | Medical records | PSG | Residual OSA post-AT |
| 2010 Urschitz MS et al.57 | 7-12 | 1144/712 | Cross-sectional | Physician-diagnosed | HPO Questionnaire | OSA |
| 2010 Li S et al.58 | 5–14 | 6369/2823 | Cross-sectional | Self-reported | Self-reported | Snoring |
| 2009 Sogut A et al.59 | 12-17 | 1030/36 | Case controlled | Questionnaire | Questionnaire | Snoring |
| 2008 Liukkonen K et al.60 | 1-6 | 1471/236 | Case controlled | Self-reported | SDSC | Snoring |
| 2008 Petry C, et al.61 | 9-14 | 998/299 | Cross-sectional | Questionnaire | Questionnaire | SDB |
| 2005 Sogut A et al.62 | 3-11 | 1198/332 | Case controlled | Self-reported | Self-reported | Snoring |
| 2005 Ng DK et al.63 | 6–12 | 3047/1242 | Cross-sectional | Self-reported | Self-reported | Daytime sleepiness, OSA |
AHI, Obstructive apnea–hypopnea index; AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; AT, adenotonsillectomy; ESPRINT-15, AR health-related quality of life questionnaire for children; ESS, Epworth Sleepiness Scale; HPO, Home pulse oximetry; OSA 18, Obstructive Sleep Apnea-18 survey; OSA, obstructive sleep apnea; PDSS, Pediatric Daytime Sleepiness Scale; PSG, polysomnography; PSQ, Pediatric Sleep Questionnaire; PSQI, Pittsburgh Sleep Quality Index; RAST, in vitro allergy testing; REM, rapid eye movement; RLS, restless legs syndrome; SDB, sleep-disordered breathing; SDSC, Sleep Disturbance Scale for Children; SPT, allergy skin-prick testing.
The authors surmise that improved delineation of the criteria for AR definition and use of PSG and other objective criteria for sleep assessments will be required in future prospective studies to more compellingly demonstrate or refute the impact of AR on sleep and vice-versa.
It was demonstrated in a few PSG studies in children that the sleep architecture may be altered by AR. The percentage of time spent in the REM sleep stage was lower among children with AR and SDB, without sleep apnea.37,38
AR and sleep questionnairesEach sleep disturbance detection method has its own reliability and precision as well as its particular indication according to the intended objective. Among the different available tools, the most commonly used for screening sleep quality, sleep habits and disorders, are validated questionnaires. This option have a very low cost, is easy to administer, and does not require a sleep center. Furthermore, it could have a relevant role as an adjuvant for diagnostic assessment when polysomnography is not available. Another advantage of the use of questionnaires is the allowance of self-administration with high accuracy, as shown in different studies.64
Among the different surveys and questionnaire-based tools investigating sleep habits in children are the Children's Sleep Habits Questionnaire (CSHQ). This is a retrospective parent-report questionnaire that was developed in the United States to evaluate sleep behavior in children. The questions were selected to include the symptom presentations of the most common pediatric sleep disorders, according to the International Classification of Sleep Disorders. The Portuguese version of the CSHQ (CSHQ-PT) showed psychometric properties that were comparable to versions from other countries and adequacy for the screening of sleep disturbances in children from 2 to 10 years old.65
A case-control study reported that children with AR have higher scores on the CSHQ than the scores of healthy children, indicating a greater probability of sleep disorders being present.46 Using the previously reported CSHQ score cutoff, 83% of the children with persistent moderate-to-severe AR would be classified as having sleep disorders, with significantly higher CSHQ scores being found among those children with uncontrolled AR.46
Chronic rhinitis has also been associated with habitual snoring in a questionnaire-based survey in Greece on 3.680 children, in whom habitual snoring was present in 5.3%, 4%, and 3.8% in the children of 1-6, 7-12, and 13-18-year age-groups, respectively.66
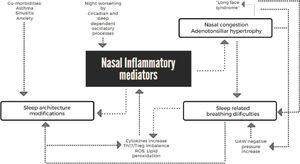
Interactions between allergic rhinitis and sleep problemsAR can affect sleep via several mechanisms (Figure 1). In light of the increased nasal airflow resistance, it is not surprising that AR is considered a risk factor for OSA. In addition, AR can foster enlargement of tonsils and adenoids due to the chronic inflammation in the nasal cavity and nasopharynx and can also promote the evolution of an elongated face due to oral breathing rather than nasal breathing. Taken together, all these factors result in a higher risk for the presence of a smaller and more collapsible upper airway.32
ATH is by far the major pathophysiological contributor to SDB in children, and its frequency is higher in children with the allergic disease compared to controls,67 and allergic disease is also considered a risk factor for adenotonsillar regrowth in children after AT.68
Worsening of AR during sleep may partially related to the typical recumbent position. This position can worsen nasal obstruction due to an increase in nasal turbinate edema.69
The nose, when obstructed, can act as an opposing force to favorable upper airway airflow. Inspiration generates negative intrathoracic pressures compared with atmospheric pressure, thereby sucking air into the lung cavity. Nasal obstruction secondary to nasal congestion influences this pressure differential by limiting airflow and velocity. When the force generated by the oropharyngeal dilator and abductor muscles is exceeded by a critical amount, the negative airway pressure increases and facilitates compliant pharyngeal tissues to collapse by acting as a Starling resistor.70 This mechanism is recognized in disturbed sleep caused by nasal obstruction.71
Furthermore, several inflammatory mediators were reported to be associated with poor sleep, including histamine, CysLTs, IL-1β, IL-4, IL-10, bradykinin, and substance P.27 Increased mediators induced by rhinitis may contribute to disturbances of sleep architecture in OSA patients.25 Histamine, a relevant modulator of the sleep-wake cycle, also influences mechanisms related to arousal, cognition, and memory.25 The typical increase in histaminergic function associated with different allergic conditions and the evidence of sleep architecture improvements with anti-histaminic therapy reinforce the significance of the relationship between AR and sleep.72 An exploratory study demonstrated that levels of IL- 1β, IL-4, and IL-10 were higher in AR patients, compared with non-allergic patients, and correlated with increased latency to REM sleep, decreased time spent in REM sleep and decreased latency to sleep onset.73 Likewise, OSA has also been shown to be associated with increased cytokines such as IL-1, IL-4, IL-6, and Tumour Necrosis Factors (TNF) that promote T helper type 2 (Th2) cell phenotypes and enhance inflammation leading to nasal congestion.74-76 One additional consequence of the intermittent hypoxia and sleep fragmentation is oxidative stress, with reactive oxygen species production and lipid peroxidation being found to increase in OSA patients.74 The association between chronic inflammation and oxidative stress is well documented. Oxidative stress results from the imbalance between the oxidative forces and the antioxidant defense systems, which is believed to favor an oxidative injury that has been implicated in the pathogenesis of AR.77
Th17/Treg ratios are elevated in OSA patients and are positively associated with the Apnea-Hypopnea Index (AHI).25 Similarly, it was shown that the Th17/Treg ratio was significantly higher in OSA children with AR compared to OSA children with ATH but without AR.78 Th17 positive cells play critical roles in the development of autoimmunity and allergic reactions through IL- 17 production. Treg cells orchestrate the overall immune response and play a role in immune tolerance. Th17 and Treg imbalance caused by AR and OSA,79,80 respectively, may mutually promote each other and lead to further imbalance. Thus, regardless of which pathological changes appear first, both AR and OSA will facilitate manifestations of Th17/Treg imbalance.78
The trigeminocardiac reflex (TCR) has been advanced as a putative mechanism linking AR to sleep disorders, as TCR can be activated by stimulation of the trigeminal nerve anywhere along its course, eliciting from mild to severe negative cardiorespiratory changes, such as bradycardia, asystole, hypotension or apnea, either due to sympathetic withdrawal or to parasympathetic overactivity via the vagus nerve.81 Further, AR is known to induce neuronal hyperresponsiveness of the upper airways to stimuli that activate nasal afferents.
Dysregulation of nasal neural pathways plays a relevant role in the pathogenesis of rhinitis and causes nasal, respiratory, and cardiac changes. Nasal discharge or nasal congestion can be potential stimuli for nasal trigeminal afferents in rhinitis and TCR, and may also contribute as a mechanism underlying the pathogenesis of sleep disorders in AR.81
When examining the impact of AR on all dimensions of sleep quality, it is important to consider the potential impact of co-existing factors such as anxiety and depression or of frequent comorbidities such as asthma. Actually, in early studies, it was estimated that 15 to 40% of AR patients had asthma, and 6% to 85% of asthmatic patients reported AR.82,83
The authors would be remiss if we did not mention that sleep quality exerts an important influence on host immune system homeostasis. A night of sleep deprivation or 5 days of sleep restriction will alter the TH1/TH2 balance.84 Furthermore, environmental (e.g., allergens and irritants,85 individual (e.g., sleep hygiene and adherence to treatment) and family/cultural factors (e.g., race, ethnicity, factors related to urban poverty, beliefs and family sleep practices), in combination or on their own, exert influence on both allergic diseases and sleep and need to be considered when working with patients who have allergic disorders.86
Treatment of AR and effects on sleepAlthough sleep is crucial for optimal child growth, development, and learning ability, a survey of Pediatric Allergies in America indicated that 40% of parents claimed that nasal allergy affected their child sleep: 32% claimed their child had difficulty falling asleep, 26% claimed their child awakened at night due to sleep problems, and 29% claimed their children had a lack of good sleep.6 This relationship between allergic diseases and sleep disorders in the pediatric age has also been confirmed in some Latin American countries.87
Therapies that reduce nasal congestion may improve sleep and daytime sleepiness and, thereby, improve quality of life. In addition, those therapies that reduce inflammation, often those that also decrease congestion, may have a positive influence by reducing the levels of inflammatory mediators, such as TNF, which can cause constitutional symptoms. However, not all treatments for AR are effective in this respect.88 The authors also point out that data regarding the effect of allergen avoidance on sleep in AR patients is limited.89 The effects of medication and isotonic or hypertonic saline were evaluated to assess how AR treatment may affect sleep and SDB.75,89,90 These trials included intranasal corticosteroids, leukotriene receptor antagonists, and topical nasal decongestants. Noteworthy, there were few studies in children, and relevant evidence is limited since most of the studies assessing treatments effects included a small number of subjects and were not conducted in a randomized double-blinded placebo-controlled fashion.75
Topical decongestants reduce nasal congestion but may have adverse effects on sleep because of their stimulatory effects and also because of the risk of rhinitis medicamentosa (“rebound” congestion), such that they should not be used for extended periods of time.88 Sedating antihistamines are generally contraindicated, particularly for those children already experiencing daytime somnolence, fatigue, and functional impairments. Non-sedating oral antihistamines are widely used to treat AR and relieve nasal symptoms such as rhinorrhea, sneezing, and pruritus but have less effect on nasal congestion.88 Some studies have shown an improvement in sleep and quality of life following therapy with an oral or topical antihistamine in adults.88 Data regarding intranasal antihistamines and effects on sleep are mixed, with some studies demonstrating improvement in quality of sleep and proportion of mouth breathing, while others indicate increased daytime somnolence as a side effect of these agents.91
A prospective randomized, double-blind controlled trial of PSG-diagnosed OSA in 57 children, ages 2-10 years, with or without rhinitis, who were treated with either oral montelukast or placebo, demonstrated that montelukast for 16 weeks effectively reduced the severity of OSA.92 Montelukast markedly improved quality of life in children with mild OSA.93 Nevertheless, the current concerns about potential undesirable side-effects with this drug raise substantial barriers to initiation of such therapy in the age group.94 In recent years, there has been an increased focus on neuropsychiatric adverse drug events (ADE) associated with leukotriene antagonists, owing to a 2008 US FDA alert on possible neuropsychiatric ADE.95 A 2016 review of pediatric psychiatric disorders associated with Montelukast using the VigiBase demonstrated age-variant neuropsychiatric ADE, with infants and children developing sleep disturbances and predisposing to depression/anxiety and psychotic reactions in adolescents.96
Multiple placebo-controlled clinical trials have shown that intranasal corticosteroids are effective against the most common symptoms of AR, including sneezing, itching, rhinorrhea, and congestion in both adults and children.89 Intranasal corticosteroids are considered first-line therapy when nasal congestion is a major symptom. Small studies in children with AR found that intranasal budesonide reduces nasal congestion, subjective daytime somnolence, and fatigue and improves patient sleep and life quality.88 Indeed, during treatment of children with AR with intranasal budesonide, nasal symptoms decreased, objective sleep studies were improved, and subjective symptoms relating to poor sleep quality were diminished.97 Treatment with intranasal budesonide may also improve concentration and performance at school in children with rhinitis.98
Besides the effects of topical corticosteroids on rhinitis control, ATH and OSA may respond favorably to anti-inflammatory agents such as corticosteroids and montelukast via their lymphocytic, anti-proliferative or anti-inflammatory effects, all of which might reduce adenotonsillar hypertrophy. Intranasal corticosteroids reduce cellular proliferation and the production of pro-inflammatory cytokines in a tonsil and adenoid mixed-cell culture system.99 As a corollary of such findings, randomized controlled trials of intranasal corticosteroids have resulted in significant improvements or resolution of mild OSA in children.100,101
Adequate treatment of AR could prevent the occurrence of OSA, reduce the severity of existing OSA, and potentially prevent the emergence of the maxillofacial growth abnormalities that predispose children to OSA.102 Multivariate analysis has also identified rhinitis as an independent predictor for the failure of AT and the presence of residual OSA.103
AR treatment drugs such as Azelastine nasal spray and Mometasone Furoate Aqueous Nasal Spray were found to be useful in decreasing adenoid pad size and the severity of symptoms related to adenoidal hypertrophy in children.104,105 So far, therapeutic studies with azelastine hydrochloride and fluticasone propionate in a single delivery system using objective sleep metrics in children and adolescents with AR are still lacking. Therefore, the advantage of the combination treatment, although promising, remains uncertain.
Concerns and future directionsSleep problems are a commonly associated morbidity in people with AR, and disrupted sleep homeostasis has many well-documented consequences; patients with AR have increased daytime and night-time symptoms related to sleep disorders; although the relative strength of studies results is low, AR has a significant association with both snoring and OSA among children, and intranasal corticosteroids or montelukast improve both AR and SDB.
Although a substantial number of studies have analyzed the relationship between AR and OSA either in the context of pathophysiological, epidemiological or therapeutic pathways, several methodological issues turn difficult the generalization of some important findings. In this context, one important issue is that most of the available literature support findings in adults which is therefore hard to transpose to pediatric ages. Also, the use of distinct assessment tools leads to a harder interpretation and uniformization of findings. Future research would benefit from increased attention on those topics.
Further studies are needed to more compellingly demonstrate or refute the impact of AR on sleep and vice-versa with improved delineation of the criteria for AR definition and the use of PSG for sleep assessments; to substantiate that allergic sensitization might increase SDB severity in children and verify the effects of AR treatment in children with sleep disorders, using large double-blind placebo-controlled clinical trials.
ConclusionsAlthough conflictive, there is currently a great body of evidence precluding firm statements on the interactive roles between AR and sleep. Still, identifying and treating rhinitis in clinical practice have been pointed out as an important step toward improving the symptoms and preventing sleep quality deterioration in children with SDB/OSA before and after undergoing AT. The future of pediatric clinical research will certainly benefit from further exploration of such a relationship between AR and sleep disorders which seems to persist as a clinically relevant interaction in modern days.