To restate the epidemiological importance of Shigella in acute diarrhea with blood, providing an overview of the treatment and stressing the need for the correct indication of antibiotic therapy.
Sources of DataA search was carried out in the Medline and Scopus databases, in addition to the World Health Organization scientific documents and guidelines, identifying review articles and original articles considered relevant to substantiate the narrative review.
Synthesis of DataDifferent pathogens have been associated with acute diarrhea with blood; Shigella was the most frequently identified. The manifestations of shigellosis in healthy individuals are usually of moderate intensity and disappear within a few days. There may be progression to overt dysentery with blood and mucus, lower abdominal pain, and tenesmus. Conventional bacterial stool culture is the gold standard for the etiological diagnosis; however, new molecular tests have been developed to allow the physician to initiate targeted antibacterial treatment, addressing a major current concern caused by the increasing resistance of Shigella. Prevention strategies include breastfeeding, hygiene measures, health education, water treatment, and the potential use of vaccines.
ConclusionsAcute diarrhea is an important cause of mortality in children under 5 years and shigellosis is the leading cause of acute diarrhea with blood worldwide. The current concern is the increase in microbial resistance to the recommended antibiotics, which brings an additional difficulty to therapeutic management. Although no vaccine is yet available against Shigella, several candidates are undergoing clinical trials, and this may be the most cost-effective preventative measure in future.
Reiterar a importância epidemiológica da Shigella na diarreia aguda com sangue, fornecer uma visão geral do tratamento e ressaltar a necessidade da correta indicação da antibioticoterapia.
Fontes dos dadosRealizada pesquisa nos bancos de dados Medline e Scopus, além de documentos científicos e diretrizes da Organização Mundial da Saúde, com a identificação de artigos de revisão e artigos originais considerados relevantes para fundamentar a revisão do tipo narrativa.
Síntese dos dadosDiferentes patógenos têm sido associados à diarreia aguda com sangue, a Shigella é o mais frequente. As manifestações da shigelose em indivíduos saudáveis são geralmente de intensidade moderada e desaparecem em poucos dias. Pode haver progressão para disenteria franca com sangue e muco, dor em abdome inferior e tenesmo. A coprocultura bacteriana convencional é o padrão-ouro para o diagnóstico etiológico, porém novos testes moleculares foram desenvolvidos, os quais permitem ao médico iniciar tratamento antibacteriano direcionado, sanar uma grande preocupação atual, devido à crescente resistência da Shigella. Estratégias de prevenção incluem aleitamento, medidas de higiene, educação em saúde, tratamento da água e o potencial uso de vacinas.
ConclusõesA diarreia aguda é uma importante causa de mortalidade em crianças com menos de cinco anos e a shigelose é a principal causa de diarreia aguda com sangue em todo o mundo. A preocupação atual é o aumento da resistência microbiana aos antibióticos preconizados, o que traz uma dificuldade adicional ao manejo terapêutico. Embora ainda não exista vacina disponível para Shigella, várias candidatas estão em fase de testes clínicos, podem futuramente ser a medida preventiva mais custo-efetiva.
Acute diarrhea (AD) is an important cause of mortality in children under 5 years. Although the mortality rate from diarrhea in this age group has decreased by more than 30% over the past 15 years, AD remains the second leading cause of death.1 Despite the decrease in mortality, morbidity in developing countries (especially in sub-Saharan Africa and South Asian regions) remains very high.2
AD (also called gastroenteritis, acute enteritis, or enteral infection) should be suspected when there is a decrease and abrupt onset of stool consistency and/or an increase in evacuation frequency, associated or not with sudden onset of vomiting, eventually with the presence of blood. In most children, AD typically lasts less than 7 days and no more than 14 days.3 In medical texts, the term dysentery often appears as a synonym for AD with blood (bloody stools).4 Others characterize dysentery as the presence of stools with blood and/or mucus, associated with fever and abdominal cramps.5
Different pathogens have been isolated in AD with blood, and Shigella is the most commonly isolated enteropathogen worldwide, with a higher incidence in developing countries. Children under 5 years are the most frequently affected, accounting for approximately 70% of all cases of diarrhea from Shigella and 60% of deaths.6
A multicenter study published by Kotloff et al. in 2013, the Global Enteric Multicenter Study (GEMS), identified in stool cultures, among 22 intestinal pathogens, that rotavirus, Shigella, enterotoxigenic Escherichia coli (ETEC), and Cryptosporidium accounted for 70% of the diarrhea cases in children under 4 years.7 Afterwards, the study samples were reanalyzed with quantitative PCR, and the incidence of diarrheal disease attributed to Shigella was over two-fold the initial evaluation. In addition to being the leading cause of AD with blood, Shigella was also the second pathogen most frequently isolated in children with watery diarrhea, which restates the need to be alert to the presence of this pathogen, even in the absence of blood.8
In this narrative literature review, the emphasis will be on the therapeutic aspects of AD with blood, presumably associated with Shigella, considering that Shigellais the most frequently isolated enteropathogen from the stool of children with AD with blood in regions where AD is endemic.
Shigella: epidemiological aspectsThe most affected groups, apart from children in endemic regions of developing countries, are children in daycare centers and schools in developed countries, travelers to developing countries and, a few years ago, a new at-risk group, male homosexuals, was identified. Shigellais highly transmissible, with a small number of colony forming units (CFU), 10–18, depending on the serotype, being capable of triggering infection.9
Fecal-oral contact is the main form of transmission. Other less common forms of transmission are contaminated food and water and, more recently, the house fly has been identified as a vector, suggesting a significant reduction in Shigella infections in areas with high density of flies after their adequate control.9,10
Discovered in 1897 by Japanese microbiologist Kiyoshi Shiga, during an epidemic of dysentery in Japan with high mortality, Shigella, initially called Shiga bacillus, comprises an antigenically diverse genus, consisting of four species, which are also called groups or subgroups: Shigella flexneri, Shigella sonnei, Shigella dysenteriae, and Shigella boydii. The bacillus isolated by Shiga was later identified as S. dysenteriae type 1, which causes more severe and prolonged disease, producing the Shiga cytotoxin.9
S. dysenteriae, rarely isolated in the last two decades, has been associated with four pandemics between the 1960s and 1990s, with a high mortality rate.9S. boydii causes milder disease and is commonly restricted to African countries and Southeast Asia.11 Historically, S. sonnei has been described as endemic in developed countries, while S. flexneriis endemic in developing countries with poor hygiene conditions, by mechanisms not yet fully explained.9 In recent years, however, a shift in the distribution pattern of Shigella has been noticed, with a significant increase in S. sonnei also in developing countries. Some hypotheses have been described to justify this change.11
The first and foremost hypothesis is the fact that populations living in places with poor hygiene conditions are immune to S. sonnei due to exposure to water contaminated with Plesiomonas shigelloides, an enterobacterium that contains an O-antigen identical to that of S. sonnei, the immune system's main target during Shigella infection. With improved hygiene and adequate water treatment in some developing countries, the contact with P. shigelloides was reduced, making individuals more susceptible to S. sonnei.12,13 A second hypothesis is that S. sonnei is phagocytized by a free-living amoeba, Acanthamoeba castellanii, finding an intracellular environment, protected from water treatment and chlorination process, while S. flexneri is lethal to A. castellanii and cannot use this same mechanism for its dissemination.14 Finally, S. sonnei has greater ability to mobilize genetic material from other enterobacteria than S. flexneri, acquiring greater resistance to antibiotics and, consequently, greater survival potential.11
A study carried out in the city of Belo Horizonte, Brazil, with a low-income population, demonstrates this change. Of the 157 children evaluated, Shigella species were identified in 17 children (approximately 11%), with S. sonnei being identified in 15 children (88.2%) and S. flexneri in there maining. A previous study carried out in the same city, but with an interval of almost three decades, observed a similar profile to that of other developing countries, with a predominance of S. flexneri. The authors suggest that improved hygiene and sanitation conditions are responsible for this epidemiological change.15
This fact, a similar distribution to that observed in developed countries, was also observed in the city of Salvador, Bahia, by Diniz-Santos et al., where of 141 children with AD and positive culture for Shigella, 80% had S. sonnei, and the other 20% had S. flexneri.16
AD with blood: clinical aspectsDuring the anamnesis of children with AD with blood, history of abdominal surgeries, radiation exposure, and recent use of antibiotics should be investigated.17 It is important to thoroughly investigate the sexual history of adolescents, because receptive anal sex and oral-anal contact may increase the risk of fecal pathogen transmission, particularly Shigella, Salmonella, Campylobacter, Escherichia coli, Entamoeba histolytica, and Giardia.18
Frequently, the first manifestations of shigellosis are fever, headache, malaise, anorexia, and vomiting, which are followed, several hours later, by watery diarrhea and, later, possibly bloody stools. In healthy individuals, the disease is usually mild or moderate in intensity and the symptoms subside within a few days. Other times, there is a progression – within hours to days – to overt dysentery with small-volume but frequent stools containing blood and mucus, accompanied by pain in the lower abdomen and tenesmus. Patients with severe infection can evacuate more than 20 times a day. Abdominal pain, often a prominent characteristic, can simulate appendicitis or, in newborn infants and young infants, intussusception or necrotizing enterocolitis.9
Shigella rarely causes invasive infections such as meningitis, osteomyelitis, arthritis, and splenic abscess. Shigella sepsis, rare in healthy people, can occur in severely malnourished infants and children, with a poor prognosis.19 The intestinal complications of shigellosis are uncommon and include rectal prolapse, intestinal obstruction, toxic megacolon, and perforation; all these complications are more often associated with type-1 S. dysenteriae infections.20
Several extra-intestinal manifestations related to Shigella diarrhea have been reported: erythema nodosum, hemolytic uremic syndrome, reactive arthritis, encephalopathy, glomerulonephritis, and post-infective irritable bowel syndrome. Encephalopathy is one of the most common extra-intestinal manifestations of diarrhea caused by Shigella21 and is associated with high fatality rates. The most common manifestations of encephalopathy are seizures, altered level of consciousness, and coma, which is usually reversible but can be fulminant and fatal. A lethal form of encephalopathy, known as Ekiri syndrome, is characterized by rapid onset of seizures and coma, associated with mild bloody diarrhea.22
Another potentially severe extra-intestinal complication is hemolytic uremic syndrome (HUS), a thrombotic microangiopathy characterized by non-immune hemolytic anemia, thrombocytopenia, and acute kidney injury.23 Microangiopathic thrombi mainly affect the kidney and may result in long-term sequelae. Other organs (such as the intestine, central nervous system, and heart) may also be affected. Shiga toxin-related HUS – produced by some bacterial strains (S. dysenteriae type 1; Enterohemorrhagic E. coli[EHEC]; Shiga toxin-producing Escherichia coli[STEC]) – is a frequent cause of acute kidney injury in childhood, and comprises approximately 90% of all cases of HUS in this population.24In vitro tests have shown that several antibiotics increase the production of Shiga toxin by E. coli; therefore, it is postulated that the use of these medications would increase the risk of HUS.24
In addition to their short-term effects, repeated episodes of AD are associated with a linear growth deficit,25 which predisposes children to new episodes of infectious disease. There is also evidence linking child growth deficits to several important lifelong outcomes, including reduced cognitive function, decreased purchasing power, and increase in chronic disease.26
AD with blood: diagnosisConventional bacterial stool culture remains the gold standard for the etiologic diagnosis of AD caused by enterobacteria, including Shigella infection. The stool culture allows the identification of antibiotic susceptibility, which is very important in this era of increased antimicrobial resistance.9 However, the test has low sensitivity and important technical difficulties. The culture can be impaired by inconsistent bacterial loading, loss of bacterial viability during sample transportation and storage, and requires specific reagents that are labile and sometimes are unavailable. Moreover, they require operator training and experience.
Over the past 10 years, new molecular diagnostic tests have been developed with a polymerase chain reaction (PCR) multiplex panel. They are faster than traditional tests, have higher sensitivity,27 and are able to test a wide range of agents simultaneously. Molecular diagnosis would allow the physician to initiate timely and targeted antibiotic therapy. However, there is little data on how the availability of molecular testing would affect the clinical conduct, cost, and outcomes of the tested patients.28 Thus, its use is still limited to scientific research.
Therefore, in clinical practice, the etiological diagnosis is presumptive, based on clinical and epidemiological data. For most patients, even those in developed countries who have AD, no testing is required if the presentation is suggestive of a viral cause or if severity is mild to moderate.28 The main reason to perform stool cultures is to ensure that treatment, when necessary, is based on susceptibility results in areas at high risk of multidrug-resistant shigellosis.28 Moreover, the test could identify cases with Shiga toxin-producing organisms, in which antibiotic therapy could increase the risk of HUS, although this is still a controversial topic.29
A complete blood count (CBC), with total and differential counts of leukocytes, may be useful and suggest whether the infectious diarrhea is bacterial, viral, or parasitic. When AD is caused by invasive bacteria, the total leukocyte and neutrophil count usually increases. In Shigella infection, a leukemoid reaction and monocytosis can be observed in intracellular pathogenic infections, such as infections caused by Salmonella. However, these findings are nonspecific, and peripheral leukocyte counts should not be routinely performed to establish an etiology of bloody diarrhea. The evaluation of the presence of leukocytes in the stool, fecal calprotectin measurement, and detection of lactoferrin are also not recommended.5
AD with blood: treatmentOverall, the therapeutic approach to AD (with or without blood) is categorized as proabsorptive, anti-secretory, anti-motility, and anti-inflammatory.30 The clinical management of AD to prevent or treat dehydration is predominantly proabsorptive and involves fluid replacement with oral rehydration salts (ORS) or parenteral rehydration therapy. Drug treatment in AD, even with episodes of bloody diarrhea, is not routinely necessary, only in special clinical situations.
Oral rehydration therapy (ORT) and intravenous rehydration therapy (IRT) can directly prevent deaths from diarrhea caused by dehydration, so global programs aiming to make ORT widely available at low cost are among the most important interventions to save lives.31
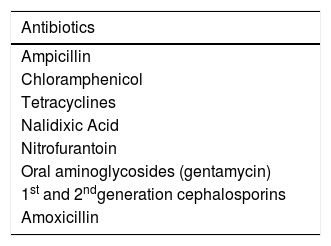
Although antibiotics are useful in some cases of acute bacterial diarrhea, they should be used with caution to avoid pathogen resistance and opportunistic infections.24 Certain enteric organisms, such as Salmonella, should not be treated with antibiotics, as they may prolong disease duration32 (Table 1).
Antibiotic therapy in AD with blood.
| Pathogen | Indication for antibiotic therapy |
|---|---|
| Shigella spp | Proven or suspected shigellosis |
| Salmonella spp (non-typhoid) | Antibiotic therapy is indicated only to reduce the risk of bacteremia and extraintestinal manifestations in high-risk children. Antibiotic use may increase fecal excretion of the bacteria. |
| Campylobacter spp | Antibiotic therapy is recommended mainly for dysentery and to decrease transmission in institutionalized children. |
| Shiga toxin-producing E. coli | Antibiotic therapy is not recommended. It may increase the risk of HUS. |
HUS, hemolytic uremic syndrome.
Furthermore, antibiotics do not immediately prevent fluid and electrolyte loss, the most common cause of death from diarrhea.
Antimicrobials in shigellosis – when and which should be indicated?Empirical treatment is indicated for immunocompetent children who present documented fever, abdominal pain, and dysentery (small-volume bloody stools several times a day and tenesmus); i.e., a clinical picture suggestive of Shigella infection. It would also be indicated for patients with a clinical picture of sepsis caused by enteropathogenic organisms after the collection of blood, stool, and urine cultures.5
When bacterial gastroenteritis is suspected, antibiotic therapy is indicated for children under 3months of age, those with immunodeficiency, severe malnutrition, cancer, inflammatory bowel disease (IBD), achlorhydria, or anatomical or functional asplenia, or those receiving corticosteroid or immunosuppressive therapy.33
It is important to emphasize that no specific antibiotic, or class of antibiotics, is universally effective for Shigella diarrhea.34 The absence of placebo-controlled clinical trials limits the conclusions about the overall efficacy of different antibiotics for treating dysentery.4
The World Health Organization (WHO) guidelines for the treatment of Shigella and dysentery, published in 2005, and ratified in 2013, recommend ciprofloxacin as the first-line treatment.35 The currently recommended dose is 15 mg/kg/day for three days. There are no data to support the use of a higher dose (20 mg/kg), and it may increase the risk of adverse events.36 The second-line treatment would be azithromycin and ceftriaxone. This therapeutic regimen is also recommended by the Brazilian Society of Pediatrics.37
Due to the growing worldwide resistance of Shigella and the adverse effects of fluoroquinolones, the American and European Societies of Infectious Diseases recommend the use of azithromycin (10 mg–20 mg/kg/day, once daily for 3 days) as the first-line therapy.5 Ceftriaxone (50–100 mg/kg/day IV for 3–5 days) should be the first option in children under 3 months and in severe cases with systemic impairment requiring hospitalization.5,37Table 2 presents the antimicrobials that should not be used to treat shigellosis.
Scientific evidence demonstrates that antibiotic resistance is a growing challenge in the therapeutic management of shigellosis. The WHO explicitly listed fluoroquinolone-resistant Shigella as one of its main concerns in the current international focus on antimicrobial resistance.38 The development of antibiotic resistance is common in all Shigella species, particularly in S. sonnei, which can also acquire resistance genes directly from E.coli, through horizontal gene transfer.11
Currently, several studies have described fluoroquinolone-resistant Shigella strains. A systematic review assessed the change in the ciprofloxacin-resistance patterns and found increasing resistance in the Asian-African regions (analyzed together), from 0.6% in 1998–2000 (95% CI: 0.2–1.3%) to 29.1% in 2007–2009 (95% CI: 0.9–74.8%), a 49-fold increase over a period of 12 years. This increase was more pronounced in children than in adults.39 Complete resistance to ciprofloxacin (MIC ≥ 4 mg/L) has been recently reported in domestic cases of S. sonnei in the United States, Vietnam, and other countries.40,41
The WHO recommends ceftriaxone and azithromycin for the treatment of infection by fluoroquinolone-resistant Shigella species.35 However, ceftriaxone- and azithromycin-resistant isolates have also been reported in some places. Cephalosporin resistance is also a matter of concern, due to the production of extended-spectrum β-lactamases (ESBLs), which confers resistance to all β-lactamases (except cephamycin and carbapenemics), being increasingly common, including in Shigella.42 A review43 that compared resistance to third-generation cephalosporins (ceftriaxone, cefotaxime, and ceftazidime) between 1999 and 2012, found a marked increase in the Asian-African regions; ceftriaxone resistance reached 14.2% (95% CI: 3.9–29.4%) in 2012.
An increase in the number of studies describing azithromycin-resistant Shigella spp. strains has been observed, including studies by the Center for Disease Control and Prevention (CDC).44 Therefore, antimicrobial resistance of Shigella has become a threat and a matter of major concern. It may also lead to the inadequate choice of antimicrobials as the initial therapy, and force doctors to choose more toxic or more expensive drugs. A systematic review carried out in 2010 with 1,748 pediatric and adult patients found no statistically significant differences regarding the presence of adverse events between patients receiving fluoroquinolones, macrolides (including azithromycin), or β-lactams, concluding that all classes of antibiotics currently available for shigellosis treatment are safe.34 However, these antimicrobials can increase the risk of arrhythmias, especially torsade de pointes; this adverse effect appears to be more often observed when there is a combination of genetic vulnerability to poor metabolism of CYP3A4 inducing medications and co-administration with other CYP enhancers.45
In 2016, the Food and Drug Administration (FDA) issued a statement specifically addressing the risk of peripheral neuropathy associated with oral fluoroquinolones.46 The neurotoxic mechanism is believed to occur through the inhibition of GABA (gamma-aminobutyric acid) receptors, which occurs after days of use and may be permanent in some cases.47 Regarding ciprofloxacin, specifically, the relative risk was 1.93 (95% CI: 1.32–2.82), a significant increase. However, the research was based on a cohort of men with a mean age of 68 years, and it is difficult to extrapolate these data to the pediatric population.48
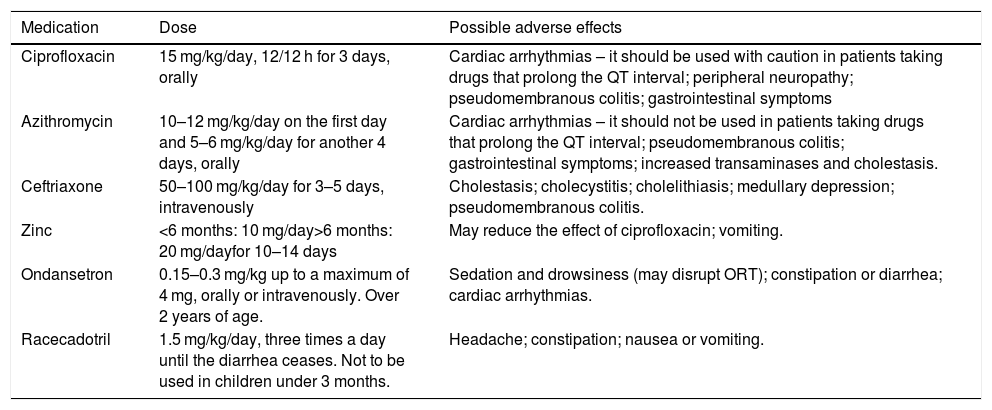
Table 3 shows the main drugs that can be used in AD with blood.
Medications used to treat diarrhea with blood.
| Medication | Dose | Possible adverse effects |
|---|---|---|
| Ciprofloxacin | 15 mg/kg/day, 12/12 h for 3 days, orally | Cardiac arrhythmias – it should be used with caution in patients taking drugs that prolong the QT interval; peripheral neuropathy; pseudomembranous colitis; gastrointestinal symptoms |
| Azithromycin | 10–12 mg/kg/day on the first day and 5–6 mg/kg/day for another 4 days, orally | Cardiac arrhythmias – it should not be used in patients taking drugs that prolong the QT interval; pseudomembranous colitis; gastrointestinal symptoms; increased transaminases and cholestasis. |
| Ceftriaxone | 50–100 mg/kg/day for 3–5 days, intravenously | Cholestasis; cholecystitis; cholelithiasis; medullary depression; pseudomembranous colitis. |
| Zinc | <6 months: 10 mg/day>6 months: 20 mg/dayfor 10–14 days | May reduce the effect of ciprofloxacin; vomiting. |
| Ondansetron | 0.15–0.3 mg/kg up to a maximum of 4 mg, orally or intravenously. Over 2 years of age. | Sedation and drowsiness (may disrupt ORT); constipation or diarrhea; cardiac arrhythmias. |
| Racecadotril | 1.5 mg/kg/day, three times a day until the diarrhea ceases. Not to be used in children under 3 months. | Headache; constipation; nausea or vomiting. |
The WHO recommends routine therapy with 20 mg of zinc per day for 10 days for all children with diarrhea, regardless of the diarrhea type. Infants aged 6 months or younger should receive 10 mg per day for 10 days.49 Multiple studies carried out in low-income countries have reported its effectiveness in reducing both the duration and severity of diarrhea. These effects are most pronounced in malnourished children and in persistent diarrhea.50
A 2016 Cochrane review assessed 33 studies including 10,841 children and concluded that current evidence does not support the use of zinc supplementation in well-nourished children and in places where children are at low risk of zinc deficiency. Moreover, vomiting was more frequently reported in children who received zinc than in those who did not (OR 1.54, 95% CI: 1.28–1.85).50
Antiemetic drugs are usually not necessary in the treatment of acute bacterial diarrhea and, as some have sedative effects, they can make ORT difficult.49 These drugs can be administered especially to children older than 2 years and adolescents, in cases of AD associated with incoercible vomiting (≥three episodes, within a short period of time) to facilitate the tolerance to oral rehydration and prevent dehydration.5 Oral ondansetron is the most studied medication and, if not tolerated, intravenous ondansetron would be indicated. Both presentations are considered equally effective. There is no evidence to support the use of other antiemetic drugs.
Some older antiemetic drugs, such as promethazine, have significant extrapyramidal side effects. The main concern regarding ondansetron use is cardiac arrhythmia, especially QT prolongation, which may predispose to a potentially fatal heart rhythm, including torsade de pointes.50 For this reason, the FDA recommends electrocardiogram monitoring in patients receiving the medication and who have electrolyte disturbances. Moreover, more commonly, ondansetron significantly increases evacuation in treated patients, when compared with placebo.50
Anti-motility drugs, such as loperamide, are contraindicated for use in children, as they have been shown to increase disease severity and the number of complications, particularly in children with bloody (invasive) diarrhea.49 In children under 3 years of age, malnourished, moderately or severely dehydrated, with systemic diseases or with bloody diarrhea, the adverse events outweigh the benefits, even at low doses (<0.25 mg/kg/day).
Racecadotril is an enkephalinase inhibitor, and thus increases the endogenous encephalin levels. These drugs potentially inhibit intestinal secretion of water and electrolytes, with little effect on motility.51 Most clinical trials with racecadotril have not been performed in patients with acute bacterial diarrhea (such as shigellosis), and several studies have specifically reported its efficacy in rotavirus-positive populations.51 Therefore, there is no consensus for its indication in AD with blood.
Probiotics are living microorganisms that, when consumed in adequate amounts, have beneficial health effects health. However, these effects are specific to each strain and need to be analyzed in human studies. Different probiotic strains, including L. reuteri ATCC 55730, L. rhamnosus GG, L. casei DN-114 001, and Saccharomyces boulardii, have been reported as being useful in reducing the severity and duration of acute infectious diarrhea in children.49 It is worth mentioning that the existing evidence on the benefits in viral gastroenteritis is more convincing than the evidence on bacterial or parasitic infections. There is a scarcity of studies in underdeveloped countries. There is still no consensus on the benefits of its use in watery AD, as well as in AD with blood. Due to the modest effects of probiotics and their high cost, the WHO does not recommend their use in resource-limited settings, especially in developing countries.49
Other medications – under studyShigella decreases the concentration of cathelicidin, an antibacterial peptide produced by the host. In animal models, this mechanism was reversed by treatment with oral sodium butyrate and resulted in disease improvement. A randomized study in children with severe shigellosis found that eating cooked green bananas (a substrate for the production of butyrate by intestinal bacteria) significantly improved the clinical picture when compared with a rice-based control diet.52
Other studies are researching the role of the extra cellular calcium-sensing receptor (CaSR). This receptor works primarily in maintaining intestinal calcium homeostasis. The receptor activation would decrease cellular cyclic nucleotides and reverse all four pathological changes that occur in diarrhea: excessive secretion, malabsorption, increased intestinal motility, and an increased inflammatory response. Early clinical studies yielded positive results, demonstrating that the activation of CaSR associated with ORS would be safe, affordable, and possibly more effective than the current gold standard. The treatment based on CaSR is considered promising for the management of childhood diarrhea, but there is no scientific evidence of its efficacy in AD with blood.30
AD associated with Shigella: preventionStrategies to prevent acute diarrheal disease include exclusive breastfeeding for six months, measures to improve infrastructure to ensure basic sanitation and clean drinking water, health education focused on hygiene measures, and vaccines.53 Especially in shigellosis, adequate waste treatment and handwashing after handling waste play a very important role in prevention, given its high transmissibility and the fact that person-to-person is the main route of contamination.54
Although there is no vaccine for Shigella yet, even with over 60 years of ongoing efforts to create a vaccine, there are currently 13 candidates under development; almost half of them are in phase 1 or 2 of clinical trials and one is in phase 3. Studies and consortia for the development of a Shigella vaccine have been furthered by new funding for the renewed acknowledgement, in recent years, of shigellosis morbidity and lethality.55,56
It is expected that, with a number of innovative strategies based on a better understanding of the virulence mechanisms and the Shigella immune response observed in recent decades, an effective vaccine will soon be available.56 However, despite advances, there is still no consensus on which immune response will result in effective protection, because there is no adequate animal model for this evaluation. Most of the candidate vaccines currently under study are attenuated, hybrid (with other live vectors, e.g. E. coli), glycoconjugates, and subunit vaccines.55,56
Some other biological and technical issues, however, will define the applicability of a vaccine for Shigella. The vaccine must be adequate, effective, and provide lasting protection, which can be a biological challenge when considering the different risk groups for the disease: children between 0 and 5 years of age in developing countries, with vulnerable immunological status and, in contrast, immunocompetent travelers from developed countries. Moreover, considering the need for a global scale use of the vaccine, stability, easy administration, need for as few applications as possible, and cost will determine the use of a Shigella vaccine in the poorest and most needy regions of the planet.55,56
ConclusionsAD with blood still show a high prevalence, especially in regions where environmental conditions are unfavorable and good-quality water and sanitation services are not yet available to a large part of the population. In the specific case of Shigella, where transmission occurs mainly person-to-person, food and personal hygiene measures, especially handwashing, are of great importance in breaking the cycle of fecal-oral contamination. Improving environmental conditions is a key step in reducing its incidence.
Another important aspect is the adequate treatment of the acute diarrheal episode to avoid short- and medium-term complications, namely dehydration and the impact on nutritional status.
Most of the time, the infectious process causes mild or moderate disease and it is self-limited within five to seven days. Adequate management to prevent or treat dehydration, adequate food supply, and non-use of medications are the basis for the treatment.
In selected cases, there is an indication for the use of antibiotics. The current concern is the increase in microbial resistance to the recommended antibiotics, which brings an additional difficulty to the therapeutic management. Symptomatic medications have restricted and non-consensual indication.
There are no commercially available vaccines to date, but several studies are being conducted. In underdeveloped regions, investing in the improvement of environmental conditions and health education programs is of great importance for controlling AD with blood.
Conflicts of interestThe authors declare no conflicts of interest.