To analyze the effectiveness, safety, outcomes, and associated factors of tuberculosis preventive treatment (TPT) in children and adolescents in Paraná, southern Brazil.
MethodThis was an observational cohort study with a retrospective collection of secondary data from the TPT information systems of the state of Paraná from 2009 to 2016, and tuberculosis in Brazil from 2009 to 2018.
ResultsIn total, 1,397 people were included. In 95.4% of the individuals, the indication for TPT was a history of patient-index contact with pulmonary tuberculosis. Isoniazid was used in 99.9% of the cases with TPT, and 87.7% completed the treatment. The TPT protection was 98.7%. Among the 18 people who had TB, 14 (77.8%) became ill after the second year of treatment, and four (22.2%) in the first two years (p < 0.001). Adverse events were reported in 3.3% of cases, most of them were gastrointestinal and medication was discontinued in only 2 (0.1%) patients. No risk factors associated with the illness were observed.
ConclusionsThe authors observed a low rate of illness in pragmatics routine conditions in TPT for children and adolescents, especially within the first two years after the end of treatment, with good tolerability and a good percentage of adherence to the treatment. TPT should be encouraged to achieve the goals of the End TB Strategy of the World Health Organization as an essential strategy to reduce the incidence rate of the disease, but studies with new schemes must continue to be carried out in real-life scenarios.
According to the End TB Strategy proposed by the World Health Organization (WHO), the treatment of tuberculosis infection (TBI) is essential to reduce the disease incidence rate.1 In 2022, the United Nations high-level meeting recommended that six million tuberculosis preventive treatments (TPT) be offered to people living with HIV, 4 million children < 5 years of age, and 20 million contacts ≥ 5 years of age, as these are the groups that contribute to the largest number of new TB cases.2 The Brazilian Ministry of Health aims to reach less than 10 cases per 100,000 inhabitants by 2035,3 implementing a TPT surveillance system in 2018, incorporating new diagnostic tests for TBI in children and people living with HIV, and incorporating a regimen for treatment with 12 weekly doses of isoniazid and rifapentine.
In randomized clinical trials, different regimens for TPT substantially reduced the risk of illness in people with TBI, both in those living with HIV4,5 and in those without HIV infection.6 It is questionable whether adherence to the treatment in the routine of the programs would be different from that found in clinical trials since TPT effectiveness under routine conditions has been little studied. This study aimed to analyze the effectiveness, safety, and outcomes of TPT as well as the factors associated with its effectiveness in children and adolescents in the state of Paraná, southern Brazil, where a surveillance system implemented since 2009 allows access to quality secondary data.
Materials and methodsStudy designThis was an observational cohort study, with a retrospective collection of secondary data from the TPT information systems of the state of Paraná (TILTB – PR database) from 2009 to 2016 and of tuberculosis from Brazil (TB – SINAN Brazil database) from 2009 to 2018.
Study population and settingThe State of Paraná has 399 municipalities, with a mean TB incidence of 20.5/100,000 hab. Vaccination with BCG is recommended for all children during the first month of life. Through the study period, the national recommendations for children and adolescents in home contact with bacilliferous patients were investigated using a tuberculin test, thoracic radiography, and clinical evaluation. In individuals with confirmed TBI, performing TPT with 6 months of isoniazid (180 doses) until 2010 and from 2010, 6 or 9 months (180 or 270 doses of isoniazid) was recommended.7
Inclusion criteria: all children and adolescents (3 months to 18 years, 11 months, and 29 days) registered in the TILTB – PR database who were treated for TPT from 2009 to 2016 who were asymptomatic, had a normal thorax radiography and a positive tuberculin skin test (≥ 5 mm) or history of contact with a patient with pulmonary tuberculosis.
Exclusion criteria: patients diagnosed with tuberculosis disease up to three months after the start of TPT or incomplete notifications that did not allow the analysis of the case.
ProceduresTILTB – PR (2009-2016) and TB – SINAN Brazil (2009-2018) were linked using OpenReclink™ software, which uses the probabilistic relation method of records8 to identify patients who underwent TPT and developed tuberculosis disease, regardless of follow-up (lost to follow-up or not). The variables of reference for pairing were the name, date of birth, and mother's name.
Data collectionThe outcome of the study was the incidence of TB, considered when individuals who underwent TPT were notified as patients in the database TB – SINAN Brazil. This notification was considered a TPT failure.
The data analysis period to identify the illness ranged from two to nine years after TPT. As this observation period varied among individuals, it was expressed in person-years. This measure is represented by the fraction where the numerator is constituted by the total number of incident cases in each period and the denominator by the sum of individual collaboration (multiplying each person by the time they were under observation).
TB is a notifiable disease, all cases are reported on specific forms in the TB – SINAN Brazil, which is considered a quality database. Thus, it was assumed that those not found on SINAN did not have tuberculosis.
The difference in disease risk was compared between groups.
Outcomes were compared according to the complete regimen (adherence), adjusted for demographic characteristics, indication for TBI treatment, data from the source patient, laboratory tests such as HIV and tuberculin tests, medication use, and presence of adverse events.
Complete treatment was considered an exposure variable. It was defined as taking at least 180 doses of isoniazid (within 9 months of treatment) or if in the absence of this information, the notification terminated as complete treatment.7
Data analysisTo characterize the sample, qualitative variables were expressed as absolute and relative frequencies, whereas quantitative variables were expressed as median, maximum, and minimum values. Comparisons between the proportions of the infected and sick groups were performed using Fisher's exact test. The Wilcoxon-Mann-Whitney nonparametric test was used to analyze continuous variables. Statistical significance was set at p < 0.05.
Potential risk factors for TB were analyzed using a semi-parametric Cox model. Risk ratios and confidence intervals (95%) were estimated to control for confounding variables. Data organization, cleaning, and analysis were performed using RStudio software.
The study was approved by the Human Research Ethics Committee (CAAE:9852518.4.0000.5225).
ResultsIn Paraná, from 2009 to 2016, 15,179 patients with pulmonary tuberculosis of all ages were reported in the TB – SINAN-Brazil database, with a record of 59,573 contacts, of which 52,194 (87.6%) were investigated.9 During the same period, 5,612 individuals (corresponding to 10.8% of those investigated) were reported in the TILTB-PR database, of which 1,418 were children or adolescents. Of these, 4 were excluded because they developed TB up to 3 months after the introduction of TPT and 17 due to incomplete data, which precluded the analysis. The final sample consisted of 1,397 individuals.
TPT was indicated due to a history of contact with tuberculosis in 1,333 (95.4%) people, immunosuppression by drug or disease and tuberculin reactor test in 18 (1.3%), and another unspecified risk factor and tuberculin reactor test in 46 (3.3%). The authors don't have information about the treatment of index-case, nor about the temporal aspects of exposure.
The total number of people reported and analyzed who evolved to tuberculosis disease was 18 (1.3%), obtaining protection of 98.7%, when considering TPT intention, 14 (77.8%) became ill after 2 years of treatment, whereas 4 (22.2%) evolved within the first 2 years (p < 0.001). When assessing the number of people monitored over time, 1,397 children and adolescents were monitored, representing 7,105 person-years of follow-up, and of these, 18 became ill, corresponding to 0.002 person-years (in one year of follow-up, 0.002 people became ill). The distribution of the number of people who underwent TPT and those who developed TB was similar over the years.
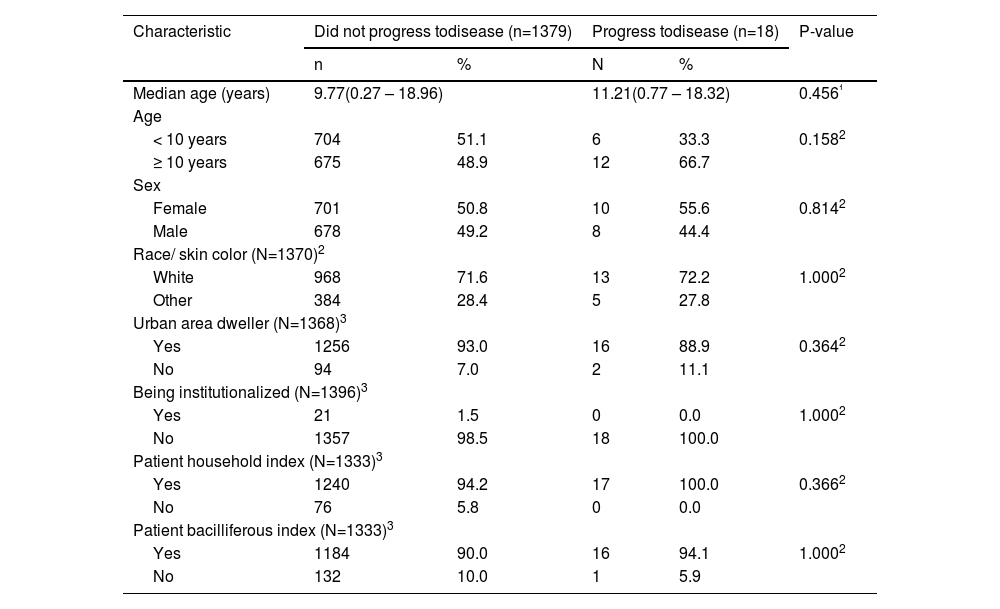
Table 1 describes the demographic characteristics of the individuals in the groups who did not progress to disease compared with those who did. Considering these characteristics, the groups were considered homogeneous. By evaluating the illness specifically by age group, the authors observed that 3 (16.6%) were < 2 years, 1 (5.5%) between 2–4 years, 2 (11.1%) between 5–9 years, 7 (38.9%) between 10–14 years, and 5 (27.9%) between 15–19 years.
Demographic characteristics according to the evolution or not of tuberculosis in children and adolescents subjected to the tuberculosis preventive treatment in Paraná, from 2009 to 2016.
| Characteristic | Did not progress todisease (n=1379) | Progress todisease (n=18) | P-value | ||
|---|---|---|---|---|---|
| n | % | N | % | ||
| Median age (years) | 9.77(0.27 – 18.96) | 11.21(0.77 – 18.32) | 0.456¹ | ||
| Age | |||||
| < 10 years | 704 | 51.1 | 6 | 33.3 | 0.1582 |
| ≥ 10 years | 675 | 48.9 | 12 | 66.7 | |
| Sex | |||||
| Female | 701 | 50.8 | 10 | 55.6 | 0.8142 |
| Male | 678 | 49.2 | 8 | 44.4 | |
| Race/ skin color (N=1370)2 | |||||
| White | 968 | 71.6 | 13 | 72.2 | 1.0002 |
| Other | 384 | 28.4 | 5 | 27.8 | |
| Urban area dweller (N=1368)3 | |||||
| Yes | 1256 | 93.0 | 16 | 88.9 | 0.3642 |
| No | 94 | 7.0 | 2 | 11.1 | |
| Being institutionalized (N=1396)3 | |||||
| Yes | 21 | 1.5 | 0 | 0.0 | 1.0002 |
| No | 1357 | 98.5 | 18 | 100.0 | |
| Patient household index (N=1333)3 | |||||
| Yes | 1240 | 94.2 | 17 | 100.0 | 0.3662 |
| No | 76 | 5.8 | 0 | 0.0 | |
| Patient bacilliferous index (N=1333)3 | |||||
| Yes | 1184 | 90.0 | 16 | 94.1 | 1.0002 |
| No | 132 | 10.0 | 1 | 5.9 | |
In 118 (29.6%) municipalities, there was at least one notification of TPT: 280 (20.0%) were performed in the capital and 264 (18.9%) in the port area, where the highest incidence of TB was found in the state.
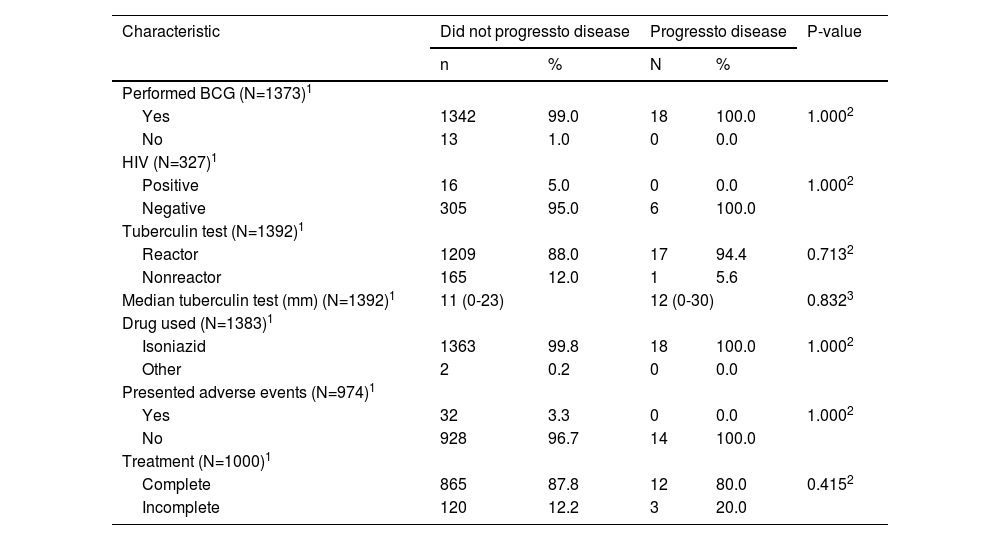
Table 2 describes the clinical, laboratory, and therapeutic characteristics according to disease evolution. These characteristics were similar in both groups.
Clinical characteristics according to the evolution or not of tuberculosis in children and adolescents subjected to the tuberculosis preventive treatment in Paraná, from 2009 to 2016.
| Characteristic | Did not progressto disease | Progressto disease | P-value | ||
|---|---|---|---|---|---|
| n | % | N | % | ||
| Performed BCG (N=1373)1 | |||||
| Yes | 1342 | 99.0 | 18 | 100.0 | 1.0002 |
| No | 13 | 1.0 | 0 | 0.0 | |
| HIV (N=327)1 | |||||
| Positive | 16 | 5.0 | 0 | 0.0 | 1.0002 |
| Negative | 305 | 95.0 | 6 | 100.0 | |
| Tuberculin test (N=1392)1 | |||||
| Reactor | 1209 | 88.0 | 17 | 94.4 | 0.7132 |
| Nonreactor | 165 | 12.0 | 1 | 5.6 | |
| Median tuberculin test (mm) (N=1392)1 | 11 (0-23) | 12 (0-30) | 0.8323 | ||
| Drug used (N=1383)1 | |||||
| Isoniazid | 1363 | 99.8 | 18 | 100.0 | 1.0002 |
| Other | 2 | 0.2 | 0 | 0.0 | |
| Presented adverse events (N=974)1 | |||||
| Yes | 32 | 3.3 | 0 | 0.0 | 1.0002 |
| No | 928 | 96.7 | 14 | 100.0 | |
| Treatment (N=1000)1 | |||||
| Complete | 865 | 87.8 | 12 | 80.0 | 0.4152 |
| Incomplete | 120 | 12.2 | 3 | 20.0 | |
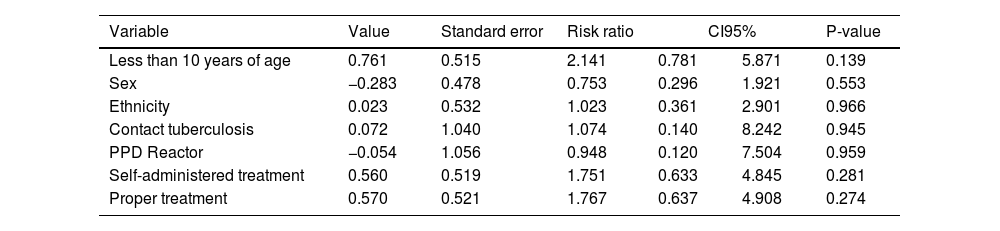
Table 3 presents the analysis of the possible risk factors for illness. No association was observed between the variables of exposure or adjustment for the outcomes.
Possible risk factors for tuberculosis in children and adolescents subjected to tuberculosis preventive treatment in Paraná, from 2009 to 2016, adjusted for the multivariate model of proportional risks by Cox.
Regarding HIV investigation, the test was not performed in 1008 (72.2%) people, 24 (1.7%) were in progress, and 38 (2.7%) had this information ignored. Of the 327 performed, 16 (4.9%) were infected with HIV, and of these, 5 (1.5%) were newly diagnosed because of an investigation of contact with TB.
Of all included, 5 (0.4%) did not undergo the tuberculin test. Among those who underwent the test, 1,226 (88.1%) were reactors, and in 24 (2.0%), the test was positive after a second test that showed a difference from the initial test.
Adverse events were reported in the database by 3.3% of the total number of people included in the study, all in the group that did not progress to disease. The symptoms were as follows: 23 (71.9%) gastrointestinal, 2 (6.3%) cutaneous, 1 (3.1%) convulsive crisis, and 6 (18.7%) others. In 2 patients (0.1%) suspension of the regimen was necessary because of adverse events, one due to convulsive crises, and the other due to gastric intolerance.
Regarding the outcome of TPT, as described in the TILTB – PR database, of the 1,397 individuals, 877 (62.8%) completed the treatment, 112 (8.0%) abandoned it, 34 (2.4%) were transferred, 9 (0.6%) were suspended by medical indication, 2 (0.1%) by adverse events, and in 363 (25.9%), the outcome was ignored. Excluding those transferred and with unknowing treatment (28.4%) and grouping the other outcomes as complete (877; 87.7%) or incomplete (123; 12.3%) treatment, the evolution to illness was later compared and no association was found (Table 2). The number of treatment doses used in both groups could not be determined.
Among the 112 individuals who were registered as loss of follow-up, the median age was 12.24 years (0.42 to 11.82), 71 (63.4%) were older than 10 years, 58 (51.8%) were male, 84 (77.8%) were white and no patient was institutionalized. Information on HIV infection status was described in 23 people and 1 (4.4%) was infected. Of the 90 individuals who had information about the presence of an adverse event, this was present in 4 (4.4%). Of all who lost the follow-up, 2 (1.8%) developed TB 2 years after the onset of TPT. No information is available regarding the stage at which the treatment was abandoned.
Among the 18 children who became ill, the median time of illness in relation to the onset of TPT was 3.73 years (0.72 to 6.26). Their age at the time of illness was 14.86 years (ranging: 1.74 to 21.84 years). Two children (11.1%) were < 5 years of age, 3 (16.7%) were between 5–10 years old, and the others were > 10 years old (72.2%). Pulmonary TB occurred in 16 (88.9%) patients, and ganglionic and bone TB occurred in one patient (5.6%). The child with bone TB was the only one with underlying disease (Crohn's disease and juvenile rheumatoid arthritis) on immunosuppressive therapy and had TPT suspended by medical order, 4.17 years before falling ill. Regarding the outcome, 15 individuals progressed to cure, two had no treatment outcome, and one had been transferred. Tuberculostatic sensitivity tests were performed in seven patients, six of whom were sensitive to all drugs and one was resistant to streptomycin.
DiscussionThe authors found high protection of the TPT in this study, corresponding to 98.7% when considering the intention to treat in a real-life context. The authors are unaware of similar studies in routine conditions of services for data comparison.
According to WHO, the expected percentage of infected people in the world population, regardless of contact with bacilli, is around 25%.10 Meta-analyses and systematic reviews have described a TBI detection rate of around 50% among TB contacts.11,12 In the present study, based on the number of notified TB contacts, a higher percentage of cases with TPT was estimated, but only 10.8% of the estimated number was identified. Therefore, this demonstrates a low number of reported cases of TPT, which can be attributed to underreporting or mistakes in the indication of TPT.
The database showed a relatively high number (28.4%) of cases without a description of the outcome at the end of treatment. A systematic review and meta-analysis of TBI in migrants globally found treatment initiation rates ranging between 23% and 97% and completion treatment rates between 7% and 86%.13 Verma et. al. identified that the main gaps in the success of TPT were referral and initiation of treatment,14 probably similar to the present study that shows only 10,8% of the estimated cases of TPT.
In the present study, participants without a description of the outcome were excluded from the analysis of adherence to the treatment and evolution of the disease. The percentage of people who got sick was higher in the group with incomplete treatment when compared to the group with complete treatment (2.5% × 1.4%, respectively), but the difference was not statistically significant. Some factors may have influenced the absence of statistical significance, such as the low rate of illness in the studied population and the lack of administered therapeutic doses, leading to incorrect classification of groups. In the present study, all evaluated individuals were submitted to some therapeutic scheme, even if it was incomplete. The authors were unable to accurately determine the number of doses administered to each individual. It should be noted that the number of doses taken is the most important factor for therapeutic effectiveness, not the time of treatment.15 One hypothesis that can be raised is that treatments, although partial, may have a residual effect and be more effective than no treatment at all. This would justify the high level of protection found in that study, with no difference in the adherence to the treatment. However, this finding can also be explained by other biases and limitations of the study.
In the present study, no HIV investigation was performed in more than 70% of cases. Five new cases of HIV infection were identified among those investigated. If this proportion of HIV-infected patients were maintained in the non-investigated, the authors would have approximately 12 more cases with a missed opportunity for an HIV diagnosis. There is a formal recommendation that HIV-infected patients with TB contacts, even with negative PT, should receive TPT. Because HIV-infected people are much more likely to get sick.4,7
The adherence to the treatment, among the notifications including this information, as described in 87.7% of the sample, and rates in the literature vary according to different scenarios (33.0 to 80.0%).16-18 Regarding the characteristics of people who lost follow-up, most were adolescents, as reported by de Oliveira et al. who evaluated the treatment of the disease.19 This population has specific characteristics, and different strategies are required. The authors emphasize that the presence of adverse events or being HIV-positive should not have been determining factors for the abandonment of therapy since these factors were described in only approximately 4% of both situations in the group that discontinued therapy.
Among the few patients who developed tuberculosis in this study, the median duration of illness was 3.73 years, with a statistically higher risk of illness 2 years after the end of TPT. A period longer than expected if considering the natural history of the disease, which would be mainly within six months, extending up to approximately two years.20-22 This subsequent illness can be interpreted not as treatment failure but as reactivation or reinfection, especially because most of those who became ill were adolescents with a median age of 14.86 years. The illness may have been secondary to new exposure or reactivation of the quiescent focus, as in the pathophysiology of the disease in adolescents who develop post-primary TB, instead of a failure of the TPT.23,24
Among those who became ill, the authors were able to define flaws in three patients’ TPT processes, which would justify a failure in protection. Two patients abandoned treatment, with illness occurring two years after abandonment. The authors found no report on the number of doses that these patients had received, making it impossible to infer the existence of any degree of protection for these drugs or a delay in the time of illness. The third patient fell ill after four years of medical therapy suspension, despite immunosuppression and a positive tuberculin skin test, which must have been a mistake in the conduct. There was no report of new exposure to TB. These facts demonstrate the importance of periodic technical training and alignment of healthcare professionals in TPT.1,15,24
The safety of TPT was demonstrated by the low incidence of adverse events. And considering the drug resistance induced by TPT, among the patients who became ill and were tested, resistance to the drugs used was not found, as described in the literature.15,25
This study has limitations inherent to its design, owing to the retrospective use of secondary databases. The failure to complete the information, lack of data, and loss of follow-up can lead to difficulties in interpretation and analysis. However, the authors believed that these limitations did not derail the importance of the results obtained. As some patients with tuberculosis may not have been diagnosed and the cross-examination with the infant mortality database was not performed, the TPT protection rate may have been overestimated. Despite the possible underdiagnosis, underreporting in the studied country is unlikely. Because once the diagnosis has been made, treatment is only provided upon notification of the case. However, the pediatric TB case detection gap in Brazil is not significant, as estimated by the WHO report of 2020 before the COVID-19 pandemic.26
This study showed a low rate of illness in children and adolescents who underwent TPT in a real-life context, especially within the first two years after the end of treatment. Additionally, the authors provided evidence for safe therapy and a good rate of adherence to the treatment. However, it is essential to maintain a periodic review of the guidelines that have been changing with advances in the diagnosis, treatment, and prevention of tuberculosis and the constant training of healthcare teams for the proper implementation of these measures. A robust notification system with consistent information that can be periodically analyzed to fund public health policies is also essential. TPT should be encouraged to achieve the goals of the End TB Strategy of the WHO as an essential strategy to reduce the incidence rate of the disease, but studies with new schemes must continue to be carried out in real-life scenarios.
Funding sourcesThis article did not receive any funding.
The authors are grateful to Anete Trajman for their intellectual support and to Betina Mendez Alcantara Gabardo who was indirectly fundamental in this work, as one of the authors in the implementation of the TPT notification in Paraná, with its own database, a pioneer in Brazil.
Institution or service with which the work is associated for indexing in Index Medicus/MEDLINE, City, Country. Postgraduate Course in Child and Adolescent Health, Pediatrics Department, Federal University of Paraná.