Upper respiratory tract infections in children generally have significant morbidity and mortality. There is little data available about functional immaturity of the immune system and the child's susceptibility to infections at the beginning of their lives, thus, justifying a more specific immunological analysis.
MethodAnalysis of hemograms and innate and adaptive immune responses in 95 children between age 1 to 6 years with episodes of recurrent respiratory infections (test group n = 39) and without these episodes (control group n = 56) was carried out. The production of reactive oxygen intermediates by peripheral blood cells stimulated by phorbol myristate acetate was analyzed. Additionally, the number of B lymphocytes, auxiliary T lymphocytes, and cytotoxic cells was determined using flow cytometry.
ResultsResults from both groups did not show statistically significant differences in red blood cells, total leukocytes count, and the differential neutrophils, eosinophils, basophils, lymphocytes, and monocytes count. The analysis of the number of B lymphocytes, auxiliary T lymphocytes (LTCD4), and cytotoxic cells (LTCD8) also did not show any difference between both groups. However, the production of radical oxygen intermediates was significantly reduced in the test group as compared to the control group (p < 0.05).
ConclusionsThere was no difference in the analysis of hemograms, leukograms, or the number of lymphocytes, LTCD4, LTCD8, or LTCD19. The reduced production of oxygen intermediates in the affected group suggests that these children's microbicide capacity is compromised, which may be related to their recurrent respiratory infections.
Respiratory infections (RI) involving the upper respiratory tract (URT) are one of the main causes of children's medical appointments and hospitalizations with significant morbidity and mortality all over the world.1-4 The recurrence and frequency of these infections in children are important challenges to pediatricians.2,5,6
RI represents the most frequent pathologies from the age 6 months to 6 years. Etiologic agents are not always identifiable, but viruses, such as respiratory syncytial virus, rhinovirus, adenovirus, and influenza are the main causal agents responsible for more than 80 % of the recurrent respiratory infections (RRI).1,7 Among bacteria, the most common are S. pneumoniae, M. pneumoniae, H. influenza, and S. pyogenes.8 Epidemiologic studies suggest that approximately 6 % of under 6-year-old children have RRI. In developed countries, up to 25% of children younger than one year old and 18% of children between 1 to 4 years old have this diagnosis.9
These children do not usually have a previous disorder that can justify the occurrence of infectious conditions. They have normal immunity but are also more susceptible to exposure to infectious agents due to genetic background and environmental factors during their first years of life. They are healthy children with normal growth and development and exhibit good health between infectious episodes, which, in most cases, do not have prolonged or complicated courses. On the other hand, children with primary immunodeficiencies usually have recurrent respiratory infections caused by specific microorganisms or by less virulent germs, with prolonged clinical evolution, inadequate response to conventional antibiotic therapy, and high risk of complications.10,11
No clear consensus exists in the literature about the definition of RRI. As a criterion for standardizing scientific terminology, most authors consider RRI if there are six or more episodes a year.2,3,7,9,12 Approximately 50% of the children affected by RRI are healthy, 30% are atopic, 10% suffer from another disorder, and 10% can be immunodeficient.8
Most children suffer from infections of URT, but about 10 – 30% of the children suffer from lower tract infections. In 60 % of the cases, there is bacterial growth after ten days. There are two RRI incidence peaks, the first one between 6 and 12 months of age, after the consumption of passively transferred maternal immunoglobulins (Ig), with low concomitant own antibody synthesis, and when children go to day nursery or school.9
Several studies investigate white blood cell and red blood cell alterations in children with RRI. Some of them did not observe any association between deficiencies in lymphocytes subpopulation and neutrophils function and the number of CD4, CD8, CD9, and NK (natural killer) cells.13 On the other hand, others demonstrated a significant association between anemia and recurring lower tract RI.14,15
The immune system is composed of non-specific mechanisms, known as innate immunity, and the specific ones called adaptive immunity. The innate immunity includes mechanical barriers (skin, mucous membranes), lysozymes, lactoferrin, fibronectin, complement system, interferons (IFNs), the phagocytic system (macrophages and neutrophils), and NK and dendritic cells. It develops independently of the presence of infections and does not have any specificity towards a particular microorganism.16
The adaptive immunity is composed of T and B lymphocytes, which are antibody producers. Adequate interaction of both the innate and adaptive immunities leads to the prevention and elimination of infections.17
The first mechanism to eliminate invading microorganisms is phagocytosis. Compared to normal adults, chemotaxis and bactericidal activity of newborn neutrophils and phagocytes show reduced quantitative and functional capacity.18 Esposito et al. (2013) characterized immune immaturity in childhood as decreased neutrophil chemotaxis, reduced phagocytosis, decreased production of reactive oxygen species (ROS), and reduced number of CD4+, CD8+, CD19+, and NK lymphocytes.
The Macrophage-monocyte system is fundamental to phagocytosis for the removal of foreign particles and antigen processing and presentation, which are essential for antibody synthesis and cell-mediated reactions. After phagocytosis, ROS, such as O2−, H2O2, and OH− are generated, which are essential for the destruction of intracellular pathogens. These microbicides intermediate are even greater in neutrophils that possess peroxidase activity.19,20
Adaptive immunity is responsible for controlling infections, eliminating infectious agents, and granting the organism protection against reinfection by the same agent thus, inducing protective immunity, the same mechanism used by vaccines.1,16,20
In this study, the authors investigated some parameters of the innate and adaptive immune response in children with RRI and compared with a control group without RRI.
MethodsSample characteristicsPeripheral blood samples (5 mL) were collected by venipuncture from 113 children between 1 and 6 years old, cared for in the general pediatrics clinic at Federal University of Triângulo Mineiro (UFTM), in Uberaba, Brazil. They were separated into two groups. The first group, defined as ‘test’, constituted of children, regardless of gender or ethnicity, with a history of RRI, excluding those with underlying pathologies (such as primary and secondary immunodeficiencies, cystic fibrosis, respiratory tract malformations, immotile-cilia syndrome), and whose parents accepted to participate in the study, and answered a questionnaire about obstetric, family, morbid and nutritional antecedents, life habits and conditions, and the vaccinal history. The number of individuals in the test group corresponds to half of the patients registered in the pediatric service with the current diagnosis of recurrent upper respiratory tract infection. The second group, defined as ‘control’, constituted of healthy children with no history of RRI and whose parents agreed to participate in the study and answered the same questionnaire. This project was approved by the ethics in research committee and was given number 61396916.0.0000.5154.
Among all the collected samples (113), 50 (44.25 %) were female children and 63 (55.75 %) male. The test group constituted 47 children, 22 (46.8 %) female, and 25 (53.2 %) male. In comparison, the control group constituted of 66 children, 28 (42.4 %) female and 38 (57.6 %) male. After collecting, processing, and analyzing the samples, 95 patients were selected: 39 in the test group and 56 in the control group.
The following criteria were used to diagnose RRI in general: 6 or more respiratory infections a year; 1 or more respiratory infections a month, involving the upper respiratory tract, from September to April, or three or more respiratory infections a year involving the upper respiratory tract.2
Clinical criteria applied by the pediatrician during routine procedures (registered in medical charts) and the questionnaire specially developed for this study, answered by the child's parents or legal guardians, were used to determine if children were healthy or had underlying disorders, allergic conditions, chronic pathologies or immunodeficiencies.
Peripheral Blood Cells preparationPeripheral blood cells (PBC) were separated using a density gradient in Ficoll-Hypaque (1.114) (GE Health Care, Uppsala, Sweden), centrifuged at 400 × g at 21°C for 30 min. They were resuspended in RPMI 1640 (GE) containing 50 mM Hepes (GIBCO, Grand Island, NY, USA), 5% inactivated fetal bovine serum (GIBCO), 2 mM L-glutamine (GIBCO), 40 μg/mL gentamicin (Neoquímica, Anápolis, State of Goiás, Brazil), and 1 mL 2β-mercaptoethanol (Merck, Darmstadt, Germany), in a final concentration of 2 × 106 cells/mL, and cultured in 96-well plates (FALCON, San Jose, CA, USA).
For analysis of lymphocytes number, 5 × 105 PBCs were resuspended in 100 µL Hank's medium (Sigma, St. Louis, MO, USA), supplemented with 10 % inactivated human AB+ serum. After that, the samples were labeled with respective surface antibodies for B and T cells. The cells were washed three times to remove excess antibodies, fixed with the addition of 500 µL HANKS medium containing 0.5 % paraformaldehyde, and stored at 4 °C in the dark until flow cytometry analysis. The samples were labeled with antibodies (BD Biosciences, San Jose, CA, USA) for B cells (CD19), for T helper cells (CD3 and CD4), and for cytotoxic T cells (CD3 and CD8). A FACS Calibur cytometer (Becton-Dickinson, Mountain View, CA, USA) was used for the acquisition of events (100,000 events/tube), and the data were analyzed using the FlowJo software (Three Star USA).
Oxygen intermediate productionFor analysis of ROS levels, a ROS-Glo kit (Promega, Madison, Wisconsin, USA) was used according to the manufacturer's instructions. Briefly, 5 × 104 cells resuspended in 80 µL per well in a 96 flat bottom plate were incubated overnight at 37 °C. After that 5 ng of phorbol myristate acetate and H2O2 substrate solution (20 µL) were added and incubated at 37 °C for 4 h. Then, 100 µL of detection solution containing Luciferin was added. The assay detects intra and extra-cellular ROS production. Luminescence was measured in a Multimode plate reader EnSpire (PerkinElmer, Waltham, Massachusetts, USA). All experiments were performed in duplicate, and the results were expressed in luminescence units.
Statistical analysisStatistical analysis was performed using GraphPadPrism 7.0 (GraphPad Software, La Jolla, CA). The distribution of the quantitative variables was verified by the D'Agostino & Pearson normality test. The variables presented normal distribution and homogeneous variance. The student's t-test was used to compare the two groups. Data were expressed as a mean with standard deviation or standard error. The results were considered statistically significant when p < 0.05.
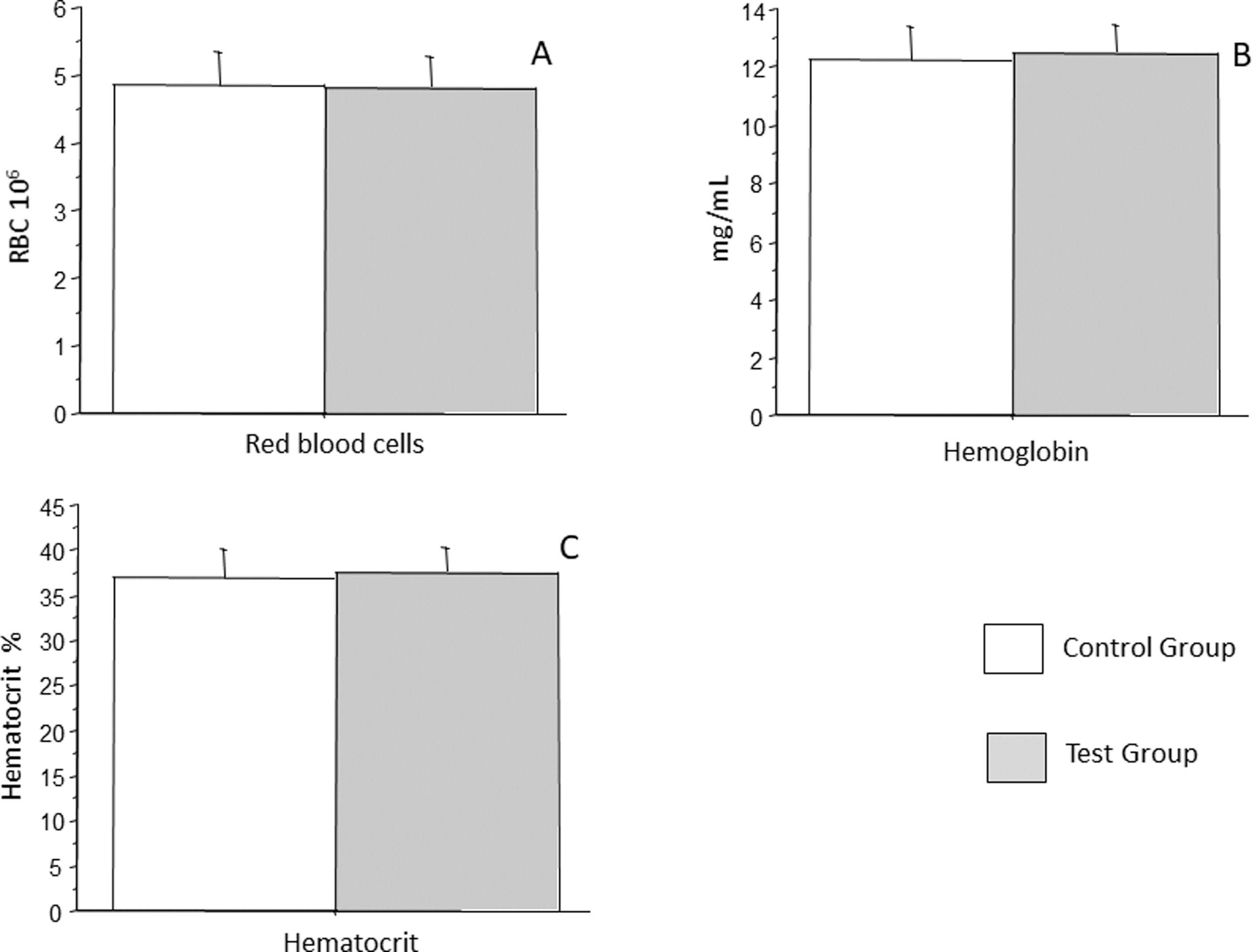
ResultsIn the present study, 95 children were selected from the initial 113 due to incomplete data, such as the absence of complete blood count or insufficient sample to perform all the experiments. Of these, 39 formed the ‘test’ group, and 55 formed the ‘control’ group. Children with a history of recurrent respiratory infections had on average 6 to 11 episodes of infections, tonsillitis being the most frequent followed by sinusitis, and in smaller numbers, common cold and otitis media. Table 1 summarizes demographic data. All the children were healthy, as they did not present a specific disease that justified the recurrence of the infections, had adequate growth and development, were well during the infectious episodes, which had clinical evolution without complications, and responded adequately to conventional therapies, however, were more susceptible to exposure to infectious agents. Red blood cells were analyzed by hemogram for erythrocytes, hemoglobin, and hematocrit count. It was observed that the control group had an average of 4.83 million/mm3 (± 0.5) erythrocytes, whereas the test group had an average of 4.80 million/mm3 (± 0.47) erythrocytes, which was not a statistically significant difference (p = 0.7587) (Figure 1A). Hemoglobin count in control group was an average 12.24 g/dL, (± 1.2 g/dL), whereas that in test group was an average 12.46 g/dL (± 1.0 g/dL), which was not a statistically significant difference (p = 0.3687) (Figure 1B). Similarly, the hematocrit count in the control group was an average of 36.94 % (± 3.1 %), whereas that in the test group was an average 37.52 % (± 2.8 %), which was not a statistically significant difference (p = 0.3885) (Figure 1C).
Demographic characteristics of the study population.
Analysis of the number of erythrocytes (A), hemoglobin (B), and hematocrit (C) in patients with recurrent respiratory infections (test group) and patients from the control group. Bars represent the average, and vertical lines represent the standard deviation. No statistically significant difference was observed between the two groups. p = 0.7587, p = 0.3687, and p = 0.3885 respectively.
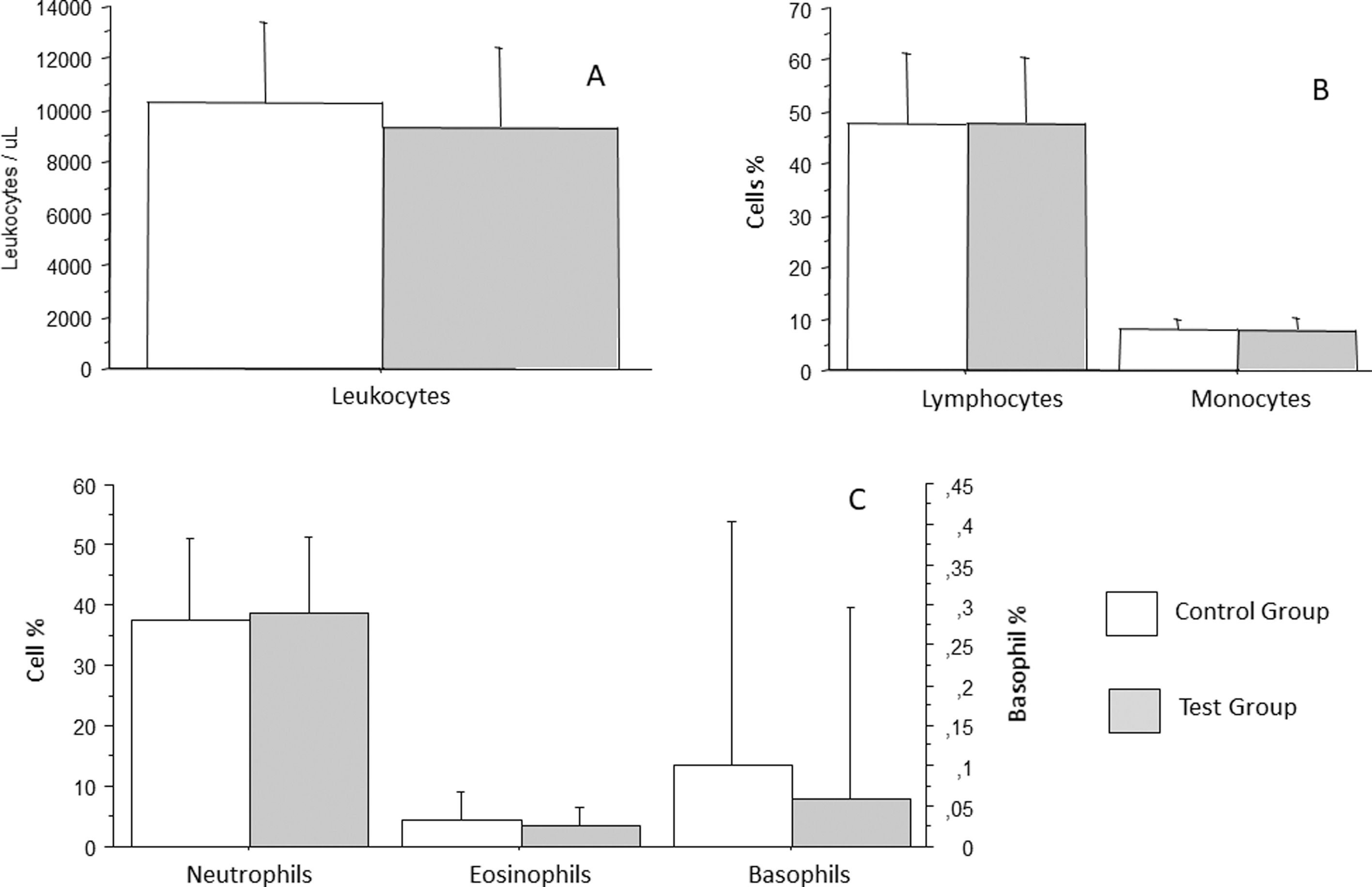
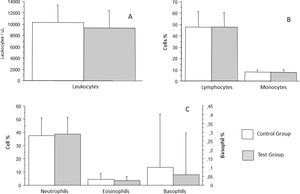
White blood cells were analyzed as total leukocytes count and leukocyte differentials. Leukocytes count in the control group was an average of 10296/mm3 (± 3143/mm3), whereas that in the test group was an average of 9357/mm3 (± 3062/mm3), which was not a statistically significant difference (p = 0.1782) (Figure 2A). White blood cells differentials revealed that segmented neutrophils of patients from the control group were an average 37.36 % (± 13.5 %), whereas those of patients from the test group were an average 38.61 % (± 12.7 %), which was not a statistically significant difference (p = 0.6691). Eosinophil count in the control group was an average of 4.26 % (± 4.81 %), whereas that in the test group was an average of 3.5 % (± 3.15 %), which was not statistically significant difference (p = 0.4205). Basophil count in the control group was an average of 0.1 % (± 0.3 %), whereas that in the test group was an average 0.06 % (± 0.24 %), which was not a statistically significant difference (p = 0.5086). Lymphocyte count in the control group was an average of 47.82 % (± 13.56 %), whereas that in the test group was an average of 47.94 % (± 12.61 %), which was not a statistically significant difference (p = 0.9671). Monocyte count in the control group was an average of 8.02 % (± 2.0 %), whereas that in the test group was an average of 7.73 % (± 2.3 %), which was not a statistically significant difference (p = 0.5491) (Figures 2A and B).
Analysis of total leukocytes count (A), lymphocytes and monocytes (B), neutrophils, eosinophils, and basophils (C) in patients with recurrent respiratory infections (test group) and patients from the control group. Bars represent the average, and vertical lines represent the standard deviation. No statistically significant difference was observed between the groups.
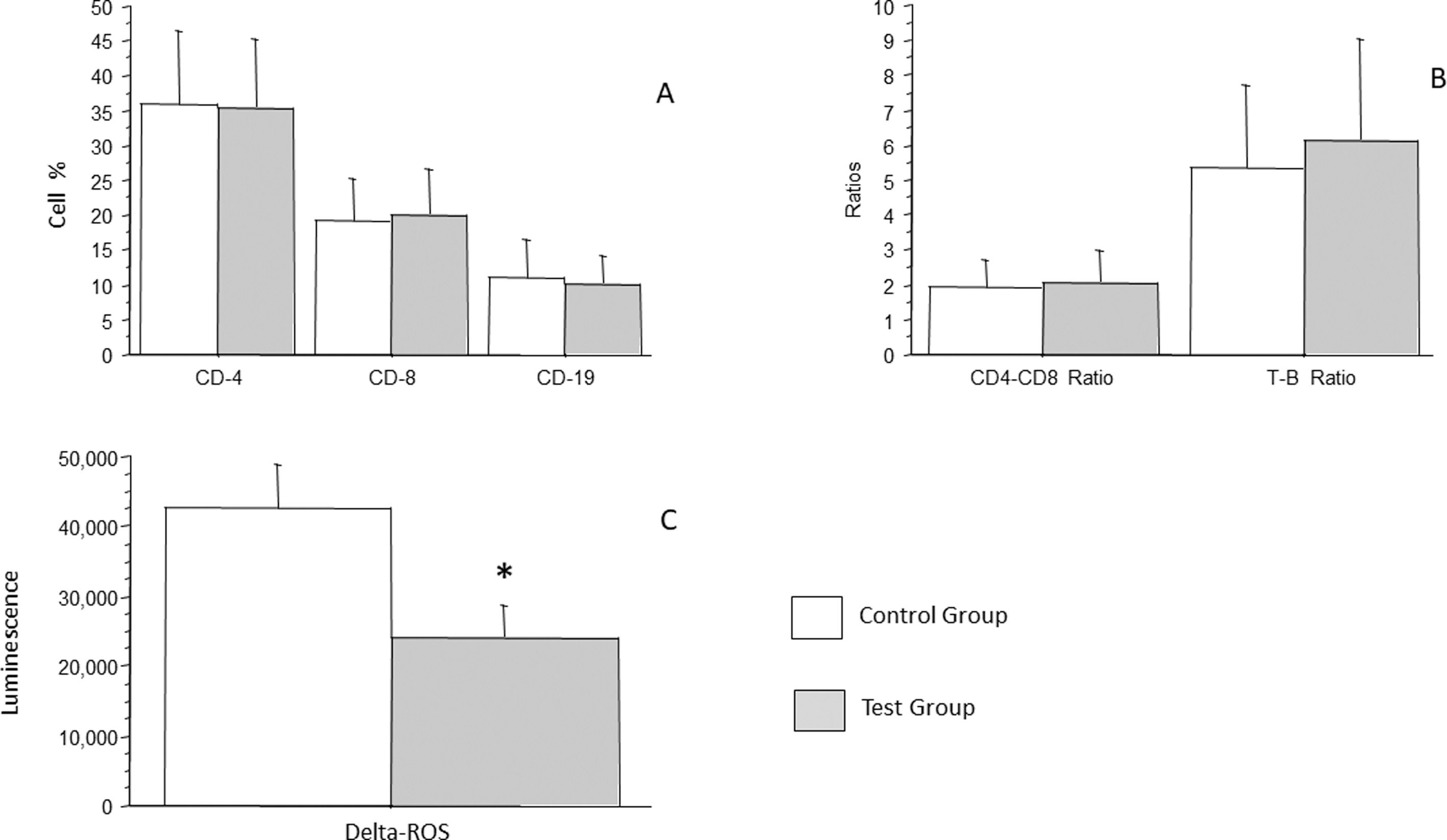
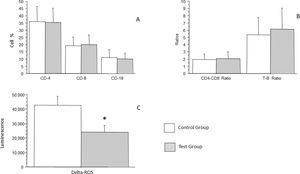
Lymphocytes were analyzed in total and separately as LTCD4, LTCD8, LTCD19, LTCD4/LTCD8 ratio, and L/LB ratio. LTCD4 count in the control group was an average of 36.06 % (± 10.3 %), whereas that in the test group was an average of 35.53 % (± 9.8 %), which was not a statistically significant difference (p = 0.8007). LTCD8 count in the control group was an average of 19.36 % (± 5.97 %), whereas that in the test group was an average of 20.0 % (± 6.63 %), which was not a statistically significant difference (p = 0.6406). LTCD19 count in the control group was an average of 10.88 % (± 5.6 %), whereas that in the test group was an average of 10.08 % (± 4.0 %), which was not statistically significant difference (p = 0.4392) (Figure 3A). LTCD4/LTCD8 ratio in the control group was an average of 1.92 (± 0.8), whereas that in the test group was an average of 2.05 (± 0.9), which was not a statistically significant difference (p = 0.5016). LTCD4/LTD8 ratio in the control group was an average of 5.39 (± 2.34, whereas that in the test group was an average of 6.16 (± 2.9), which was not a statistically significant difference (p = 0.1758) (Figure 3B).
Analysis of the percentage of LTCD4, LTCD8, LBCD19 and analysis of the production of oxidant radicals by cells of patients with recurrent respiratory infections (test group) and patients from the control group (A), CD4/CD8 ratios and LT/LB ratios are shown in panel B. Bars represent the average, and vertical lines represent the standard deviation. No statistically significant difference was observed between the groups. In C, analysis of the production of oxidant radicals by cells of patients with recurrent respiratory infections (test group) and patients from the control group. Bars represent the average, and vertical lines represent the standard error. A statistically significant difference (p = 0.0321) was observed between the groups *.
Analysis of the production of oxidant radicals was performed with chemiluminescence. The control group had an average of 42.628 (± 6.306), whereas the group of patients with recurrent infections had an average of 24.004 (± 4.616). A statistically significant low potential to produce oxidant radicals was observed in children with recurrent infections control compared to the group (p = 0.0321).
DiscussionIt is an undeniable fact that there is a marked discrepancy in the number of respiratory infections among children. While some children have 1 or 2 episodes a year, others have 10 to 11 episodes a year. Thus, it is important to characterize these patients, be attentive to the environmental, hygienic, genetic, socioeconomic, cultural, and nutritional factors, anatomic and immune conditions, pondero-statural and neuropsychomotor development, and signs of immunodeficiency.
As there is little data available in the scientific literature regarding functional immaturity of the immune system in children and susceptibility to RRI at the beginning of their lives, this study aimed to perform a more specific immunological analysis of this population. In order to achieve that, two groups of children were studied. One group constituted of children with a history of RRI, except those with underlying pathologies justifying the infections, called the ‘test’ group. The second group, called ‘control’, constituted of children who were healthy, with no history of RRI. Blood samples were collected from both the groups, and the blood elements, as well as the production of intermediate oxygen radicals were analyzed.
In some children, it is possible to identify minor or partial alterations in the immune system, which strengthen the theory of a transient secondary immune depression, and a post-infectious stage. Transient decrease in CD4+ lymphocytes, cytokines, or neutrophil chemotaxis, IgA partial defect or IgG subclass alterations and deficiency of anti-polysaccharide antibody are examples of changes identified in healthy children with recurrent respiratory infections.5,12,21-25
Many factors play an important part in the genesis of recurrent respiratory infections episodes. In some children, it is possible to detect transient or permanent deficiencies in the immune system. However, real immunodeficiency is rare, and the main recurrent respiratory infections cause is the infant's own susceptibility, as both cellular and humoral immunities reach their efficiency peak in the fifth or sixth year of age. Some children can have low levels of immunoglobulin isotypes or rarely of any specific cell type of the defense system.5,26
In the present study, routine test results from both the groups did not show statistically significant differences regarding red blood cells (erythrocytes, hemoglobin or hematocrit count) or white blood cells (total leukocytes and leukocyte differentials, including neutrophils, eosinophils, basophils, lymphocytes, and monocytes). The number of helper T lymphocytes (CD4), cytotoxic T cells (CD8), B lymphocytes (CD19), as well as an LTCD4/LTCD8 ratio and LT/LB ratio did not show significant differences as per flow cytometry analysis. Consistently, the study performed by Roxo Jr (2009) demonstrated that children with RRI do not normally show an underlying disorder that justifies the successive infectious episodes. Another study of children from 6 months to 6 years of age showed that anemia, especially iron-deficiency anemia.25 Similar studies on RI of the lower tract found that anemic children are 5.75 times more risk than the control group, leading to the conclusion that preventing anemia would reduce the incidence of such infections.14
In the present study, the production of ROS was significantly lower in the group of children with RRI (test group) when compared to the group of children with no RRI (control group). ROS production by phagocytic cells is an important microbicide mechanism. In these cells, the respiratory bush is activated by bacteria-derived molecules and pro-inflammatory cytokines, like TNF-α and IL-1β.27,28 Similarly, a study including 90 children with RRI showed a reduction in neutrophil activity, as measured by phagocytosis assay, in 15.5 % of children. These results are comparable with Raniszewska et al. (2015) study, in which no association was found between defects in lymphocytes subpopulations and the function of neutrophils.
Neutrophils and other phagocytic cells are equipped with an electron transfer chain consisting of a multi-component enzyme known as NADPH oxidase. This enzyme is responsible for producing microbicidal oxidants in phagocyte activation. For the activation of NADPH oxidase, at least five different proteins are necessary: two subunits of the cytochrome related to b558, gp91-phox, and p22-phox membrane, and three cytosolic proteins, p47-phox, p67-phox, and a low molecular weight GTP-binding in rac-1 (in macrophages) or rac-2 (in neutrophils).29 ROS produced by NADPH oxidase plays an important part in host antimicrobial defense; hence, its deficiency can result in recurrent bacterial infections, whereas its unregulated release can result in excessive inflammation.30
A high concentration of ROS helps in the control of invasive bacteria. Host immune cells, such as neutrophils and macrophages, release high amounts of ROS at the infection site after the surface receptors, such as receptors attached to G protein, toll-like receptors, and cytokine receptors, are activated. Meanwhile, Fcγ-receptor and integrin activation directly induce the production of high levels of ROS. Moreover, GPCRs, which bind to peptide analog of a bacterial peptide FMLP, a neutrophil chemoattractant, may stimulate cells and unleash production of low levels of ROS.30
Inside PMNs, ROS released by NADPH oxidase complex can activate granular proteases and induce the formation of extra-cellular neutrophil traps. Furthermore, ROS can pass through bacterial membranes and damage the nucleic acids, proteins, and cellular membranes. However, the pathogens also use different intrinsic and extrinsic mechanisms to escape from such host defenses.30
The authors of the present study put in evidence that a lower capacity to produce ROS is associated with recurrent respiratory infections. Thus, suggesting that the reduced production of oxygen intermediates in the affected children affects their microbicide capacity, which may be related to their recurrent respiratory infections. This situation appears to be transient and limited to childhood since RRI are not observed in adolescence.
Study limitationsAll laboratory evaluations were performed simultaneously, whereas the data used to group children as ‘test’ or ‘control’ was gathered from medical charts and reports from parents and legal guardians. In addition, the size of the sample of the studied subject may be a limiting factor.
FundingFAPEMIG – REDE 313/2016; CNPq - PQ309.104/17.