To translate, cross-culturally adapt, and validate a specific questionnaire for the evaluation of celiac children and adolescents, the celiac disease DUX (CDDUX).
MethodsThe steps suggested by Reichenheim and Moraes (2007) were followed to obtain conceptual, item, semantic, operational, and measurement equivalences. Four pediatric gastroenterologists; a researcher with tool validation background; three English teachers; and 33 celiac patients, aged 8–18 years, and their caregivers participated in the study. The scores of celiac patients and those obtained from their caregivers were compared. Among the patients, the scores were compared in relation to gender and age.
ResultsAll items were considered relevant to the construct and good semantic equivalence of the version was acquired. During measurement equivalence, the exploratory factor analysis showed appropriate weight of all items and good internal consistency, with Cronbach's α of 0.81. Significant difference was found among the final scores of children and their caregivers. There was no difference among the final scores in relation to gender or age.
ConclusionThe questionnaire was translated and adapted according to all the proposed steps, with all equivalences adequately met. It is a valid tool to access the QoL of celiac children and adolescents in the translated language.
traduzir, adaptar transculturalmente e validar um questionário específico para avaliação de qualidade de vida (QV) de crianças e adolescentes com doença celíaca (DC), o Celiac Disease DUX (CDDUX).
MétodoForam seguidas as etapas descritas por Reichenheim e Moraes (2007) para obtenção de equivalências conceitual, de itens, semântica, operacional e de mensuração. Participaram do estudo quatro gastroenterologistas pediátricos, um profissional com experiência em validação de instrumentos, três professores de inglês e 33 pacientes celíacos, entre oito e 18 anos, com seus responsáveis. Foram comparados os escores de QV obtidos dos pacientes com os obtidos através dos seus responsáveis. Dentro do grupo de pacientes, compararam-se os escores em relação ao sexo e idade.
ResultadosTodos os itens foram considerados pertinentes ao construto, e foi atingida boa equivalência semântica da versão. A análise fatorial exploratória demonstrou carga fatorial adequada de todos os itens e boa consistência interna, com α de Cronbach de 0,81. Foi evidenciada diferença significativa entre o escore final do CDDUX de crianças e seus pais. Não houve diferença do escore final do questionário em relação ao sexo ou à idade.
ConclusãoA tradução e adaptação seguiram adequadamente as etapas propostas, com a equivalência sendo atingida de maneira satisfatória. O instrumento traduzido mostrou-se válido para avaliação da QV de crianças e adolescentes com DC.
Celiac disease (CD) is an autoimmune enteropathy triggered by gluten, which has a great variety of clinical manifestations and occurs in genetically susceptible individuals. The treatment consists of removing gluten from the diet throughout life.1 The gluten-free diet is strict and, therefore, difficult to accept and follow, as it leads to modification of eating habits and lifestyle, which affects patient quality of life (QoL).2 Once treatment is established, celiac individuals are asymptomatic, showing no external sign indicating the presence of the disease. However, the need to follow a special diet gives visibility to the disease, putting patients at risk of denigration and stigmatization within the social context, with a negative impact on the QoL of these patients.3
The operational assessment of QoL is commonly performed through questionnaires, which can be generic, encompassing several domains and wide-ranging health problems, or specific, evaluating issues inherent to a certain group of individuals or disease.4
Several QoL assessment studies in children and adolescents with CD have been published in the last decade,5–13 with the validation of two specific questionnaires. The first one, the celiac disease DUX (CDDUX)7 was developed by a Dutch pioneer group in QoL studies in celiac individuals and, in 2013, Jordan et al.13 validated a questionnaire for U.S. children and adolescents.
The development of an assessment tool is a time-consuming and costly task; therefore, performing the translation and adaptation of an existing questionnaire is a good option, following specific methodological recommendations, which allows its use in different cultural contexts.14–16
The lack of a QoL assessment tool for celiac children and adolescents validated for use in Brazil motivated this study, which aimed to translate, adapt, and validate the (CDDUX),7 a disease-specific questionnaire for health-related QoL assessment for children with CD.
MethodsThis is a methodological study of translation, cultural adaptation, and validation of a questionnaire for QoL assessment in celiac children and adolescents. The process of cultural adaptation, performed from March of 2012 to November of 2013, followed the equivalence steps proposed by Reichenheim and Moraes,15 as follows: (a) conceptual, (b) items, (c) semantics, (d) operational, and (e) measurement.
The first two steps were carried out by a committee consisting of four pediatric gastroenterologists that conceptually verified the relevance of the tool and the items it comprised.
The semantic equivalence was performed in five steps: (a) translation; (b) back-translation; (c) semantic equivalence of versions; (d) cultural adaptation; and (e) pre-testing. The translation of the original tool from English into Portuguese was independently performed by two professionals fluent in English. The first was a pediatric gastroenterologist and the second a children's English teacher with a college degree, who created the final version of the translation together. The translated questionnaire was back translated into English by two North-American college graduates who were also English teachers, fluent in Portuguese. After the consensus, a final version was produced. At the semantic evaluation, the denotative and connotative meanings of the words were verified, as the literal correspondence of a term does not imply that the same emotional reaction is evoked in different cultural contexts.
The pre-final version was tested with a group of five patients and their parents/guardians, to assess the understanding of the tool, and they were asked about the existence of any unintelligible words or questions. These five were included in the final group, which consisted of 33 celiac individuals, aged between 8 and 18 years, and 33 parents/guardians who participated in the final stages of the cross-cultural adaptation process of the tool. Among the celiac patients, most were aged between 8 and 11 years (66.7%), were women (72.7%), had good reading skills (81.8%), and reported they were following a gluten-free diet (91.9%). Among the parents/guardians, most of the participants were mothers (84.8%), had good reading skills (81.8%), and had a per capita family income <½ minimum wage at the time of the study (69.7%).
All patients were followed at the Pediatric Gastroenterology Clinic of Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), located in the city of Recife, which only treats patients from the Brazilian Unified Health System (SUS) who have the diagnosis confirmed by a pediatric gastroenterologist and are instructed to follow a gluten-free diet.
The evaluation of the questionnaire format, the order of appearance of questions, and the manner and location of questionnaire application comprised the operational equivalence, performed by two pediatric gastroenterologists, one with experience in research with this group of patients.
The verification of the psychometric properties of the questionnaire corresponded to the measurement equivalence, focusing on the dimensional validity assessment, adequacy of items by domain, and the tool's internal consistency.
The CDDUX consists of 12 items comprising three domains: Diet (6), Communication (3), and having celiac disease (3). A five-point Likert scale is used for the answers, aided by a picture diagram with faces expressing different emotional states related to the answers. There is also a questionnaire version to be answered by the parents/guardians about the children, containing the same questions and answer options.7
The scores per item corresponding to the picture-answers ranged from 1 to 5; the original authors used a correction factor in each question to obtain a mean final score ranging from 1 to 100, with 1–20 being considered very poor QoL, 21–40 poor, 41–60 neutral, 61–80 good and 81–100, very good.7
Data were tabulated using Epidata software, release 3.1 (Epidata Assoc. – Odense, Denmark), and all analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, USA).
Sample adequacy was assessed using Kaiser-Meyer-Olkin (KMO) index, resulting in a value of 0.64, with values >0.50 considered acceptable.17 Bartlett's test of sphericity was used to verify whether the correlation matrix was an identity matrix.
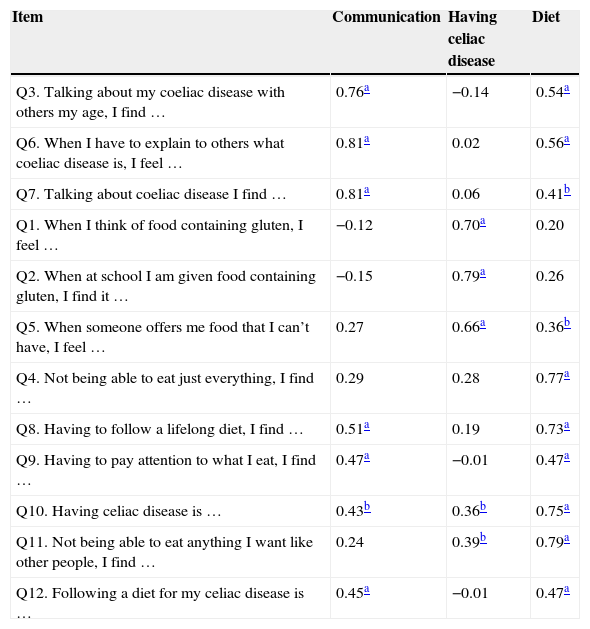
Additionally, the authors sought to identify the existence of multicollinearity (high correlation, r>0.80) and singularity (perfect correlation, r=1) between questions, using Spearman's correlation coefficient. The correlation was performed to meet two objectives: to verify whether the variables were linked to the others that comprised the domain, but without having an r>0.80, as this would mean the suppression of one of the items and identifies in which domain each item should remain. The items with the highest correlation (p<0.05) remained in their respective domain.
An exploratory factor analysis was performed using promax rotation to verify the factor loading of each item of the scale. Eigenvalues≥1.0 were accepted, in addition to items with factor loading >0.4, in order to define the factors obtained in the analysis. Internal consistency was determined by Cronbach's α, considering α values≥0.7 to be satisfactory.
Student's t-test was used to compare the mean score of the questionnaires applied to the children and their parents/guardians. ANOVA was used to verify the variance of patients’ means in relation to gender and age. For this purpose, patients were categorized in relation to age into two groups: between 8 and 11 years and between 12 and 18 years.
This study was approved by the Research Ethics Committee of Instituto de Medicina Integral Prof. Fernando Figueira (IMIP) (process no. 3420-13). All participating children signed the informed consent and their parents/guardians also signed the informed consent before the start of the study. The cross-cultural adaptation was authorized by the researcher responsible for the original questionnaire.7
ResultsThe committee of gastroenterologists evaluated all items as relevant to the domains and construct (QoL) in the cultural context of the target population and chose to maintain all questions in the following steps.
The semantic equivalence steps were performed satisfactorily. The translations showed to be equivalent, and there was a consensus between the translators in the development of the final translation. Table 1 shows the original questionnaire and the final translated and adapted version. The back-translation showed good resemblance to the original questionnaire, with a few modified terms. The term “find,” which appears in several questions, was translated as “achar” in Portuguese, but it was decided to replace it by “sentir” (feel). In the instructions, both translators showed difficulty when translating the term “faces” and, after a consensus, it was decided to translate it as “carinhas” (little faces). Also in the instructions, the phrase “there are no wrong answers” was removed, initially translated as “não existem respostas erradas” in Portuguese, so as not to denote a sense of evaluation, i.e. that the patient was being tested. In the wording of the questions, the term “express” was translated by T1 as “expressar” (express) and by T2 as “mostrar” (show). The term “mostrar” was chosen to facilitate understanding.
Original questionnaire and final translated and adapted version of CDDUX.
| Original version | Final translated and adapted version |
|---|---|
| We would like to know how you feel these days | Nós gostaríamos de saber como você tem se sentido esses dias |
| Therefore, could you please indicate how you feel in different situations? | Você poderia indicar como se sente em diferentes situações? |
| You can do that by circling in each question one of the faces that fits you best. There are no wrong answers; it's about what you feel | Você pode fazer isso circulando em cada pergunta a carinha que mostre como você se sente. Não há respostas certas ou erradas; trata-se de saber como se sente |
| Please express how you’ve been feeling lately. | Por favor, circule a carinha que mostra como você tem se sentido ultimamente. |
| 1. When I think of food containing gluten, I feel … | 1. Quando você pensa em comida com glúten, você se sente … |
| 2. When at school I am given food containing gluten, I find it … | 2. Quando na escola lhe dão comida com glúten, você se sente … |
| 3. Talking about my coeliac disease with others my age, I find … | 3. Quando precisa falar para outros da sua idade sobre sua doença celíaca, você se sente… |
| 4. Not being able to eat just everything, I find … | 4. Não poder comer tudo faz você se sentir … |
| 5. When someone offers me food that I can’t have, I feel … | 5. Quando alguém lhe oferece comida que você não pode comer, você se sente … |
| 6. When I have to explain to others what celiac disease is, I feel … | 6. Quando você tem que explicar aos outros o que é doença celíaca, você se sente … |
| 7. Talking about celiac disease I find … | 7. Quando se fala sobre doença celíaca, você se sente … |
| 8. Having to follow a lifelong diet, I find … | 8. Ter que seguir uma dieta por toda a vida faz você se sentir … |
| 9. Having to pay attention to what I eat, I find … | 9. Ter que prestar atenção ao que você come faz você se sentir … |
| 10. Having celiac disease is … | 10. Ter doença celíaca é … |
| 11. Not being able to eat anything I want like other people, I find … | 11. Não poder comer tudo que você quer, como as outras pessoas, faz você se sentir … |
| 12. Following a diet for my celiac disease is … | 12. Ter que seguir uma dieta por causa da sua doença celíaca é … |
No expressions were observed that required adaptation and no words showed to be inappropriate for the local culture. However, it was decided to change the subject form from the first-person to the third-person singular, because in the operational equivalence step, the questionnaire application was chosen as an interview.
Also at this stage, it was decided to keep the original questionnaire format and the order of appearance of items. The questionnaires were applied in the Gastroenterology Department consultation offices where patients are followed, as patients were already familiarized with the environment.
At the measurement equivalence phase, the sample adequacy test (KMO) showed a value of 0.64. Analyses of singularity and multicollinearity showed adequate correlation values, as shown in Table 2, and all items were maintained for the exploratory factor analysis.
Spearman's correlation coefficient between the items of the translated version of CDDUX and its domains.
| Item | Communication | Having celiac disease | Diet |
|---|---|---|---|
| Q3. Talking about my coeliac disease with others my age, I find … | 0.76a | −0.14 | 0.54a |
| Q6. When I have to explain to others what coeliac disease is, I feel … | 0.81a | 0.02 | 0.56a |
| Q7. Talking about coeliac disease I find … | 0.81a | 0.06 | 0.41b |
| Q1. When I think of food containing gluten, I feel … | −0.12 | 0.70a | 0.20 |
| Q2. When at school I am given food containing gluten, I find it … | −0.15 | 0.79a | 0.26 |
| Q5. When someone offers me food that I can’t have, I feel … | 0.27 | 0.66a | 0.36b |
| Q4. Not being able to eat just everything, I find … | 0.29 | 0.28 | 0.77a |
| Q8. Having to follow a lifelong diet, I find … | 0.51a | 0.19 | 0.73a |
| Q9. Having to pay attention to what I eat, I find … | 0.47a | −0.01 | 0.47a |
| Q10. Having celiac disease is … | 0.43b | 0.36b | 0.75a |
| Q11. Not being able to eat anything I want like other people, I find … | 0.24 | 0.39b | 0.79a |
| Q12. Following a diet for my celiac disease is … | 0.45a | −0.01 | 0.47a |
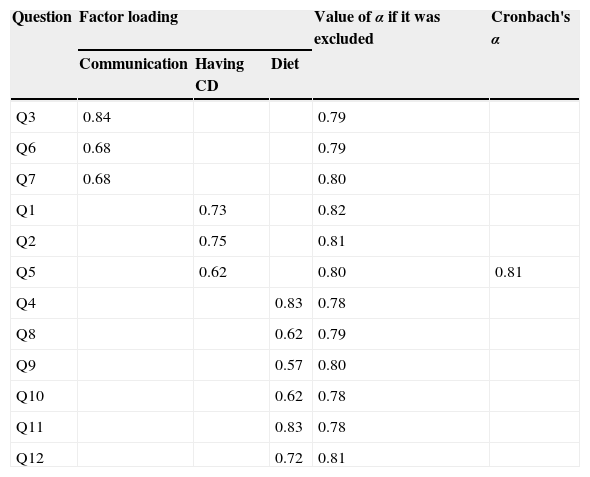
When analyzing the main components, the domains explained 70.7% of the scale variance, with good internal consistency, as shown in Table 3, and the exclusion of only one of the items would determine a modest increase in Cronbach's α value, which was not significant.
Factor analysis of the translated version of CDDUX.
| Question | Factor loading | Value of α if it was excluded | Cronbach's α | ||
|---|---|---|---|---|---|
| Communication | Having CD | Diet | |||
| Q3 | 0.84 | 0.79 | |||
| Q6 | 0.68 | 0.79 | |||
| Q7 | 0.68 | 0.80 | |||
| Q1 | 0.73 | 0.82 | |||
| Q2 | 0.75 | 0.81 | |||
| Q5 | 0.62 | 0.80 | 0.81 | ||
| Q4 | 0.83 | 0.78 | |||
| Q8 | 0.62 | 0.79 | |||
| Q9 | 0.57 | 0.80 | |||
| Q10 | 0.62 | 0.78 | |||
| Q11 | 0.83 | 0.78 | |||
| Q12 | 0.72 | 0.81 | |||
CD, celiac disease.
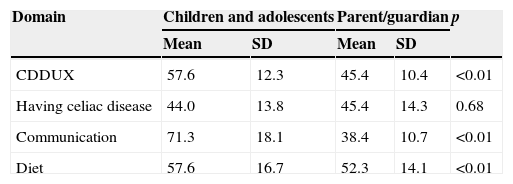
A significant difference was observed between the mean final score of the questionnaire answered by the children and their parents/guardians. When assessing the domains separately, only the domain “Having celiac disease” was not significantly different between the two groups, as shown in Table 4.
Mean final score and by domains of the translated version of CDDUX applied to children and adolescents with celiac disease and their parents/guardians.
| Domain | Children and adolescents | Parent/guardian | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| CDDUX | 57.6 | 12.3 | 45.4 | 10.4 | <0.01 |
| Having celiac disease | 44.0 | 13.8 | 45.4 | 14.3 | 0.68 |
| Communication | 71.3 | 18.1 | 38.4 | 10.7 | <0.01 |
| Diet | 57.6 | 16.7 | 52.3 | 14.1 | <0.01 |
There was no difference between the means in the celiac group aged between 8 and 11 years (59.4±11.2) and between 12 and 18 years (54±14.3), with p=0.24; and between the means of female (56.8±13.1) and male patients (59.9±10.3), with p=0.53.
DiscussionBased on the proposal of Reichenheim and Moraes,15 the translation and cultural adaptation of CDDUX was carried out, and it was only available questionnaire for QoL assessment in children and adolescents when the study started. It is known that a good translation may not be enough to maintain a good conceptual level between different cultures and adaptations are often necessary.14,15,18
In an attempt to answer specific questions of celiac children and adolescents, van Doorn et al.7 developed CDDUX, a disease-specific tool, divided into three domains: having celiac disease, Communication, and Diet.
Davis et al.,19 in a systematic review of the conceptual basis of QoL assessment tools in children, suggested that, given the great diversity in the conceptualization used and the small number of QoL theories that have been identified, in addition to the time required to develop new theories, the appropriate choice of domains and items is required.
In the authors’ opinion, the CDDUX domains comprehend important aspects of the assessment of QoL of patients with CD, and all the items were assessed as relevant by the committee of gastroenterologists. However, other aspects are not addressed, such as the difficulty for obtaining access to gluten-free foods, especially during times of social interaction. This fact is highlighted by Jordan et al.,13 who, when developing a questionnaire for U.S. celiac children, highlighted other relevant issues in the original cultural context, such as the regular consumption of food produced outside the home and difficulties eating in leisure situations, such as when travelling.
During the semantic equivalence, the choice of replacing the term “find” by “feel” was based upon the understanding of the translators and gastroenterologists about the need for the question to evoke not only an opinion, but the patient's feeling in relation to the addressed issue. In the instructions, it was decided to use “little faces” replacing the term “faces”, as the use of the diminutive form makes the explanation friendlier.
From the operational point of view, the CDDUX format, with answers provided through a face diagram, facilitates its application, avoiding problems with words that require a higher cultural level, as faced by other authors.20 Unlike the original questionnaire, which was self-administered and mailed, it was decided to perform an interview. It is possible that the performance of the interview by the attending physician in the consultation environment may have affected the final score of the questionnaire.
At the measurement equivalence, the observed results indicated good correlation between the items and their domains, as well as internal consistency. All items showed a significant correlation (p<0.01) with the originally proposed domains. In some, such as question 3, a significant correlation with another domain can also be observed, because they are somehow interrelated, but this correlation was found to be always less than or equal to that of the domain to which the item is assigned. It is also noteworthy that although several items not assigned to a domain are correlated with it, most of the items that comprise the domain have higher values.
The tool showed good internal consistency (α=0.81), although at a lower value than the evaluation of the original tool (α=0.85). Regarding the factorial analysis, only question 9, “Having to pay attention to what you eat, makes you feel …”, which belongs to domain “Diet,” showed an item value <0.6. As the exclusion of this question would result in a decreased α and its value was above the recommended one (0.4), it was decided to retain it.
Reproducibility cannot be assessed due to the small number of patients that attended the retest, although they were all invited to do so. Returning to perform the retest seems to be more easily achieved with more symptomatic clinical conditions.20
Traditionally in QoL studies, parents and guardians are responsible for their children, but they are seen as unreliable responders, as they lack language and cognitive capabilities.21 However, in this analysis of QoL, the authors realize the need for subjective evaluation of the individual in question. Hence, the agreement between responses obtained from children and their parents/guardians through measurement tools has been investigated by some authors.21,22 The low agreement between the answers, which some researchers consider to be due to tool inadequacy, may reflect a natural disagreement between parents and children in many aspects. The parents’ capacity to assess the QoL of their children depends on what QoL domains are being evaluated, as they have difficulty assessing questions internalized by the children, such as sadness and anxiety.22
Although parents/guardians and CD patients classified QoL in the neutral range (40–60), the final score of the parents/guardians was significantly lower, in agreement with what was described by Upton et al.21 in a systematic review, showing that parents of children with chronic diseases tend to underestimate their children's QoL. When the original tool was applied, the final score was also significantly lower in the parents’ assessment and even showed to be in another classification range.7
However, the difference found in scores of parents and children in the communication domain is noteworthy. Talking about the disease and giving it visibility can have a negative impact on the social lives of these children, due to possible stigmatization. However, knowledge about the disease can make the act of living and eating in environments outside the home an easier task, because celiac individuals do not have to justify the fact that they have different eating habits.3 In this sense, explaining and talking about CD can become a benefit to celiac children and adolescents, unlike what was predicted by their parents. This fact was not observed in the original study,7 possibly because the level of knowledge about CD in European countries is higher.
As described in the literature, no differences were observed in the final score of the translated version of CDDUX in relation to patient gender and age.10,11 However, the use of the same tool in patients within a wide age range is debatable, as they face different situations and perform different social roles.13 In the development of the original questionnaire through focal groups, only four adolescents participated in the study; this limitation is emphasized by the researchers.7
Some aspects must be considered when using this translated and adapted version of the CDDUX. The present study interviewed a group of celiac patients followed at a specialized service, with low socioeconomic status and that included few adolescents. The context in which the adaptation process occurs interferes with the version's final result and this fact must be analyzed before its implementation.14,23
The assessment of QoL during the consultation, even in the presence of a well-established physician–patient relationship, is often difficult, especially in those patients who tend to provide socially correct answers. A QoL assessment tool can facilitate this approach and can be used to initiate communication on the subject,7 although it should not replace it.24
The final version of CDDUX in Portuguese was prepared in accordance with the steps outlined in the literature,15 with adequate psychometric properties, representing a valid tool for QoL assessment of children and adolescents with CD.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Lins MT,Tassitano RM, Brant KG, Antunes MM, da Silva GA. Translation, cultural adaptation, and validation of the celiac disease DUX (CDDUX). J Pediatr (Rio J). 2015;91:448–54.
Study conducted at Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil.