Describe the clinical and laboratory profile, follow-up, and outcome of a series of cases of acute viral myositis.
MethodA retrospective analysis of suspected cases under observation in the emergency department was performed, including outpatient follow-up with the recording of respiratory infection and musculoskeletal symptoms, measurement of muscle enzymes, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), transaminases (AST and ALT), blood count, C-reactive protein, and erythrocyte sedimentation rate in the acute phase and during follow-up until normalization.
ResultsBetween 2000 and 2009, 42 suspected cases were identified and 35 (27 boys) were included. The median age was 7 years and the diagnosis was reported in 89% in the first emergency visit. The observed respiratory symptoms were cough (31%), rhinorrhea (23%), and fever (63%), with a mean duration of 4.3 days. Musculoskeletal symptoms were localized pain in the calves (80%), limited ambulation (57%), gait abnormality (40%), and muscle weakness in the lower limbs (71%), with a mean duration of 3.6 days. There was significant increase in CPK enzymes (5507±9180U/L), LDH (827±598U/L), and AST (199±245U/L), with a tendency to leukopenia (4590±1420) leukocytes/mm3. The complete recovery of laboratory parameters was observed in 30 days (median), and laboratory and clinical recurrence was documented in one case after 10 months.
ConclusionTypical symptoms with increased muscle enzymes after diagnosis of influenza and self-limited course of the disease were the clues to the diagnosis. The increase in muscle enzymes indicate transient myotropic activity related to seasonal influenza, which should be considered, regardless of the viral identification, possibly associated with influenza virus or other respiratory viruses.
Descrever o perfil clínico-laboratorial, acompanhamento e desfecho de uma série de casos de Miosite Aguda Viral.
MétodoFoi conduzida uma análise retrospectiva de casos suspeitos, em observação em unidade de emergência, e seguimento ambulatorial com o registro de sintomas de infecção respiratória, sintomas músculo-esqueléticos, determinação de enzimas musculares, creatina-fosfoquinase (CPK), desidrogenase lática (DHL), transaminases (AST e ALT), hemograma, proteína C reativa e velocidade de hemossedimentação, na fase aguda e acompanhamento, até a normalização.
ResultadosEntre 2000 e 2009, 42 casos suspeitos foram identificados e 35 (27 meninos) foram incluídos. A mediana de idade foi 7 anos e o diagnóstico relatado em 89%, na primeira visita de emergência. Os sintomas respiratórios observados foram: tosse (31%), coriza (23%), e febre (63%) com duração media de 4,3 dias. Os sintomas músculo-esqueléticos foram: dor localizada nas panturrilhas (80%), deambulação limitada (57%), marcha anormal (40%), fraqueza muscular nos membros inferiores (71%), com duração média de 3,6 dias. Observou-se elevação importante das enzimas CPK (5507±9180) U/l, DHL (827±598) U/l e AST (199±245) U/l, e tendência a leucopenia (4590±1420) leucócitos/mm3. A recuperação complete dos parâmetros laboratoriais foi observada em 30 dias (mediana) e a recaída clínica e laboratorial em um caso após 10 meses.
ConclusãoOs sintomas típicos com enzimas musculares elevadas após diagnóstico de Influenza e o curso auto-limitado foram os indícios para o diagnóstico. A elevação de enzimas musculares indicam a atividade miotrópica transitória relacionada à influenza sazonal que deve ser considerada, à despeito da identificação viral, possivelmente associada com o vírus Influenza ou outros vírus respiratórios.
Acute viral myositis is a syndrome characterized by musculoskeletal impairment after upper airway disorders, which leads to temporary limited ambulation in children and is predominant in boys. It manifests as muscle pain and lower-limb weakness, especially in the calves and thighs.1–4 Its incidence is unknown, but it is considered rare and is described mainly during influenza outbreaks.5 The preceding respiratory symptoms are common, including fever, malaise, cough, odynophagia, headache, and rhinorrhea.
Musculoskeletal signs and symptoms – although transient and often self-limited – require emergency care, are of concern to parents, and cause some diagnostic difficulties, especially regarding limited ambulation.
There are literature reports of isolated cases, small series, or epidemic outbreaks.2,6–12 Research on its pathogenesis is still limited. The early recognition of the disease may improve emergency care and conservative treatment. The objective was to explore and describe the clinical and laboratory presentation and outcome of a series of cases of acute viral myositis.
MethodsA retrospective study of patients treated in an emergency care unit from June 2000 to 2009 was performed. The cases were initially identified by the diagnostic records during outpatient follow-up. A demographic and clinical data collection protocol was used for medical record review: 1 – demographic: date of birth, gender, initial diagnosis, origin, consulted specialties, referral, date of first appointment, date of last appointment; 2 – clinical: date of symptom onset, date of diagnosis, time to symptom resolution, clinical signs of presentation, including respiratory signs and symptoms and duration, associated diseases, family history, hospitalization and length of stay, complications; 3 – laboratory: laboratory tests at diagnosis and follow-up; 4 – outcome: treatment and disease duration. The suspected consecutive cases were observed in a public hospital by different assistant physicians; clinical evaluation, from the first to the last visit, was recorded in the chart, identifying during this interval the date of diagnosis, date of symptom onset, and the date when laboratory test normalization was observed, as well as their values. The duration of initial symptoms and complications, as well as the identification of respiratory symptoms, musculoskeletal symptoms, and recurrence were identified in outpatient visits. Possible differential diagnoses considered during the emergency care were recorded in the protocol.
The laboratory tests were requested by the attending physician according to the clinical suspicion or diagnostic referral in the emergency room and consisted of the following: measurement of muscle enzymes, creatine phosphokinase (CPK) and its isoenzyme, CK-MB fraction (CK-MB), lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine aminotransferase (ALT), performed in the routine hospital laboratory, using a colorimetric enzyme assay with results expressed in units per liter (U/L). Blood count and urinalysis were performed by routine methods, as well as erythrocyte sedimentation rate (Westergreen method), with results expressed in mm/h, while C-reactive protein was measured by an automated nephelometric assay and results were expressed in mg/dL.
As there are no standardized criteria for the diagnosis of acute viral myositis, the protocol was established according to the symptoms most often described in the literature, i.e., pain in the limbs and muscle weakness accompanied by elevated muscle enzymes. Cases that met the following criteria were included: age<18 years with clinical suspicion confirmed during outpatient follow-up, from 2000 to 2009, with clinical and laboratory assessment at diagnosis and at least one follow-up visit. Suspected cases that did not have elevated muscle enzymes were excluded, despite the symptoms of myalgia – limb pain with self-limited evolution – occurring after respiratory infection.
Variable frequency was recorded, including gender; primary diagnosis, origin; consulted specialties; complications; additional requested tests and altered laboratory tests; associated diseases; and the presence of clinical signs, mainly myalgia, pain in the calves, limited ambulation, and gait abnormality, such as walking on tiptoe and muscle weakness. The preceding respiratory symptoms, such as sneezing, nasal obstruction, cough, rhinorrhea, fever, headache, sore throat, vomiting, diarrhea, epistaxis, pharyngotonsillitis, and use of medications were also recorded. The protocol was approved by the ethics committee for institutional research on December 7, 2009 (No. 3409/2009).
Statistical analysis was performed; quantitative data are shown as medians, minimum and maximum values, and means and standard deviations; categorical variables are shown as absolute values and percentages. The frequency and the annual distribution of cases are shown as frequency histogram. The comparison between laboratory variables at clinical presentation and during symptom resolution (time variable) was performed using the paired t-test, with the significance threshold set at 5% (p<0.05). The tests were performed and the charts created using Prism Graph Pad v.4.0® (GraphPad Software Inc., California, USA).
ResultsA total of 42 cases with clinical suspicion of acute viral myositis were identified in emergency care and outpatient clinic records, from June of 2000 to December of 2009. Of these, 35 cases were included, 27 boys and 8 girls, with one case of recurrence in a second episode.
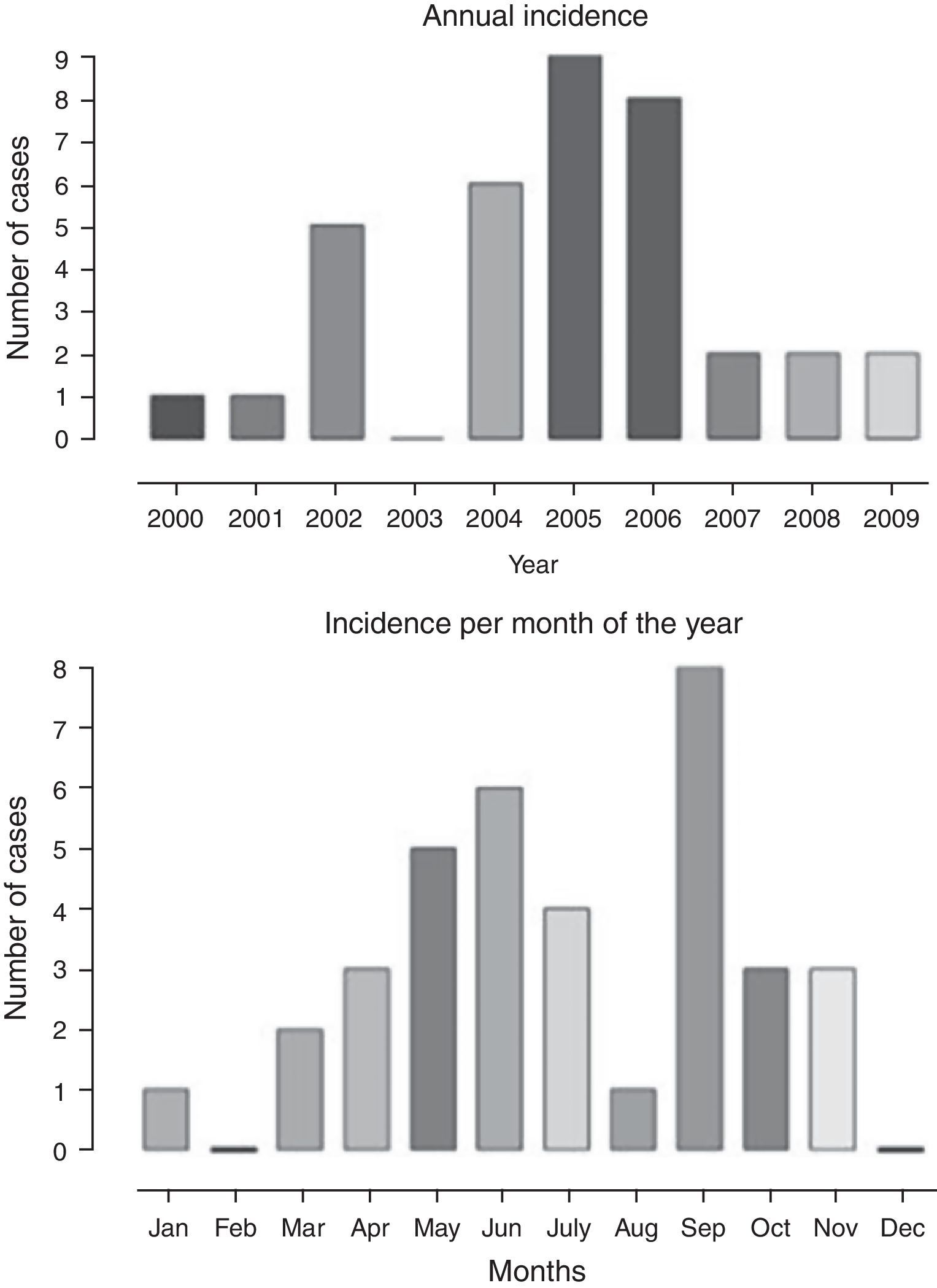
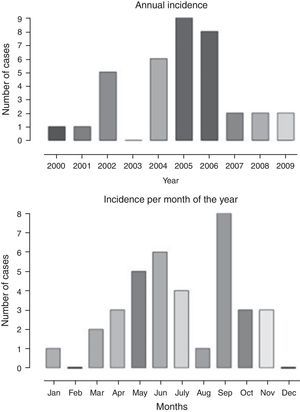
The annual distribution of cases and the frequency according to the months of the year is shown in Fig. 1, with a higher frequency in the months of May, June, July, and September, when compared to the others, corresponding to the colder months of the year. There was a higher frequency of cases diagnosed from 2004 to 2006.
The age of symptom onset ranged from 3.4 to 12.5 years, with a median of 7.5 years. The interval between symptom onset and the first consultation ranged from 1 to 6 days. The duration of respiratory symptoms ranged from 1 to 15 days, with a median of 3.5 days and mean of 4.3±2.8 days. Among the reported symptoms, fever, cough, and rhinorrhea were observed in 63%, 31%, and 23%, respectively. Table 1 shows the musculoskeletal signs and symptoms described according to the data collection protocol. Neurological examination was normal in all cases. The duration of muscle weakness ranged from 1 to 8 days, with a median of 2 days and mean of 2.7±1.9 days. Symptom resolution ranged from 1 to 16 days, with a median of 3 and mean of 3.6±3.3 days. One-to-three consultations were performed during the acute phase and 48-h hospitalization occurred in 4/35 (11%). Follow-up duration varied from 30 to 180 days, with a median of 32 days.
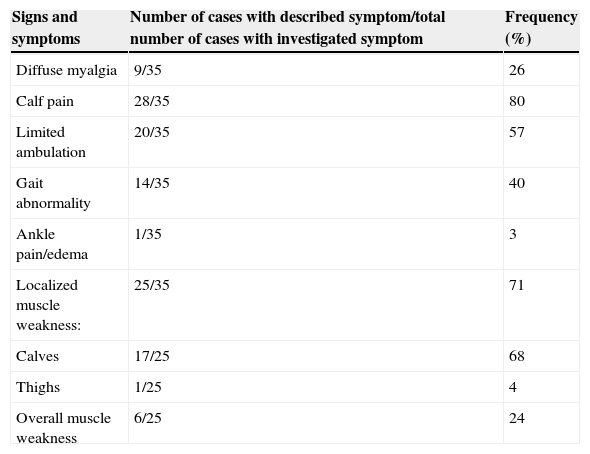
Description of musculoskeletal symptoms at presentation in a series of cases of acute viral myositis.
| Signs and symptoms | Number of cases with described symptom/total number of cases with investigated symptom | Frequency (%) |
|---|---|---|
| Diffuse myalgia | 9/35 | 26 |
| Calf pain | 28/35 | 80 |
| Limited ambulation | 20/35 | 57 |
| Gait abnormality | 14/35 | 40 |
| Ankle pain/edema | 1/35 | 3 |
| Localized muscle weakness: | 25/35 | 71 |
| Calves | 17/25 | 68 |
| Thighs | 1/25 | 4 |
| Overall muscle weakness | 6/25 | 24 |
Symptomatic treatment of respiratory and musculoskeletal symptoms included analgesics or anti-inflammatory drugs. Symptomatic use ranged from 2 to 45 days, with a median of 2 days and mean of 16.3±24.8 days. Antibiotics were prescribed in cases complicated by sinusitis or pharyngotonsillitis. Antibiotic use ranged from 5 to 15 days, with a median of 10 days and mean of 10.6±3.3 days.
Laboratory test alterations are shown in Table 2. The resolution time until normalization of muscle enzymes and blood count parameters, as well as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, varied from 30 to 180 (median of 32) days.
Laboratory parameters at presentation and at follow-up of acute viral myositis.
| Laboratory parametera | Initial value (mean±SD) | Final value (mean±SD) | Paired t-test |
|---|---|---|---|
| Creatine-phosphokinase (CPK, U/L) | (5507±9180) | (127±59) | p<0.002 |
| MB fraction (CKMB, U/L) | (96±96) | (28±57) | p<0.0003 |
| Lactate dehydrogenase (LDH, U/L) | (827±598) | (520±233) | p<0.0003 |
| Transaminase (AST, U/L) | (199±245) | (57±129) | p<0.008 |
| Transaminase (ALT, U/L) | (68±66) | (44±45) | NS |
| AST/ALT ratio | (3±1.48) | (1±0) | p<0.0001 |
| Hemoglobin (Hb, mg/dL) | (13±0.93) | (13±1) | NS |
| Leukocytes (absolute n/mm3) | (4.59±1.42)×103 | (8.07±2.72)×103 | p<0.0001 |
| Neutrophils (absolute n/mm3) | (2±0.88)×103 | (4.02±1.93)×103 | p<0.0004 |
| Lymphocytes (absolute n/mm3) | (2.02±0.76)×103 | (2.93±0.89)×103 | p<0.0001 |
| Platelets (absolute n/mm3) | (206±59)×103 | (332±164)×103 | p<0.0005 |
| ESR (mm/h) | (17±10) | (13±13) | NS |
| C-reactive protein (CRP mg/dL) | (0.4±0.8) | (1±4) | NS |
NS, non-significant.
Only three (9%) urinalysis tests were indicated, with abnormal results in one of the tests, which showed proteinuria of 2+, but none indicated myoglobinuria. Serology tests were performed, including toxoplasmosis IgM+/negative mononucleosis/Cytomegalovirus IgG+ in a single case. In this case, the initial diagnosis was unspecified limb pain. No imaging or electromyography tests were indicated.
The normalization of laboratory parameters, muscle enzymes (CPK, CK-MB, LDH, AST and ALT) and the decrease in AST/ALT ratio are shown in Table 2. A significant difference was observed at the paired t-test, when comparing initial and resolution values for CPK (p<0.002), CK-MB (p<0.0003), LDH (p<0.006) and AST (p<0.008), and AST/ALT ratio (p<0.0001), but the difference was not significant for ALT.
The normalization of blood count parameters and acute phase reactants, shown in Table 2, also indicated statistical difference between the initial and resolution parameters for the absolute count of leukocytes (p<0.0001), neutrophils (p<0.0004), lymphocytes (p<0.0001), and platelets (p<0.005), but there was no significant difference between baseline and resolution parameters for hemoglobin, C-reactive protein, and ESR.
The time interval between measurements at presentation and resolution was variable (67±78) days. Recurrence occurred in one case, with two distinct episodes, the first at 8.3 years and the second at 9.1 years. Only the first episode was considered in the analysis. The initial diagnosis was acute viral myositis in both. The clinical characteristics of the first and second episodes were myalgia, calf pain, limited ambulation, gait abnormality, and localized muscle weakness in the lower limbs. Symptom duration was 3 days in the first and 5 days in the second episode. The laboratory profile was similar when the first and second episodes were compared, with both preceded by respiratory symptoms.
In this series, the time of musculoskeletal symptom resolution ranged from 1 to 16 days. There were other manifestations, which were recorded in 20% of cases, including diagnoses of sinusitis, pneumonia, and transient hip synovitis. The differential and exclusion diagnoses recorded in the emergency room due to limited ambulation were: nonspecific synovitis, hip synovitis, Guillain–Barre syndrome, primary neuromuscular disease, and dermatomyositis.
DiscussionThis series showed a higher number of cases of acute viral myositis between 2004 and 2006, with an annual distribution in the cold months, with no other apparent explanation. There was a 3:1 predominance in the male gender and most cases were treated first in the emergency department, which indicates its impact on children's health. The recognition of respiratory manifestations associated with musculoskeletal symptoms led to the clinical suspicion, subsequently confirmed by the marked elevation in muscle enzymes, especially CPK, in addition to other muscle enzymes such as LDH and AST; however, aldolase, another enzyme with muscle inflammation specificity, was not available.
In addition to the preceding respiratory symptoms indicating a common hematological response to viral infections, prone to transient leukopenia, as well as nonspecific and subtle changes of acute phase reaction, the suspected diagnosis was confirmed by self-limited musculoskeletal symptoms, by serial measurement of muscle enzymes in cases of calf pain and refusal to walk or gait impairment, in association with influenza symptoms.
The influenza virus and other respiratory viruses can cause acute and self-limited febrile illness, occurring in outbreaks, frequently in the winter. A variety of associated complications are known, including respiratory ones, such as primary viral pneumonia with or without secondary bacterial infections, laryngitis, bronchitis, and bronchiolitis. Non-respiratory complications, such as myositis, myocarditis, aseptic meningitis, and encephalitis, occur less frequently.12
The frequency of acute viral myositis is proportionally higher in type B influenza, with male individuals more often affected than females.5 Myositis may also be caused by bacterial, fungal, and parasitic infections.13 Other infectious diseases may be associated with myalgia, with or without myositis, including, in addition to influenza, dengue, rickettsiosis, infective endocarditis, toxoplasmosis, Lyme disease, and human immunodeficiency virus (HIV) infection. The bacterial etiology by pyogenic agents is called pyomyositis and may have an acute and more severe evolution, but the involvement is localized in certain muscle groups in school-age children, clearly related to mechanical trauma in these muscle groups, such as the quadriceps and gluteus muscles.
Among the parasitic infections, cysticercosis, schistosomiasis, and trichinosis can cause symptoms such as myalgia and fever, accompanied by eosinophilia. The diagnosis of muscular involvement in these cases is most commonly performed by identification of calcified cysts on radiographs and the pseudo-hypertrophy of the thigh and calf muscles in the disseminated forms. The optimal diagnosis of acute viral myositis can be attained by viral identification, by serology, or by biopsy, in the presence of nonspecific musculoskeletal symptoms.
Other investigations, such as electromyography, have a limiting factor, i.e., the invasiveness of the technique and the cost-benefit ratio, in practice. In spite of the identification failure by serology,6,7 studies have identified the influenza virus in muscle tissue of patients affected by acute viral myositis. However, its etiopathogenic mechanisms are still unknown. There is a hypothesis of muscle damage by immune mechanism or muscle tissue invasion by viral particles, causing damage to muscle fibers. There is evidence of viral particles isolated from calf muscles biopsies with no specific degenerative alterations and myonecrosis.13 Among the complications, rhabdomyolysis, although rare, can result in kidney damage secondary to myoglobinuria. 14
Acute viral myositis associated with pandemic influenza H1N1 has been reported in children and adults,15,16 with one of the cases showing orbital muscle involvement, characterizing a rare form of orbital myositis15 in an infant. The recent pandemic, as well as all historical cycles of epidemic and seasonal influenza, have indicated the benefits of mass vaccination.
The present study was conducted during a period when vaccination for seasonal influenza had limited coverage in the public health care system and documentation of specific vaccination in this series was not discriminated. The association between the immune status for influenza vaccination and the risk of acute viral myositis is unknown. Although patients that received the seasonal influenza vaccine could admittedly have a lower risk of respiratory complications (e.g., pneumonia, otitis media), there is no clear demonstration of decrease in other secondary complications.
Among the limitations of this study, the following should be considered: the small size of the series, treatment at a specific hospital, and the retrospective study design. Ideally, prospective population studies would provide the best description of acute viral myositis incidence.5 The association with the identification of respiratory viruses was not possible.17,18 Despite these limitations, the results indicate some recommendations, such as the systematic evaluation of children with lower-limb myalgia in the presence of respiratory symptoms and follow-up with serial measurement of muscle enzymes, especially CPK, consistent with recent observations.19
Most children with acute viral myositis are treated in the emergency department,20,21 as the symptoms can be alarming for parents, with a tendency toward multiple consultations with various specialties and costly investigations.4,11,22 The pediatrician in the emergency care service should feel comfortable to discharge the patient, making the differential diagnosis with neuromuscular diseases, checking during the follow-up to review symptoms and the measurement of muscle enzymes until normalization.
Conflicts of interestThe authors declare no conflicts of interest.
CAPES – Post-graduate Program in Collective Health, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista (UNESP).
Please cite this article as: Cardin SP, Martin JG, Saad-Magalhães C. Clinical and laboratory description of a series of cases of acute viral myositis. J Pediatr (Rio J). 2015;91:442–47.
Study conducted at the Department of Pediatrics, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista (UNESP), Botucatu, SP, Brazil.