To investigate the diagnostic efficacy of serum IL-33 single indicator and combined indicators for asthma in children.
Methods132 children were initially diagnosed with asthma during acute exacerbation and 100 healthy children were included. Serum IL-33 concentration differences were compared between asthmatic and normal children. Correlations between IL-33 with pulmonary function parameters, FeNO, peripheral blood EOS counts and serum total IgE were analyzed in asthmatic children. ROC curves were used to assess IL-33 diagnostic efficacy and its combined indicators. To prevent overfitting of the predictive model, the hold-out cross-validation method was used.
Results(1) Serum IL-33 concentrations were significantly higher in children with asthma than in normal children (p < 0.001). (2) IL-33 concentration was negatively correlated with FVC z-score, FEV1 z-score and FEF75% z-score in asthmatic children (p < 0.05). (3) The area under the ROC curve of IL-33 was 0.821, which was higher than those of FeNO, FVC z-score, and FEV1 z-score. (4) Cross-validation of the combined indicators showed that IL-33 significantly improved asthma diagnostic efficacy. The combination of IL-33, FEF75% z-score, and FeNO showed the highest diagnostic efficacy, with the AUC, sensitivity, and specificity of the combined indicator being 0.954, 90.1%, and 89. 0%, respectively, and good extrapolation of the predictive model.
ConclusionSerum IL-33 is higher in children with asthma and increases with the severity of pulmonary ventilation obstruction. A single indicator of serum IL-33 demonstrates moderate diagnostic accuracy, and its combination with FEF75% z-score and FeNO significantly improves the diagnostic accuracy in childhood asthma.
Asthma is the most common chronic airway disease in children.1 Clinically, the diagnosis of asthma in children is mainly based on respiratory symptoms, signs and diagnostic examinations to confirm the existence of expiratory airflow limitation in the airway and to exclude other diseases that can cause the related symptoms, and there is no stand-alone test that can confirm the diagnosis.2 However, respiratory symptoms such as cough, wheezing and dyspnea are not specific for the diagnosis of asthma, and the temporal variability and phenotypic heterogeneity of asthma complicate the interpretation of diagnostic test results.3 Thus, it is still challenging to diagnose asthma in children, and these uncertainties make asthma under- or overdiagnosed.4 As measurable indicators, biomarkers provide objective measurements for disease prediction, diagnosis and detection.5 Exploring a new reliable biomarker for diagnosing asthma in children is urgently needed to assist physicians in clinical diagnosis.
IL-33, as a member of the IL-1 family, is a key cytokine involved in Type 2 immunity and allergic respiratory diseases.6,7 It is highly expressed in endothelial cells, fibroblasts, and epithelial cells of the lung and plays an important role in innate and acquired immunity of mucosal organs.8 Itepekimab is an anti-IL-33 monoclonal antibody that can reduce the incidence of uncontrolled asthma events and improve lung function in patients with moderate-to-severe asthma.9 IL-33 and its receptor are involved in the pathogenesis of asthma and are considered to be putative biomarkers or targets for pharmacological intervention in asthma.6-8 However, few studies have been performed on serum IL-33 in the diagnosis of children with asthma.10
IgE is a key trigger for allergic airway inflammation in asthma, and total serum IgE is elevated in the blood of patients with allergic asthma.11 Blood eosinophil counts correlate with the degree of airway inflammation and asthmatic activity.5 Recommendations for diagnostic tests in clinical guidelines include pulmonary function test and FeNO measurement.2 Pulmonary function test assesses the reversibility of airway obstruction and is an important tool for diagnosing asthma and assessing the severity and level of control of asthma.2 FeNO is a noninvasive and sensitive biological marker that reflects the inflammatory status of the airways and has a certain value in the diagnosis of asthma.12 Whether the combination of serum IL-33 with pulmonary function parameters or FeNO can improve the diagnostic efficacy of asthma has not been reported.
In this study, the authors compared serum IL-33 levels between children with an initial diagnosis of asthma during acute exacerbation and healthy children. The correlations between serum IL-33 and other indicators, such as pulmonary function parameters and FeNO, were analyzed, and the diagnostic efficacy of a single serum IL-33 and its combination with pulmonary function parameters or FeNO in children with asthma was investigated. The authors aimed to evaluate the diagnostic value of single and combined indicators of serum IL-33 in childhood asthma.
MethodsStudy populationA total of 132 children initially diagnosed with asthma at the Hospital of the University between October 2021 and September 2022 were recruited for this study. They were recruited in the emergency room and were all in the acute exacerbation phase. Asthma diagnosis was based on the Global Initiative for Asthma (GINA).2 The exclusion criteria for children with asthma were as follows: 1) children who were previously diagnosed with asthma and received asthma treatment; 2) children with respiratory infections or other infectious diseases within 4 weeks; 3) children with other diseases that can affect pulmonary ventilation function, such as congenital airway malformation, obstructive bronchitis, extraluminal compression, and congenital heart disease; 4) children with autoimmune diseases and severe systemic diseases; 5) children in need of systemic corticosteroids therapy; and 6) for asthmatic children with comorbidities such as allergic rhinitis or other allergic diseases, the authors excluded some of them whose comorbidities were not well controlled or who were given any medication that could affect the test results within 4 weeks before recruitment. Pulmonary function tests and FeNO measurements were performed on the day of the first visit for children with mild acute exacerbations. To ensure the safety of children with asthma, children with moderate and severe acute exacerbations were treated with budesonide and terbutaline nebulizer for 3 days. When the children were stable, pulmonary function tests and FeNO measurements were performed 8 hours after the last nebulization treatment to avoid the influence of drugs on the test results. Grading of acute asthma exacerbations with reference to GINA guidelines.2
One hundred healthy children who underwent routine physical examinations in the studied hospital were selected concurrently. The inclusion criteria for healthy children were as follows: (1) no cough, wheezing, shortness of breath, chest tightness, or other related respiratory tract infection symptoms 4 weeks before the visit; (2) no history of asthma, allergic diseases, autoimmune diseases, serious systemic diseases or inflammatory diseases; (3) no related diseases that may affect pulmonary ventilation function; and 4) no abnormal growth and development history.
This study was reviewed and approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University (2021-108-01). All subjects and their parents signed written informed consent forms.
Study designDetailed medical histories were taken from all study subjects, especially asthmatic children's personal and family histories of atopic diseases and the results of allergen skin prick test. In addition, fasting peripheral blood samples were collected, and pulmonary function tests and FeNO measurements were performed on the study subjects. Serum IL-33 concentration levels were measured by enzyme-linked immunosorbent assay (ELISA) in serum, then the present study focused on serum IL-33 concentration levels were compared between children with and without asthma. The correlations of IL-33 with pulmonary function parameters [forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC), mean forced expiratory flow between 25 and 75% of FVC (FEF25%-75%), and instantaneous forced expiratory flow at 75% of FVC (FEF75%)], FeNO, peripheral blood EOS count, peripheral blood neutrophils count and serum total IgE were further analyzed in asthmatic children. IL-33 was combined with pulmonary function parameters and FeNO to assess the diagnostic efficacy of IL-33 and its combined indicators in childhood asthma. To avoid overfitting the prediction model, the authors randomly divided the 232 subjects into a training set (70% of the subjects) and a testing set (30% of the subjects). The logistic regression prediction model was established in the training set to assess the diagnostic efficiency of the combined indicators, and the prediction model was validated in the testing set.
Blood processingFasting peripheral blood was sampled from the subjects in tubes treated and untreated with ethylenediaminetetraacetic acid (EDTA). Serum total IgE was measured using immune scatter turbidity in an automatic biochemical analyzer (AU5400, Beckman Coulter Inc, Calif, USA) and the reagents from Diasys diagnostic systems (Diasys, Catalog No: 17239). Peripheral blood EOS and neutrophil counts were measured using the direct counting method with a fully automatic hemocyte analyzer (BC-5800, Mindary, Shenzhen, China). The serum was isolated from peripheral blood samples and stored at -80°C until testing. IL-33 concentration levels in serum were measured by using Human ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., No: ml058087). The sensitivity of this ELISA kit was less than 1.0 pg/ml, and the measurement was performed according to the manufacturer's instructions.
FeNO measurementFeNO measurement was performed according to the American Thoracic Society/European Respiratory Society (ATS/ERS) recommended standards12 using an NO analyzer with electrochemical sensors (SV-02, Wuxi Shangwo, China). Before pulmonary function testing, FeNO measurement was performed. The subjects were seated and breathing calmly, inhaling gas without nitric oxide to near total lung volume. During the measurement, the children exhaled smoothly and slowly at 50 mL/s for 10 s. The unit of the test results is expressed in parts per billion (ppb).
Pulmonary function testingAccording to the guidelines,13 certified specialist technologists performed rigorous pulmonary function testing using a pulmonary function analyzer (Type MasterScreen IOS, Germany). Each subject performed at least 3 trials (no more than 8), and the best outcome was selected for data analysis. The subjects generally took a standing position, and those who were taller than 160 cm could take a sitting position. The Z-cores of FEV1, FVC, FEV1/FVC, FEF 25%-75% and FEF 75% were calculated with reference to the Global Lung Function Initiative (GLI-2012) reference equations for North East Asians.14 Using the GLI-2012 Desktop Software for Data Sets Version 1.3.4, the GLI reference values of each subject were calculated.
Statistical analysisIBM SPSS statistical software (SPSS 26.0, Chicago, Illinois) was used for data analysis. Continuous data are expressed as the mean ± standard deviation (SD) or median (interquartile range) [M (P25, P75)]. According to whether the data conformed to a normal distribution, the Student's t test or the Mann‒Whitney U test was used to compare the differences between groups. The enumeration data are expressed as a percentage (%), and the differences in categorical data between groups were analyzed using the chi-square (χ2) test. Pearson's or Spearman's correlation analysis was used to analyze the correlation of the data. ROC curves were used to assess the diagnostic efficacy of the variables on childhood asthma. Finally, to avoid overfitting the model, the authors used the Hold-out cross-validation method to establish the logistic regression model in the training set to evaluate the diagnostic efficiency of the combined indicators and validated it in the testing set. The area under the curve (AUC), sensitivity, specificity, and accuracy were calculated to assess the predictive accuracy of the model. Logistic regression models were adjusted for the effects of age and BMI. P < 0.05 indicated that the difference was statistically significant.
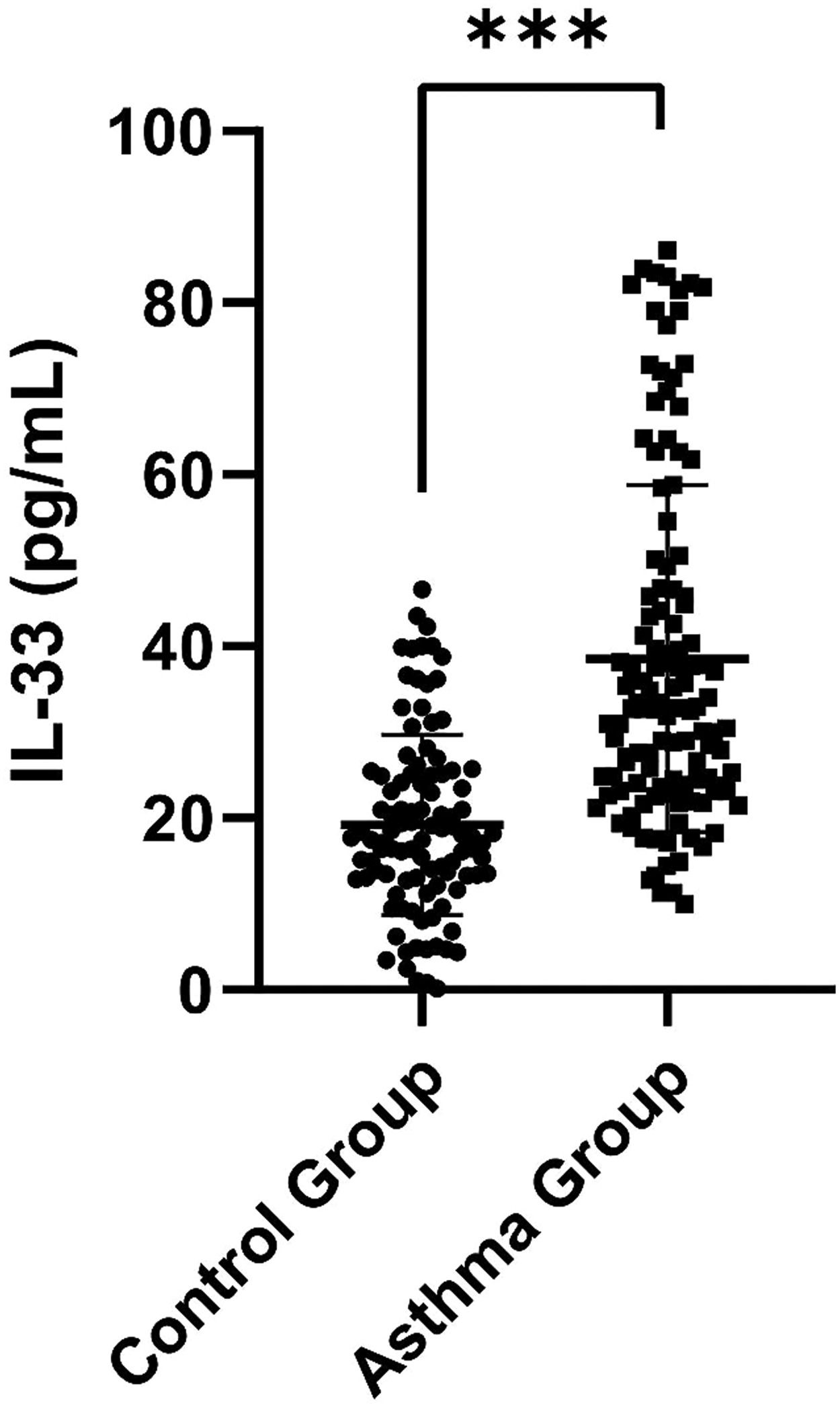
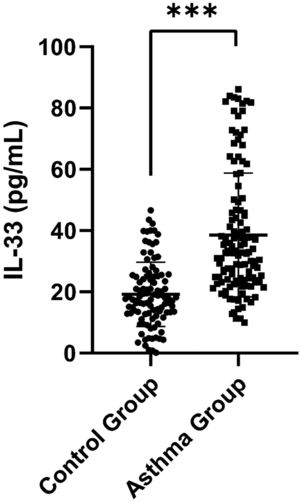
ResultsDemographic characteristics of the control and asthma groupsSerum IL-33 was significantly higher in the asthma group than in the control group [32.90 (23.78, 46.77) pg/mL vs. 17.77 (12.93, 25.11) pg/mL, p < 0.001] (Figure 1). Additionally, FeNO, EOS and IgE were elevated in the children with asthma compared to the control children (p = 0.001, p = 0.012 and p < 0.001, respectively). In terms of pulmonary function parameters, FVC z-score, FEV1 z-score, FEV1/FVC z-score, FEF25%-75% z-score, and FEF75% z-score were significantly lower in the asthma group than in the control group (p < 0.05) (Table S1).
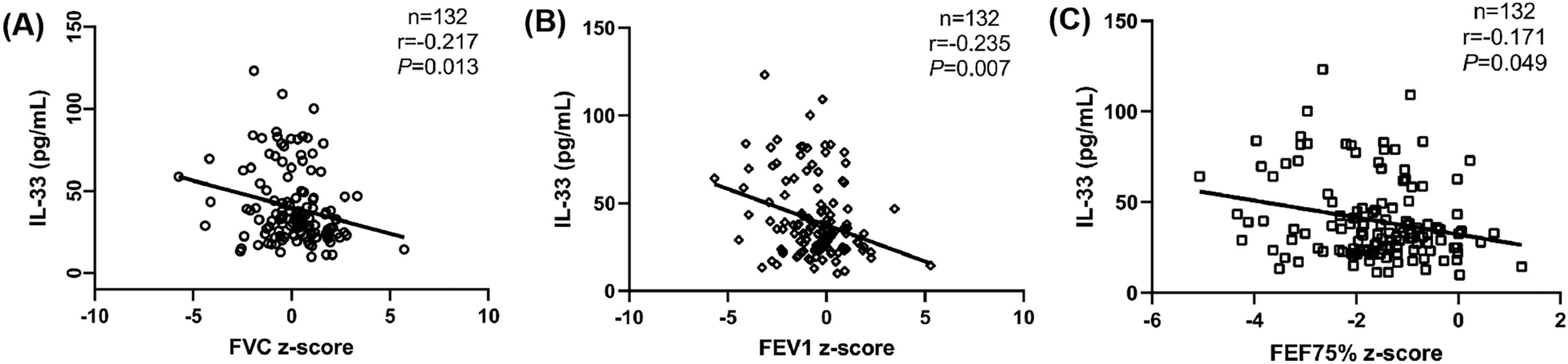
Correlation analysis between serum IL-33 and other study variables in asthmatic childrenThe authors analyzed the correlations between serum IL-33 and FeNO, pulmonary function parameters, EOS and IgE in asthmatic children. No correlations were shown between serum IL-33 and FeNO, FEV1/FVC z-score, FEF25%-75% z-score, EOS and IgE (p > 0.05) (Table S2). Negative correlations between serum IL-33 and FVC z-score, FEV1 z-score and FEF75% z-score were observed (r = −0.217, r = −0.235 and r = −0.171, respectively, p < 0.05) (Figure 2).
Correlation analysis between serum IL-33 concentrations, FVC z-score, FEV1 z-score, FEF75% z-score in asthmatic children. (A): Correlation between serum IL-33 concentrations and FVC z-score (r = −0.217, p = 0.013). (B): Correlation between serum IL-33 concentrations and FEV1 z-score (r = −0.235, p = 0.007). (C): Correlation between serum IL-33 concentrations and FEF75% z-score (r = −0.171, p = 0.049).
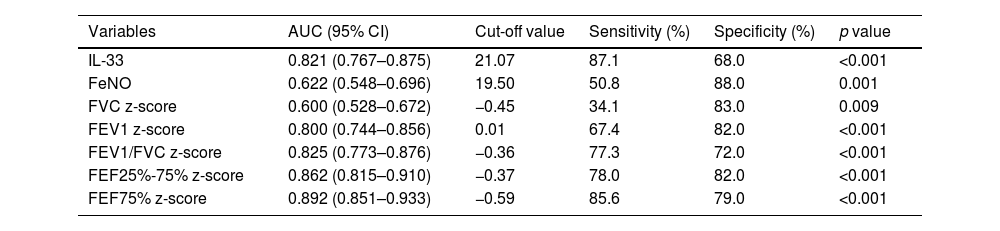
To investigate the diagnostic efficacy of each indicator, ROC curves were constructed. The AUC of serum IL-33 was 0.821, which was higher than that of FeNO (AUC = 0.622), FVC z-score (AUC = 0.600), FEV1 z-score (AUC = 0.800). The cutoff value of serum IL-33 was 21.07 pg/mL (Table 1). Among the single indicators, FVC z-score showed the worst diagnostic efficacy for asthma in children (AUC = 0.600). The AUCs for FEV1/FVC z-score, FEF25%-75% z-score, and FEF75% z-score were 0.825, 0.862 and 0.892, respectively, which were higher than those of the other indices and demonstrated better diagnostic efficacy (Table 1).
Diagnostic efficacy of IL-33, FeNO, pulmonary function indices for children with asthma.
AUC, area under the curve.
AUC = 0.5: completely worthless diagnosis; 0.5 < AUC ≤ 0.7: low diagnostic accuracy; 0.7 < AUC ≤ 0.9: moderate diagnostic accuracy; 0.9 < AUC < 1: high diagnostic accuracy; AUC = 1: completely ideal diagnosis.
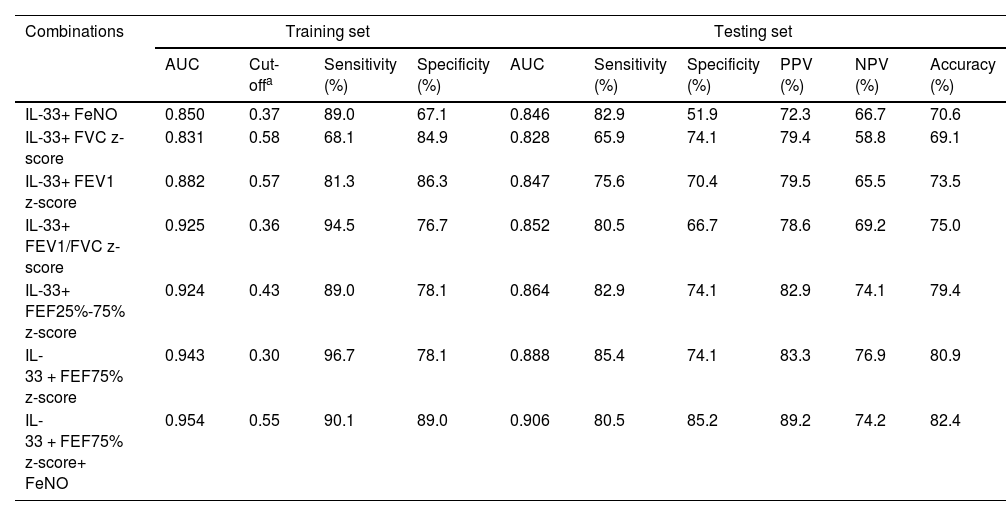
The diagnostic efficacy of serum IL-33 combined with FeNO and pulmonary ventilation function parameters was higher than that of a single indicator, and the combination increased the diagnostic accuracy of asthma (Table 2). In the training set, IL-33, FEF75% z-score, and FeNO combined had the highest diagnostic accuracy (AUC = 0.954) and better diagnostic sensitivity and specificity (90.1% and 89.0%, respectively) (Table 2). Moreover, comparing the sensitivity and specificity of the training and testing sets of each combination indicator, the smallest difference was found when IL-33, FEF75% z-score, and FeNO were combined, and the established model showed better extrapolation. In addition, the testing set had the highest AUC, specificity, positive predictive value (PPV), and accuracy when the above three indicators were combined.
The cross-validation results of IL-33 combined with other indices.
| Combinations | Training set | Testing set | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-offa | Sensitivity (%) | Specificity (%) | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| IL-33+ FeNO | 0.850 | 0.37 | 89.0 | 67.1 | 0.846 | 82.9 | 51.9 | 72.3 | 66.7 | 70.6 |
| IL-33+ FVC z-score | 0.831 | 0.58 | 68.1 | 84.9 | 0.828 | 65.9 | 74.1 | 79.4 | 58.8 | 69.1 |
| IL-33+ FEV1 z-score | 0.882 | 0.57 | 81.3 | 86.3 | 0.847 | 75.6 | 70.4 | 79.5 | 65.5 | 73.5 |
| IL-33+ FEV1/FVC z-score | 0.925 | 0.36 | 94.5 | 76.7 | 0.852 | 80.5 | 66.7 | 78.6 | 69.2 | 75.0 |
| IL-33+ FEF25%-75% z-score | 0.924 | 0.43 | 89.0 | 78.1 | 0.864 | 82.9 | 74.1 | 82.9 | 74.1 | 79.4 |
| IL-33 + FEF75% z-score | 0.943 | 0.30 | 96.7 | 78.1 | 0.888 | 85.4 | 74.1 | 83.3 | 76.9 | 80.9 |
| IL-33 + FEF75% z-score+ FeNO | 0.954 | 0.55 | 90.1 | 89.0 | 0.906 | 80.5 | 85.2 | 89.2 | 74.2 | 82.4 |
PPV, positive predictive value; NPV, negative predictive value.
Logistic regression models were adjusted for the effects of age and BMI.
This study confirmed that serum IL-33 has a moderate diagnostic accuracy, and it combined with FEF75% z-score and FeNO significantly improved the diagnostic accuracy. IL-33, a member of the IL-1 family, is a key factor involved in Type 2 immunity and allergic airway diseases.6,8 Similar to previous studies, this study found significantly higher serum IL-33 concentrations in children with asthma than in healthy children.15,16
A study in untreated patients with mild asthma found that IL-33 expression was positively correlated with FeNO,17 but their correlation was not found in this study. The reasons are as follows: First, previous studies were conducted in adults with mild asthma, but all children with different severities of acute exacerbations of asthma were included in this study. Second, previous studies analyzed the relationship between IL-33 mRNA expression and FeNO, however, this study examined serum IL-33 levels. Third, some children were given treatment before FeNO measurement and pulmonary function testing in this study. It is well-recognized that corticosteroid treatment could reduce FeNO levels.18 However, it has been found that the type 2 inflammation induced by IL-33 is steroid-resistant.19 In steroid-resistant children, IL-33 was expressed in lung tissue despite their treatment with high doses of inhaled steroids and was even higher in children who received additional oral corticosteroids.16 IL-33 may dominate the immune response in severe steroid-resistant asthma.20
Studies have investigated the negative correlation between serum IL-33 and FEV1.21,22 Thickness of the reticular basement membrane (RBM) was negatively correlated with FEV1.23 A study has shown that IL-33 promotes airway remodeling, and IL-33 levels are positively correlated with reticular basement membrane thickness.21 Saglani et al. reported that IL-33 was associated with increased RBM thickness in endobronchial biopsies from children with severe steroid-resistant asthma but not in mild asthma.16 In the present study, not only FEV1, IL-33 levels also negatively correlated with FVC and FEF75%. This provided more in-depth confirmation of the correlation between serum IL-33 and pulmonary function parameters, suggesting that serum IL-33 levels increase with increasing severity of pulmonary ventilation obstruction. IL-33 activates allergic inflammation-associated cells such as eosinophils, basophils, mast cells and group 2 innate lymphoid cells through its receptor ST2.6,24 However, one study showed no correlation between serum IL-33 and sputum EOS.21 Given the difficulty in collecting sputum specimens from children, this study collected data on peripheral blood EOS from the study subjects and similarly did not find a correlation with IL-33. In mouse models, IL-33 has been shown to induce increased IgE production.25 However, the results of some studies did not find a correlation between IL-33 and IgE,16 as did this study. These findings suggested that there may be other pathways by which IL-33 functions in asthma patients,21 and further exploration is needed.
Pulmonary ventilation function testing is one of the important objective tests in the diagnosis of asthma, which objectively assesses whether the airways are restricted.2 The single-indicator diagnostic efficacy suggested that serum IL-33 demonstrated moderate diagnostic accuracy, which was higher than FeNO, FVC z-score, and FEV1 z-score. However, FEV1 z-score, which responded to whether the airway was obstructed, did not show good diagnostic efficacy, but FEF25%-75% z-score and FEF75% z-score, which responded to small airway function, showed good diagnostic efficacy, which was consistent with the results of a Swiss cohort study.26 It has been shown that most children with asthma have normal or near normal FEV1 (≥ 80% of predicted), and there may be limitations in diagnosing asthma based on FEV1.27 Additionally, FEV1 and FEV1/FVC primarily assess the function of the large airways, and they only may detect abnormalities in cases of severe airway obstruction.28 FEF25%-75% and FEF75% both reflect small airway function in asthmatic patients, with similar clinical implications, but FEF75% is more sensitive.29 Some data suggest that small airway dysfunction occurs early in asthma before clinical symptoms become apparent28 and that small airways have more extensive inflammation and remodeling than large airways.30
The diagnostic efficacy of the combined indicators showed that when combined with serum IL-33, the diagnostic efficacy of each indicator increased, which confirmed the diagnostic efficacy of IL-33 in childhood asthma in depth. Of the combined indicators, serum IL-33 combined with FEF75% z-score and FeNO had the highest AUC value and better diagnostic sensitivity (90.1%) and specificity (89.0%), providing the highest diagnostic efficacy for childhood asthma. Comparing the differences in sensitivity and specificity between the training and testing sets of each combined indicator, the differences in IL-33 and FEF75% z-score combined were smaller than those of the other combined indicators, indicating that the diagnostic model established by the three indicators has a certain degree of extrapolation and better diagnostic stability. The predictive model developed in this study has high sensitivity and specificity for diagnosis and good extrapolation performance. It could accurately identify children with asthma for subsequent treatment. Serum IL-33 and FeNO assessed the degree of airway inflammation in children with asthma, while FEF75% assessed the degree of obstruction of small airways. The combination of these showed the best diagnostic efficacy, which also corresponds to the characteristics of chronic airway inflammation and reversible airflow limitation in asthma.2 In conclusion, serum IL-33 has some diagnostic value in childhood asthma regardless of single or combined indicators.
There are several limitations to the present study. First, even though pharmacological treatment ensured the safety of testing in children with asthma, it is undeniable that the treatment made the timing of FeNO testing and pulmonary ventilation function testing in the study subjects out of sync. Second, although the diagnostic role of serum IL-33 in childhood asthma was confirmed, it failed to explore the optimal diagnostic efficacy of childhood asthma in combination with more diagnostic tools for asthma (e.g., allergen testing). Additionally, whether IL-33 is associated with severe refractory asthma, and its relationship to corticosteroid therapy still needs to be further explored.
ConclusionIn the diagnosis of asthma in children, a single indicator of serum IL-33 demonstrates moderate diagnostic accuracy, and its combination with the FEF75% z-score significantly improves the diagnostic accuracy. Serum IL-33 is higher in children with asthma and increases with the severity of pulmonary ventilation obstruction.
FundingThis work was supported by the Joint Open Research Fund of Henan Key Laboratory of Child Brain Injury and Henan Pediatric Clinical Research Center (Project Number: KFKT2021005) and the Project of Henan Province Science and Technology Tackling (Project Number: 222102310689).
Data availability statementAll data generated or analyzed during this study are included in this article. The data that support the findings of this study are openly available in the Figshare database (https://doi.org/10.6084/m9.figshare.22233490.v1).
The authors acknowledge the Joint Open Research Fund of Henan Key Laboratory of Child Brain Injury and Henan Pediatric Clinical Research Center and the Project of Henan Province Science and Technology Tackling for supporting this study.