to establish the influence of late-onset sepsis on neurodevelopment of preterm infants with very low birth weight (VLBW), according to the etiologic agent.
Methodthis was a cohort of newborns with birth weight<1,500g and gestational age less than 32 weeks, admitted to the institutional intensive care unit (ICU) with up to 48hours of life, and followed-up at the outpatient follow-up clinic for preterm infants with VLBW until 2 years of corrected age. Exclusion criteria: death within the first 72hours of life, congenital malformations and genetic syndromes, children with congenital infection by the human immunodeficiency virus (HIV), congenital infection (STORCH), presence of early-onset sepsis and cases with more than one pathogen growth in blood cultures. Septic and non-septic infants were compared regarding neonatal outcomes and mortality. Neurodevelopment was assessed using the Bayley Scale (BSDI-II) at 18 to 24 months of corrected age.
Results411 preterm infants with VLBW were eligible; the mean gestational age was 29±2.2 weeks and mean birth weight was 1,041±281grams. Late-onset sepsis occurred in 94 preterm infants with VLBW (22.8%). VLBW infants with Gram-positive infection showed motor deficit when compared to the non-septic group, 68.8% vs. 29.3%, respectively (OR 6; 1.6-21.8, p=0.006); the cognitive development was similar between the groups. The overall mortality rate from infection was 26.7%; considering the pathogens, the rates were 18.7% for coagulase-negative Staphylococcus, 21.8% for Gram-positive bacteria, and 50% for Gram-negative bacteria and fungi.
Conclusionneonatal sepsis has a significant influence on late neurodevelopment at 2 years of corrected age in preterm infants with VLBW, and Gram-positive infections are associated with motor deficit.
estabelecer a influência da sepse tardia no neurodesenvolvimento de prematuros de muito baixo peso (MBP) recém-nascidos (RNs) de acordo com o agente etiológico.
MétodosCoorte de RN com peso de nascimento <1.500 ge idade gestacional <32 semanas, internados na UTI da instituição dentro de 48 horas de vida, e atendidos no ambulatório de MBP para até dois anos de idade corrigida. Foram excluídos: a morte nas primeiras 72h de vida, malformações congênitas e síndromes genéticas, filhos de mães HIV-positivas e infecção congênita, presença de sepse precoce, e os casos com mais de um microorganismo identificado em hemoculturas. RNs sépticos e não sépticos foram comparados quanto resultados neonatais, mortalidade e neurodesenvolvimento avaliadas através das escalas Bayley (BSDI-II) aos 18-24 meses de idade corrigida.
Resultadosum total de 411 RNs prematuros de muito baixo peso eram elegíveis, com idade gestacional=29±2,2 semanas e peso de nascimento=1.041±281g. Sepse tardia ocorreu em 94 casos (22,8%). MBP RN com infecção causada por microrganismos Gram-positivos apresentaram atraso motor, quando comparado com o grupo sem sépsis - 68,8% vs 29,3% (OR 6; 1,6-21,8, p=0,006), e atraso cognitivo, foi semelhante. Taxa de mortalidade global de infecção foi de 26,7%, e as taxas de mortalidade por grupo microorganismo foram: Staphylococcus coagulase negativa, 18,7%; Gram-positivos, 21,8%; Gram-negativas e fungos, 50%.
Conclusãoa sepse neonatal tem uma influência significativa no atraso no desenvolvimento neuropsicomotor aos dois anos de idade corrigida em prematuros de muito baixo peso RN e infecções Gram-positivas estão associadas com atraso motor.
The higher survival rate of preterm infants with very low birth weight (VLBW) in recent decades has not been accompanied by a reduction in the number of serious morbidities in neonatal intensive care units (ICUs) and during follow-up after discharge. In addition to minor morbidities, severe neurodevelopmental disorders and/or cerebral palsy are significant, with high economic and social costs, particularly in less developed countries.1
The association of neonatal sepsis with the increased risk of neurodevelopmental disorders in this population, especially learning difficulties, cognitive impairment, cerebral palsy, and visual and auditory deficits, has been a subject of interest in developed countries.1–3
Bacterial and fungal infections remain a major cause of neonatal morbidity and mortality. Approximately 21% of preterm infants with VLBW have late-onset sepsis, with high mortality rates, mainly caused by Gram-negative infections.4 However, data on the follow-up of preterm infants with late-onset sepsis are underexplored. Therefore, this study aimed to establish the influence of late-onset sepsis, according to the etiologic agent, on the neurodevelopment and mortality of preterm infants with VLBW.
MethodThis was a prospective cohort study conducted from November, 2003 to May, 2010, which included newborns with VLBW (birth weight <1,500g and gestational age less than 32 weeks), admitted to the institutional ICU with up to 48hours of life and followed at the outpatient follow-up clinic for preterm infants with VLBW. The study was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre, under registration number 10-0300. Parents or guardians of the study participants signed an informed consent.
Exclusion criteria were deaths within the first 72hours of life, congenital malformations and genetic syndromes, children with congenital infection by the human immunodeficiency virus (HIV), congenital infection (STORCH), newborns with early-onset sepsis and more than one pathogen growth in blood cultures. Patients included in the study were divided into two groups, according to the presence or absence of late-onset sepsis, defined as the presence of positive blood cultures over 72hours of life,5,6 followed by clinical signs (changes in breathing pattern, hypothermia or hyperthermia, circulatory symptoms, gastrointestinal symptoms). All blood cultures throughout hospitalization were reviewed, and the patients of the non-septic group had negative blood cultures. Positive blood cultures for bacteria considered contaminants were excluded, including Bacillus sp., Micrococcus sp., and Bacteroides sp., in accordance with the criteria of the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA).7 In cases of a blood culture positive for coagulase-negative Staphylococcus (CNS), only newborns whose clinical and laboratory findings were consistent with sepsis (leukocytosis and/or leukopenia and/or increased C-reactive protein) were included in the sepsis group.8
The following data were collected and compared between both groups: use of antenatal corticosteroids; preeclampsia; gender; gestational age (GA) determined by obstetric GA, in cases of obstetric ultrasound in the first 12 weeks of pregnancy, or by pediatric GA, using the Ballard method;9 birth weight, using the classification by weight and GA: small for GA (SGA), appropriate for GA (AGA), and large for GA (LGA), in accordance with Alexander's curve;10 type of delivery; APGAR score at 5minutes; SNAPPE II score (Score for Neonatal Acute Physiology- Perinatal Extension-II); length of hospital stay; intraventricular hemorrhage; and periventricular leukomalacia. In cases of intraventricular hemorrhage, the diagnosis was performed using cranial ultrasound, and only cases with grade 3 and 4 on the Papile classification were considered for statistical analysis, due to their association with developmental disorders.11 Ventilatory support; hyaline membrane disease (HMD); bronchopulmonary dysplasia (BPD), defined as oxygen dependency at 28 days of life;12 patent ductus arteriosus (PDA) diagnosed by echocardiography; severe retinopathy of prematurity, grades 3 and 4 (ROP 3-4) according to the international classification; neutropenia; transfusion of packed red blood cells; and seizures were also assessed.
During outpatient follow-up, neurodevelopment at 18 and 24 months of corrected age was evaluated using the Bayley Scales of Infant and Toddler Development II (BSDI-II). The psychologist was blinded to the groups during this evaluation. Scale applications were performed in the outpatient follow-up on the same day of the routine visit. The cognitive/mental scores assessed by the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) were considered normal for results greater than or equal to 85, moderate for results between 70-84, and severe for results less than or equal to 69.13 Cases of blindness, deafness, and cerebral palsy in premature infants with VLBW, whom the application of Bayley Scales (MDI and PDI) is not adequate, were considered as severe late neurodevelopment.
The primary outcomes assessed were death during neonatal hospitalization and outpatient follow-up, and diagnosis of late neurodevelopment, overall and according to the pathogens identified in blood cultures after 72hours of life.
Statistical analysisThe estimated sample size was based on the incidence of neurological events found in the group with clinical sepsis of a major multicenter study,14 which evaluated VLBW newborns stratified into different categories of infection, with a sample size of 124 patients in the non-sepsis group and 31 patients with neonatal sepsis, in order to detect a two-fold risk of higher neurological outcomes in the sepsis group compared to the group unexposed to the risk factor, considering an alpha error of 0.05 and 80% power.
In multivariate analysis, the variables selected for control were those with statistically significant difference or slightly higher than 0.05 in univariate analysis, when compared with the groups with and without neurological outcomes. Logistic regression was performed for each group of neurological outcome and considering each sepsis group by pathogen.
The Statistical Package for Social Science (SPSS) version 18.0 was used for analysis. The chi-squared test, Student's t-test, Mann-Whitney test, and logistic regression were used, considering p <0.05 as significant.
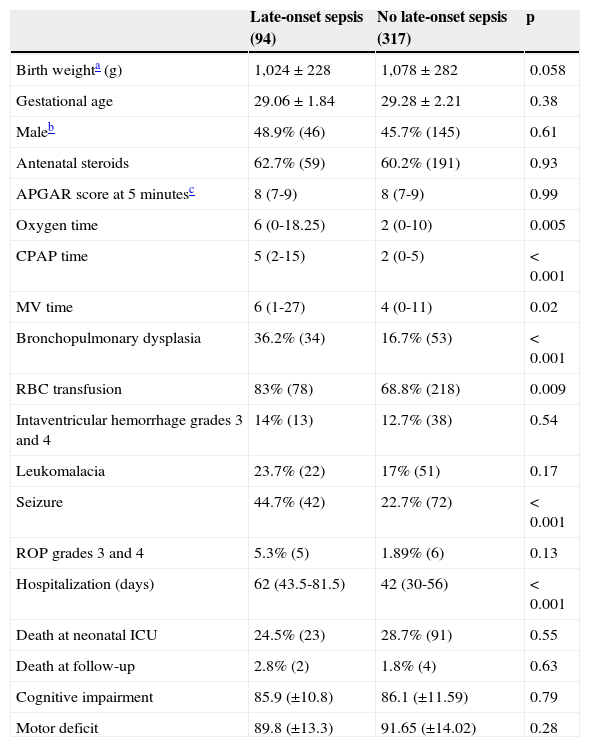
ResultsA total of 411 preterm infants with VLBW were consecutively admitted. Mean GA was 29±2.2 weeks and mean birth weight 1,041±281 grams, with predominance of the female gender (53.5%). Late-onset sepsis was present in 94 preterm infants with VLBW, an incidence of 22.8%. VLBW infants with late-onset sepsis were sicker; required longer mechanical ventilation support and hospital stay; and had higher incidence of bronchopulmonary dysplasia, seizures, and transfusion of packed red blood cells (Table 1).
Characteristics of preterm infants with very low birth weight according to the presence of late-onset sepsis.
| Late-onset sepsis (94) | No late-onset sepsis (317) | p | |
|---|---|---|---|
| Birth weighta (g) | 1,024±228 | 1,078±282 | 0.058 |
| Gestational age | 29.06±1.84 | 29.28±2.21 | 0.38 |
| Maleb | 48.9% (46) | 45.7% (145) | 0.61 |
| Antenatal steroids | 62.7% (59) | 60.2% (191) | 0.93 |
| APGAR score at 5minutesc | 8 (7-9) | 8 (7-9) | 0.99 |
| Oxygen time | 6 (0-18.25) | 2 (0-10) | 0.005 |
| CPAP time | 5 (2-15) | 2 (0-5) | <0.001 |
| MV time | 6 (1-27) | 4 (0-11) | 0.02 |
| Bronchopulmonary dysplasia | 36.2% (34) | 16.7% (53) | <0.001 |
| RBC transfusion | 83% (78) | 68.8% (218) | 0.009 |
| Intaventricular hemorrhage grades 3 and 4 | 14% (13) | 12.7% (38) | 0.54 |
| Leukomalacia | 23.7% (22) | 17% (51) | 0.17 |
| Seizure | 44.7% (42) | 22.7% (72) | <0.001 |
| ROP grades 3 and 4 | 5.3% (5) | 1.89% (6) | 0.13 |
| Hospitalization (days) | 62 (43.5-81.5) | 42 (30-56) | <0.001 |
| Death at neonatal ICU | 24.5% (23) | 28.7% (91) | 0.55 |
| Death at follow-up | 2.8% (2) | 1.8% (4) | 0.63 |
| Cognitive impairment | 85.9 (±10.8) | 86.1 (±11.59) | 0.79 |
| Motor deficit | 89.8 (±13.3) | 91.65 (±14.02) | 0.28 |
CPAP, continuous positive airway pressure; MV, mechanical ventilation; RBC, red blood cell; ROP, retinopathy of prematurity.
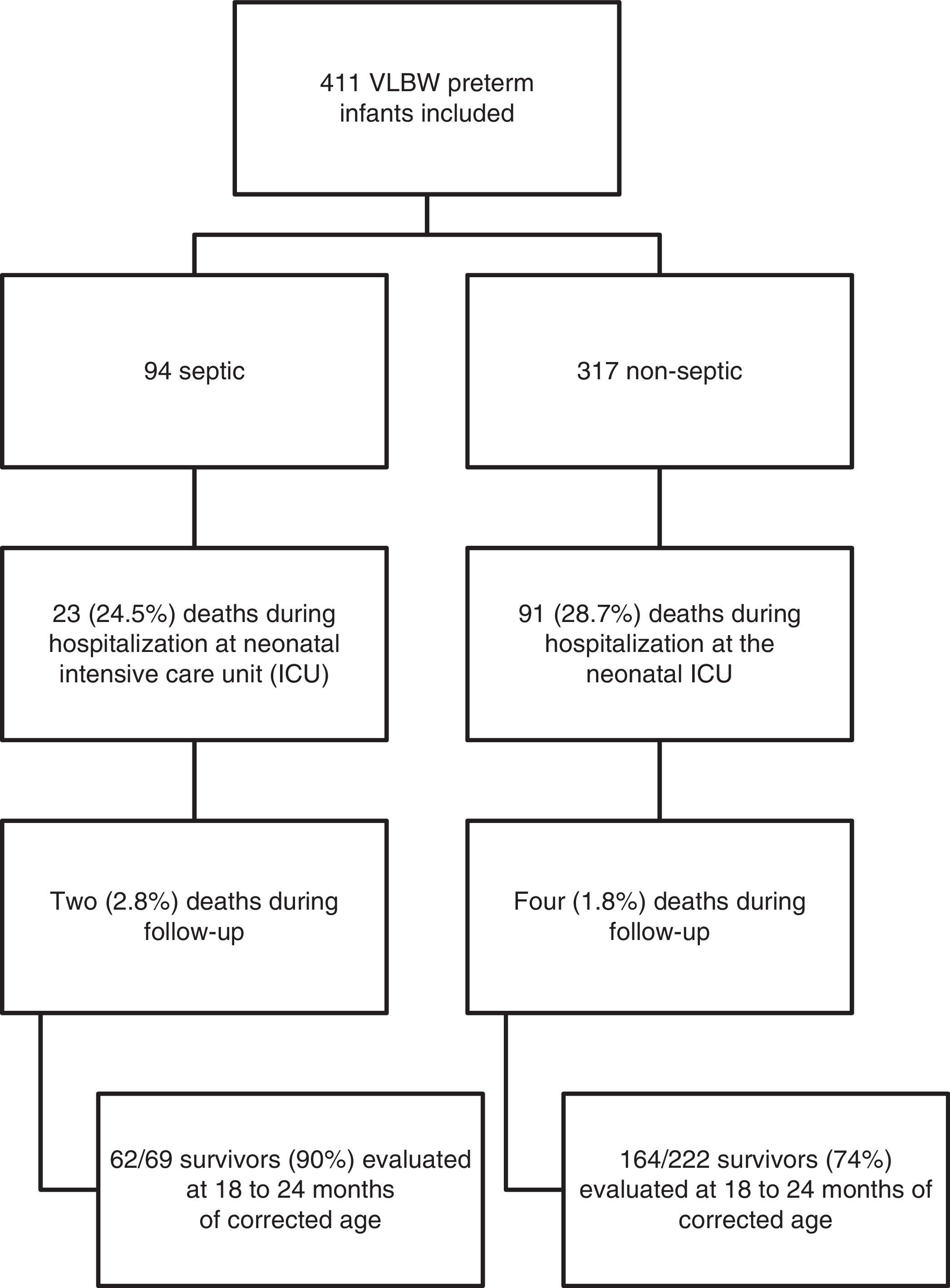
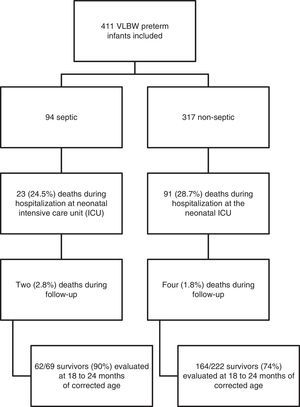
The death rate of patients included in the study was 28% (n=114) in the neonatal ICU, and 1.5% (n=6) in the outpatient clinic. Neurodevelopmental evaluation using the BSDI-II was performed on 213 patients; 90% of the sepsis and 74% of the non-sepsis groups (Fig. 1). Thirteen patients had very serious impairment, which prevented the execution of tasks during BSDI-II application due to blindness (n=3), deafness (n=3), and cerebral palsy (n=7), and were included in the group with severe neurodevelopmental impairment for analysis.
Sixty-eight infants (30%) had late motor development, of whom 22 (9.7%) were in the non-sepsis group. Changes in muscle tone were identified in 24 infants. Hypertonia was the most frequent dysfunction (17 evaluated as hypertonic and seven as hypotonic), mainly affecting the upper and/or lower limbs, or one hemisphere. In ten infants, four cases of left spastic hemiparesis, four cases of spastic quadriparesis, and two cases of spastic diparesis were identified.
Among VLBW preterm infants with higher motor impairment, deficits in gross motor developmental milestones were identified, such as trunk and head support, as two children did not acquire cervical control and two did not achieve the sitting position independently at 24 months of age. Another important functional limitation in gross motility refers to independent walking, which was not achieved by 12 children at 24 months of age; in eight of these, walking was only possible with the use of support (help from others and/or baby walker). Among those who walked independently, atypical patterns were observed in ten cases: hemiplegic gait (n=3), unsteady gait and an increase in the support polygon (n=3), valgus feet (n=2), digitigrade gait (n=1), and scissor gait (n=1). reeling gait” e “pes valgus.
The microorganisms found were CNS (n=44; 46.8%), S. aureus (n=22; 23.4%), Candida (n=11; 12%), Klebsiella (n=6; 6.4%), Enterobacter (n=2; 2%), Pseudomonas (n=2; 2%), Streptococcus agalactiae (n=2; 2%), E. coli (n=1; 1%), Enterococcus (n=1; 1%), Streptococcus viridans (n=1; 1%), Acinetobacter (n=1; 1%), Cepacia (n=1; 1%), and S. epidermidis (n=1; 1%). For analysis, patients were grouped into “Gram-positive sepsis”, “CNS sepsis”, and “Gram-negative and fungi sepsis”.
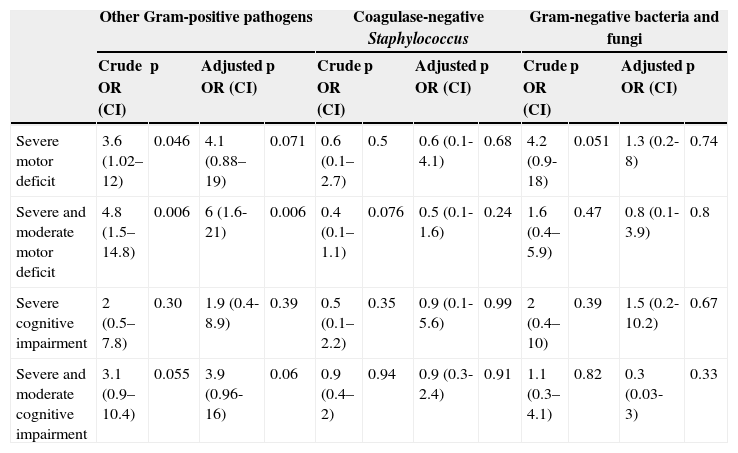
Infection caused by Gram-negative bacteria and fungi presented the worst results when the death or severe motor deficit outcome was analyzed: 10.7% of the patients with this outcome had sepsis caused by Gram-negative bacteria, versus 4.5% of patients without this outcome (p=0.046); however, this difference was not maintained in the multivariate analysis (OR 3.76; 0.77-18.30; p=0.1); lower GA and duration of hospital stay were the main risk factors. Infection by Gram-positive bacteria showed motor deficit among VLBW preterm infant survivors (68.8% vs. 29.3%), while cognitive development was similar (Table 2). This result was maintained in the multivariate analysis, where an association between motor deficit and Gram-positive sepsis was observed (OR 6; 1.6-21.8, p=0.006) (Table 3), adjusted for type of delivery, maternal pre-eclampsia, use of antenatal corticosteroids, packed red blood cell transfusions, leukomalacia, ROP, SNAPPE II, duration of hospitalization, and GA; these factors are known to be associated with late neurodevelopment.
Neurodevelopmental follow-up of very low birth weight preterm infants according to the infectious agent.
| Gram-positive (n=13) | Non-septic (n=155) | ||
|---|---|---|---|
| PDI | 83.54±13.67 | 91.65±14.02 | 0.047 |
| MDI | 84.85±12.02 | 86.11±11.59 | 0.7 |
| Gram-negative and fungi (n=9) | Non-septic (n=155) | ||
|---|---|---|---|
| PDI | 85.67±16.52 | 91.65±14.02 | 0.22 |
| MDI | 80.89±10.27 | 86.11±11.59 | 0.18 |
| CNS (n=36) | Non-septic (n=164) | ||
|---|---|---|---|
| Cognitive impairment | 17 (47.2%)c | 76 (46.3%) | 1 |
| Motor deficit | 6 (16.7%) | 48 (29.3%) | 0.18 |
| Gram-positive (n=16) | Non-septic (n=164) | ||
|---|---|---|---|
| Cognitive impairment | 12 (75%) | 76 (46.3%) | 0.054 |
| Motor deficit | 11 (68.8%) | 48 (29.3%) | 0.003 |
| Gram-negative and fungi (n=10) | Non-septic (n=164) | ||
|---|---|---|---|
| Cognitive impairment | 5 (50%) | 76 (46.3%) | 1 |
| Motor deficit | 4 (40%) | 48 (29.3%) | 0.71 |
CNS, coagulase negative Staphylococcus; MDI, Mental Development Index; PDI, Physical Development Index.
Neurodevelopment at 2 years of corrected age by group of pathogens in septic neonates - multivariate analysisa
| Other Gram-positive pathogens | Coagulase-negative Staphylococcus | Gram-negative bacteria and fungi | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR (CI) | p | Adjusted OR (CI) | p | Crude OR (CI) | p | Adjusted OR (CI) | p | Crude OR (CI) | p | Adjusted OR (CI) | p | |
| Severe motor deficit | 3.6 (1.02–12) | 0.046 | 4.1 (0.88–19) | 0.071 | 0.6 (0.1–2.7) | 0.5 | 0.6 (0.1-4.1) | 0.68 | 4.2 (0.9-18) | 0.051 | 1.3 (0.2-8) | 0.74 |
| Severe and moderate motor deficit | 4.8 (1.5–14.8) | 0.006 | 6 (1.6-21) | 0.006 | 0.4 (0.1–1.1) | 0.076 | 0.5 (0.1-1.6) | 0.24 | 1.6 (0.4–5.9) | 0.47 | 0.8 (0.1-3.9) | 0.8 |
| Severe cognitive impairment | 2 (0.5–7.8) | 0.30 | 1.9 (0.4-8.9) | 0.39 | 0.5 (0.1–2.2) | 0.35 | 0.9 (0.1-5.6) | 0.99 | 2 (0.4–10) | 0.39 | 1.5 (0.2-10.2) | 0.67 |
| Severe and moderate cognitive impairment | 3.1 (0.9–10.4) | 0.055 | 3.9 (0.96-16) | 0.06 | 0.9 (0.4–2) | 0.94 | 0.9 (0.3-2.4) | 0.91 | 1.1 (0.3–4.1) | 0.82 | 0.3 (0.03-3) | 0.33 |
CHAD, Concentrado de hemácias de adultos; CI, confidence interval; OR, odds ratio; SNAPPE, Score for Neonatal Acute Physiology- Perinatal Extension-II.
Overall mortality secondary to infection accounted for 26.7% of deaths; stratifying by pathogen, the percentages were: 18.7% in the CNS group, 21.8% in the Gram-positive group, and 50% in the Gram-negative and fungi group.
DiscussionThe present study reinforced the finding that late-onset of neonatal sepsis is associated with late neurodevelopment in the first 2 years of life in preterm infants with VLBW. Previous studies have suggested that infants with signs of sepsis were more likely to have cerebral palsy, lower cognitive and motor scores, and visual impairment.14 In this study, the particular involvement of Gram-positive infection, which presented a six-fold higher risk for motor deficit in this population, was emphasized.
Some hypotheses have been suggested to justify late neurodevelopment in newborns with sepsis, particularly early-onset sepsis. The most accepted theories associate systemic inflammatory response (with cytokine and free radical activation) with subsequent white matter damage, probably due to astrogliosis, and loss of pre-oligodendrocytes.8 The association of late-onset sepsis with unfavorable neurodevelopmental outcomes may be explained as a result of this systemic inflammatory response, increasing comorbidities associated with worse outcomes (such as hypotension, disseminated intravascular coagulation, chronic lung disease, and severe intraventricular hemorrhage) and death.2
In the present study, late-onset sepsis was detected in 22.8% of the VLBW preterm infants, similar to previous studies,2,15 as well as the predominance of Gram-positive organisms (70.2%) and the most common CNS pathogen (46.8% of all sepsis cases, 66.6% of Gram-positive infections). These findings are also very close to those of a large study conducted by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network, which observed that 70% of infections were caused by Gram-positive bacteria, and CNS accounted for 48% of infections and 68% of Gram-positive infections.16
Among VLBW preterm infants with late-onset sepsis followed-up for up to 24 months of corrected age, 6.1% had severe neurodevelopmental impairment and 3.2% had cerebral palsy. The mortality rates from sepsis and pathogens (26.7% overall mortality, 18.7% in the CNS group, 21.8% in the Gram-positive group, and 50% in the Gram-negative and fungi group) in the present study are in agreement with those observed by Stoll et al.16 (mortality rate due to sepsis, 18%; to CNS, 9%; to other Gram-positive bacteria, 11.2%; to Gram-negative bacteria 36%; and to fungi, 32%). This demonstrates that infection indicators from major international centers may be applied in the Brazilian setting.
There are conflicting data for CNS infection as a risk factor for late neurodevelopment.8,15 In the present study, no differences were observed in cognitive and motor scores for newborns who presented with late-onset sepsis by CNS, similarly to a previous study.8 Therefore, it was decided to evaluate the newborns who presented sepsis for Gram-positive infection, excluding CNS; in this scenario, a significant motor deficit was observed, even after controlling for variables such as intraventricular hemorrhage and leukomalacia. Schlapbach et al.15 found a two-fold higher risk of late neurodevelopment in the presence of sepsis caused by other Gram-positive bacteria.
In the present study, the mortality rate due to Gram-negative bacteria and fungal infection was 50%. Previous studies also observed high mortality associated with Gram-negative or fungal infection.2,17,18 Fungal infection is associated with moderate to severe cerebral palsy, higher risk of developing blindness and deafness, and late neurodevelopment at 18 months of life;18 60% of survivors of a fungal infection episode have late neurodevelopment.19 However, the highest incidence of severe motor deficit in that particular group in the present study was not maintained after the multivariate analysis, possibly due to the small number of patients with proven fungal sepsis (11 preterm infants), and future studies are needed.
The concern in determining the impact of pathogens on neurodevelopment was limited to the number of positive blood cultures and variety of pathogens found. The volume of blood collected for cultures is usually small, which interferes with the sensitivity of these cultures, particularly in early-onset sepsis.20 A limitation of the present study is the lack of an assessment of the inflammatory reaction intensity in each of the infected newborns, in order to determine a possible correlation with late neurodevelopment, as elevated levels of pro-inflammatory cytokines and late neurodevelopment were observed in a previous study.21 However, an extremely important aspect of the present study was the assessment of neurodevelopment excluding the VLBW preterm infants with early-onset sepsis, focusing on the outcomes of late-onset sepsis. Early-onset sepsis is closely related to maternal chorioamnionitis, which is related to periventricular leukomalacia, a well described cause of cerebral palsy and cognitive impairment.8,22
In this cohort of preterm infants with VLBW, late-onset sepsis was associated with late neurodevelopment; preterm infants with sepsis due to Gram-positive bacteria presented a higher incidence of motor deficit. Care in the neonatal ICU and during follow-up should anticipate therapeutic measures, and the follow-up after hospital discharge should identify early deficits in order to ensure appropriate intervention.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the statistician Luciano Santos Pinto Guimarães, MsC, for his assistance with the analysis of data; they would also like to thank the pediatrician Ana Claudia W. Benjamin, MD, and the neonatologist Rosanna Nejedlo, MD, for their valuable support in the follow-up of patients.
Please cite this article as: Hentges CR, Silveira RC, Procianoy RS, Carvalho CG, Filipouski GR, Fuentefria RN, et al. Association of late-onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight. J Pediatr (Rio J). 2014;90:50–57.
Study conducted at the Universidade Federal do Rio Grande do Sul and Hospital de Clínicas de Porto Alegre.