Report the incidence, epidemiology, clinical features, death, and vaccination status of patients with whooping cough and perform genotypic characterization of isolates of B. pertussis identified in the state of Paraná, during January 2007 to December 2013.
MethodsCross-sectional study including 1,209 patients with pertussis. Data were obtained through the Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação – SINAN) and molecular epidemiology was performed by repetitive sequence-based polymerase chain reaction (rep-PCR; DiversiLab®, bioMerieux, France).
ResultsThe incidence of pertussis in the state of Paraná increased sharply from 0.15-0.76 per 100,000 habitants between 2007-2010 to 1.7-4.28 per 100,000 between 2011-2013. Patients with less than 1 year of age were more stricken (67.5%). Fifty-nine children (5%) developed pertussis even after receiving three doses and two diphtheria-tetanus-pertussis (DTP) boosters vaccine. The most common complications were pneumonia (14.5%), otitis (0.9%), and encephalopathy (0.7%). Isolates of B. pertussis were grouped into two groups (G1 and G2) and eight distinct patterns (G1: P1-P5 and G2: P6-P8).
ConclusionThe resurgence of pertussis should stimulate new research to develop vaccines with greater capacity of protection against current clones and also encourage implementation of new strategies for vaccination in order to reduce the risk of disease in infants.
Relatar a incidência, aspectos epidemiológicos, clínicos, morte e a vacinação de pacientes com coqueluche e realizar a caracterização genotípica de isolados de B. pertussis identificados no estado do Paraná, de janeiro de 2007 a dezembro de 2013.
MétodosEstudo transversal, incluindo 1.209 pacientes com coqueluche. Os dados foram obtidos através do Sistema de Informação de Agravos de Notificação (SINAN) e a epidemiologia molecular foi realizada por PCR baseada em sequências repetitivas (rep-PCR; DiversiLab®, bioMerieux, France).
ResultadosA incidência de coqueluche no Estado do Paraná aumentou acentuadamente de 0,15-0,76 por 100.000 habitantes entre 2007-2010 para 1,7-4,28 por 100.000 habitantes entre 2011-2013. Os pacientes com menos de um ano de idade foram os mais afetados (67,5%). Cinquenta e nove crianças (5%) desenvolveram coqueluche mesmo depois de receber três doses da vacina e dois reforços com a vacina tríplice DTP. As complicações mais comuns foram pneumonia (14,5%), otite (0,9%) e encefalopatia (0,7%). Isolados de B. pertussis foram agrupados em dois grupos (G1 e G2) e oito padrões distintos (G1: P1-P5 e G2: P6-P8).
ConclusãoO ressurgimento da coqueluche vem para sugerir novas pesquisas com o objetivo se desenvolver vacinas com maior capacidade de proteção contra os clones atuais e também implementar novas estratégias de vacinação, a fim de reduzir o risco de doenças em lactentes.
Pertussis, commonly known as whooping cough, is a severe, highly contagious disease of the human respiratory tract, caused by Bordetella pertussis.1 The disease is characterized by uncontrollable coughing fits, accompanied by inspiratory stridor.2 Children and adults of any age can develop the disease; however, it is more severe in infants, especially up to 6 months of age.3 Despite good vaccination coverage, it is estimated that 50 million cases occur each year, with approximately 300,000 deaths annually, 90% of them in developing countries.1,4
The last decade showed a surprising increase in incidence rates of pertussis in several regions of the world. The causes of this disease resurgence are still unclear. Some hypotheses raised were post-vaccine immunity loss; implementation of molecular methods for diagnosis; improvement of epidemiological surveillance systems; reduction of the vaccine efficacy; or even genetic changes in the pathogen.5,6
In Brazil, pertussis was included in the notifiable diseases list in 1975, with the recommendation to investigate all disease outbreaks. In the early 1980s, there were more than 40,000 cases a year and the incidence rate was>30/100,000 inhabitants. This number has decreased sharply since 1983, with the introduction of the diphtheria-tetanus-pertussis (DTP) vaccine in the Brazilian childhood vaccination schedule; since then, it has shown a downward trend. Evidence of pertussis resurgence in Brazil was demonstrated by the detection of some outbreaks in 2010, followed by an increase in the number of cases in several Brazilian capitals.7
The monitoring of B. pertussis circulation is being performed in the Brazilian states, with the implementation of surveillance services and qualified laboratories for the isolation of the etiological agent. The methods used in the laboratory diagnosis of pertussis include culture and real time polymerase chain reaction (RT-PCR). The diagnosis of pertussis by laboratory testing was implemented in the Central Laboratory of the state of Paraná (Lacen-PR) in 2005. The test (culture) was first made available for three sentinel hospitals; two implemented in the capital city of Curitiba and another in the city of Londrina.
The first isolation of B. pertussis occurred only in 2007, in a family contact with cough, from a child with the disease symptoms. In 2011, the survey was expanded to all Basic Health Units (BHUs) and other hospitals in the city of Curitiba and the metropolitan area. These services received training that addressed clinical diagnosis, epidemiological behavior, and biological sample collection. Recently, some studies have reported the use of repetitive element sequence-based PCR (rep-PCR; DiversiLab®, bioMerieux, France) for molecular typing of microorganisms. This method uses oligonucleotide primers complementary to repetitive highly conserved DNA sequences present at numerous copies in the bacterial genome. It allows genotypic characterization, clone differentiation, and their dispersion in the community.8
The objective of this study was to describe the incidence, epidemiological and clinical characteristics, number of deaths and vaccination status, of patients with pertussis, and to perform the genotypic characterization of isolates of B. pertussis circulating in the state of Paraná, Brazil, from January of 2007 to December of 2013.
Materials and MethodsThis was an observational and cross-sectional study, consisting of patients with a confirmed diagnosis of pertussis, performed in the state of Paraná, from January 2007 to December 2013. The cases of pertussis are reported to the Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação – SINAN), in which the notifying source completes a form that provides data on location, identification, clinical signs and symptoms, death, and vaccination status of patients.
Pertussis cases were confirmed by: I. Clinical criteria - an individual, regardless of age and vaccination status, who had cough of any kind for 14 days or more associated with two or more of the following signs and symptoms: paroxysmal cough, inspiratory stridor, and/or vomiting after coughing; II. Laboratory criteria - all individuals who met the definition of a suspected case of pertussis and have B. pertussis isolated in culture or identified by RT-PCR; and III. Clinical epidemiological criteria - all individuals who met the definition of a suspected case and who had contact with a case of pertussis confirmed by laboratory testing, during the period of communicability.2
All cases of pertussis confirmed by at least one of the abovementioned criteria were included in the study. The following epidemiological and clinical variables were analyzed: age, sex, clinical signs and symptoms, complications, number of deaths, and vaccination status. This study was approved by the Research Ethics Committee of Hospital do Trabalhador/SESA/PR, CAAE 16584713.6.0000.5225.
Laboratory diagnosisClinical samples (deep nasopharyngeal secretion) of patients with suspected pertussis were referred to Lacen-PR in Regan-Lowe (RL) transport media supplemented with 10% sheep blood and 40μg/mL cephalexin. The samples were cultured in RL agar plates and incubated at 35°C (±1) in a humid environment for ten days. Suspected colonies of B. pertussis were identified through their development after three days of incubation, by demonstrating Gram-negative morphology, catalase and oxidase positivity, and compatible biochemical identification according to published studies.9 All isolates identified during the study period were stored in a freezer at -80°C.
Rep-PCR TypingB. pertussis isolates were typed by the repeated DNA sequence technique (rep-PCR; DiversiLab®, bioMerieux, France) to determine the genetic proximity between them. This technique analyzes specific regions of the bacterial genome that may or may not be present in certain strains. When present, they are amplified and can be visualized by creating bands on the virtual gel formed by the system. The differences and similarities (presence and/or intensity of bands) between isolates allow their classification in different groups and patterns. Isolates that had≥90% similarity were considered as belonging to the same group. Unique patterns (clones) were considered when isolates showed similarity≥97.0% to each other and had no different band.
Statistical analysisThe SINAN system provides data for the calculation of epidemiological indicators using the application TABWIN, version 32 (http://www2.datasus.gov.br/DATASUS/index.php?area=060805), which generates reports from the SINAN NET Postgres base or the DBF base of the NET or SINAN ONLINE versions (http://dtr2004.saude.gov.br/sinanweb/novo/relatorios/descricao.pdf). To calculate the annual incidence, coefficient tabulation was performed in the TABWIN format of the number of new cases each year (2007-2013), divided by the state of Paraná’s population. The DiversiLab software, version 1.2.66 (DiversiLab®, bioMerieux, France) analyzed the genotyping results by creating a proximity matrix, using Pearson's correlation coefficient to calculate distance matrices and create a dendrogram. The reports were generated automatically by the system. The variables were described according to their frequencies.
ResultsDuring the analyzed period, 3,451 pertussis cases were reported in the state of Paraná; however, only 1,209 were confirmed by the above criteria. A considerable increase in the incidence of this disease was observed, which ranged from 0.15 to 0.76 per 100,000 inhabitants between 2007 and 2010 to 1.7 per 100,000 in 2011, 3.83 per 100,000 in 2012, and 4.28 per 100,000 in 2013. Six hundred and sixty-five (55%) patients were females. The most affected age group was the one aged less than 1 year, with 816 (67.5%) cases, especially in infants younger than 2 months.
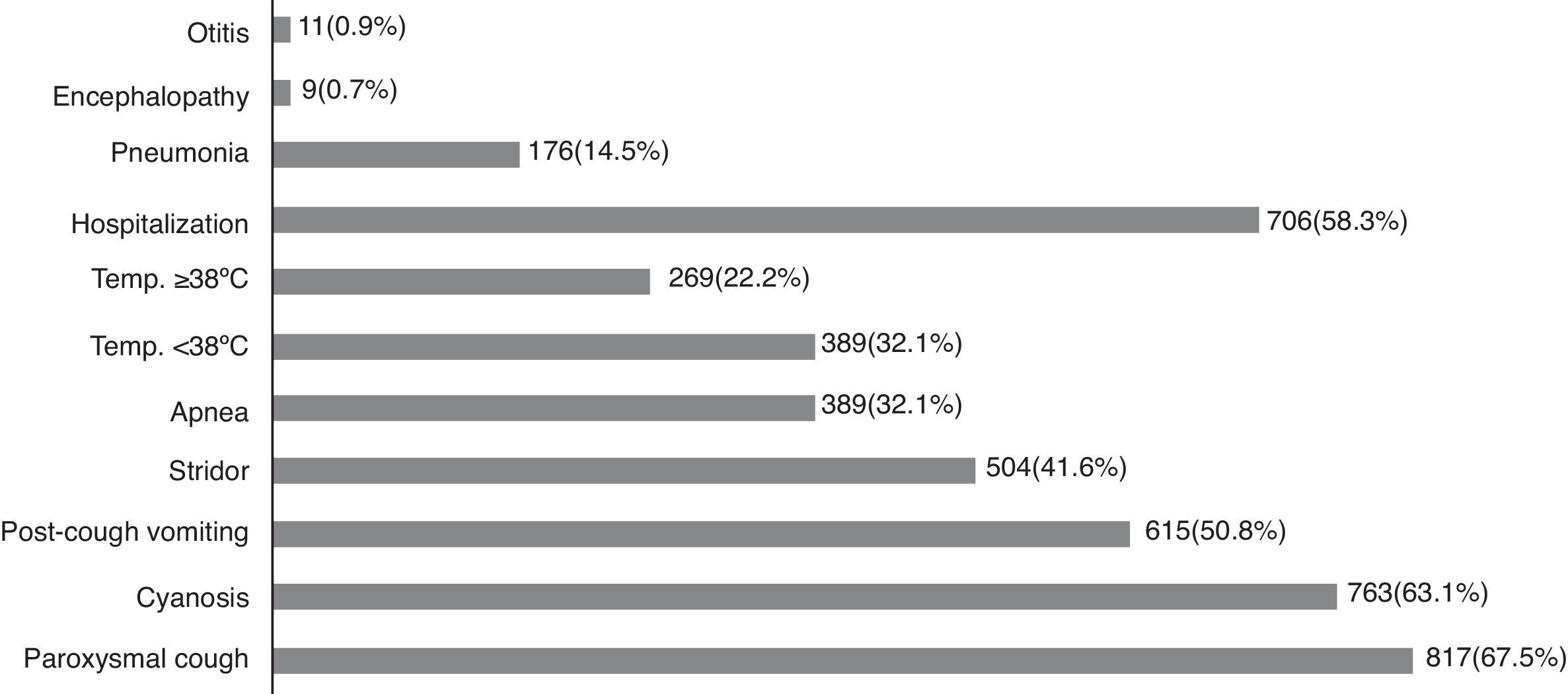
The clinical presentation of pertussis may vary and more than one sign/symptom may be present in the same patient. The most frequent signs/symptoms were paroxysmal cough, present in 817 (67.5%) patients, followed by cyanosis in 763 (63.1%), vomiting after coughing in 615 (50.8%), inspiratory stridor in 504 (41.6%), and apnea in 389 (32.1%). Temperature ≥ 38°C was found in 269 (22.2%) patients, and temperature between 37 and 38°C was observed in 389 (32.1%; Fig. 1). A significant number of patients required hospital admission and the main complication was pneumonia in 176 (14.5%). Nineteen patients died; of them, 11 were males, 17 (89.5%) were younger than 2 months of age, one was 3 months old, and another was 44 years old, with a previous diagnosis of tuberculosis.
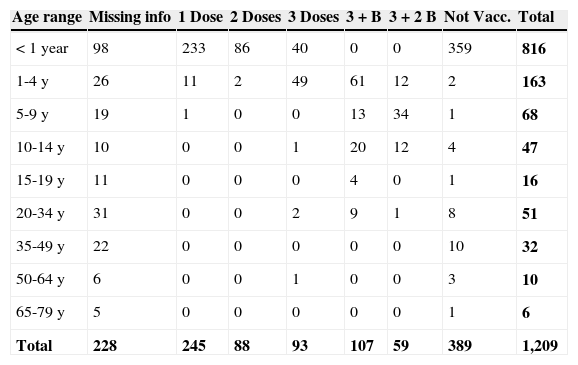
Almost half of the patients with confirmed diagnosis of pertussis, 592 (49%), had already received at least one dose of DTP vaccine and, among these, 93 (7.7%) had received three doses and 59 (5%) had completed the vaccination schedule (three doses + two boosters; Table 1).
Confirmed cases of pertussis by age range and number of diphtheria-tetanus-pertussis (DTP) vaccine doses in the state during the period of 2007-2013.
| Age range | Missing info | 1 Dose | 2 Doses | 3 Doses | 3 + B | 3 + 2 B | Not Vacc. | Total |
|---|---|---|---|---|---|---|---|---|
| < 1 year | 98 | 233 | 86 | 40 | 0 | 0 | 359 | 816 |
| 1-4 y | 26 | 11 | 2 | 49 | 61 | 12 | 2 | 163 |
| 5-9 y | 19 | 1 | 0 | 0 | 13 | 34 | 1 | 68 |
| 10-14 y | 10 | 0 | 0 | 1 | 20 | 12 | 4 | 47 |
| 15-19 y | 11 | 0 | 0 | 0 | 4 | 0 | 1 | 16 |
| 20-34 y | 31 | 0 | 0 | 2 | 9 | 1 | 8 | 51 |
| 35-49 y | 22 | 0 | 0 | 0 | 0 | 0 | 10 | 32 |
| 50-64 y | 6 | 0 | 0 | 1 | 0 | 0 | 3 | 10 |
| 65-79 y | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| Total | 228 | 245 | 88 | 93 | 107 | 59 | 389 | 1,209 |
Missing info, Missing information; 3 + B, three doses plus one booster dose; 3 + 2 B, three doses plus two booster doses; Not Vacc., Not vaccinated.
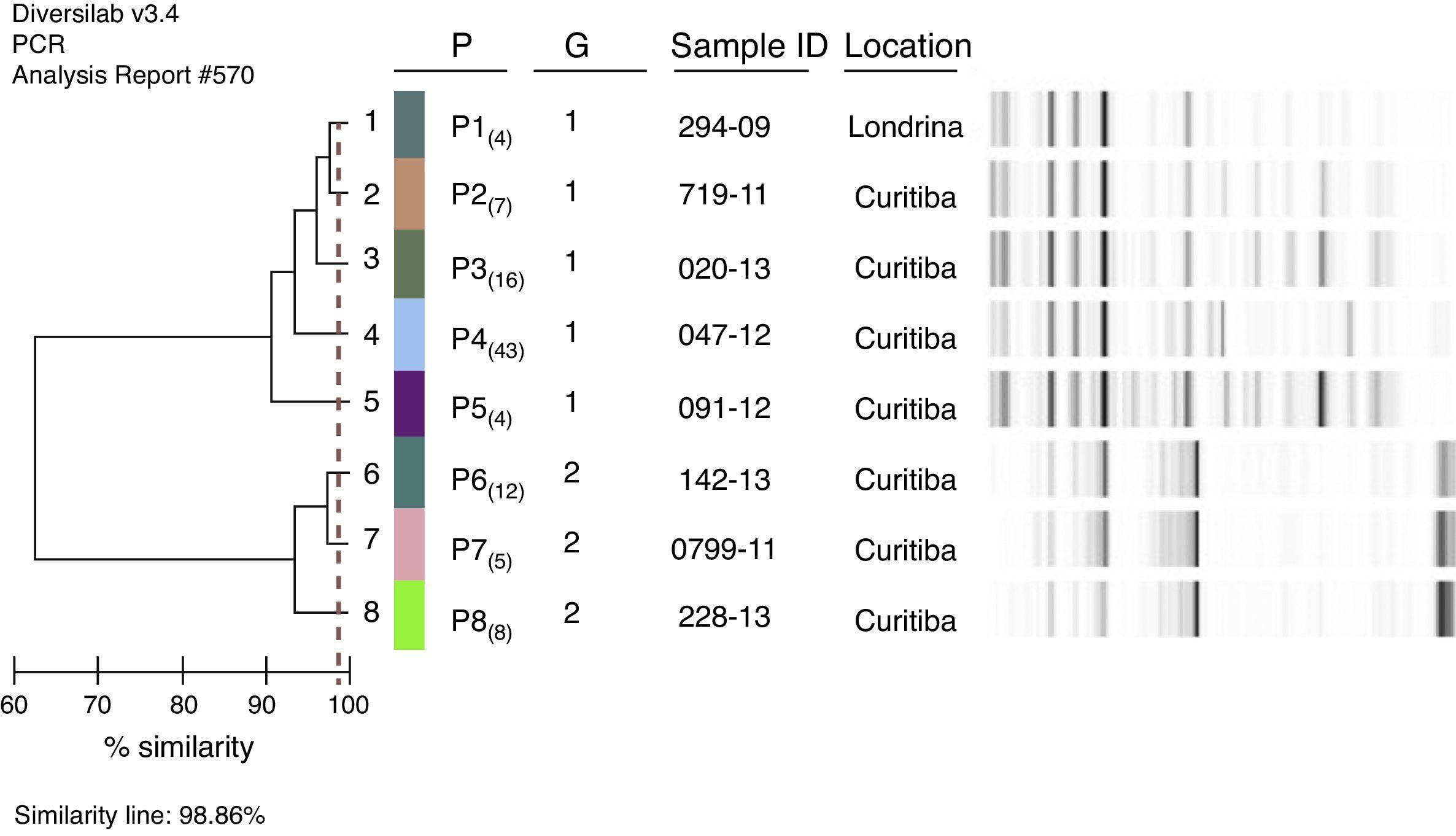
Two hundred and sixteen (17.9%) patients with pertussis had a diagnosis of pertussis confirmed by laboratory testing, after B. pertussis was isolated in culture. During the reactivation process, it was observed that only 45.8% (n=99) were viable. Molecular typing (rep-PCR) was able to detect and differentiate two major groups (G1 and G2) and 8 different patterns (P) (clones) among the 99 isolates of B. pertussis analyzed. The G1 group was the most frequent with 74 isolates and the G2 group consisted of 25 isolates. The similarity between the two groups was less than 62%.
It was possible to differentiate five different patterns [P1, P2, P3, P4, and P5] in the group G1, while only three patterns [P6, P7 and P8] were observed in G2. Each pattern showed ≥ 97% similarity to the others, with no different bands. The B. pertussis isolate identified in 2007 belonged to the most frequent clone (G1: P4). The isolates with the patterns [G1: P2, P3, P4, and P5] were found to be circulating in all analyzed years (Fig. 2). The pattern G2: P6 was first detected in 2009, and the patterns G2: P7 and G2: P8 circulated from 2011, and since then, have been disseminated in later years. The G1: P1 clone circulated only during the year 2012.
DiscussionPertussis has resurged in the state of Paraná, increasing from 16 cases in 2010 to 178 in 2011. In the subsequent years, the number of confirmed cases has continued to increase, with 400 in 2012 and 447 in 2013. This may be associated with the presence of multiple circulating clones of B. pertussis.
Brazil started the systematic control of pertussis in 1983, with the inclusion of the DTP vaccine in children's basic vaccine schedule, around the time when developed countries indicated the first signs of disease resurgence.10,11 In 1990, vaccination coverage increased to approximately 70% and the incidence of the disease decreased from 30 to 10.6/100,000 inhabitants. In the last decade, the incidence of pertussis in Brazil remained stable, ranging from 0.72/100,000 inhabitants in 2004 to 0.32/100,000 inhabitants in 2010. In 2011, there was a sudden increase in the number of confirmed cases in relation to the previous five years, increasing the incidence to 1.2/100,000 inhabitants, even though high vaccination coverage was maintained.12
The classic symptoms of pertussis are prolonged (“100-day cough”) and paroxysmal coughing, accompanied by noisy breathing (inspiratory stridor).4 Cyanosis was a very common finding in patients of the present study, in addition to the presence of the classic symptoms. Nicolai et al. reported that paroxysmal cough is a highly specific symptom for the diagnosis of pertussis, present in 63.2% of cases, whereas not found in patients with bronchiolitis caused by respiratory syncytial virus (RSV). The rate of cyanosis (52.6%) found in patients with pertussis was also higher than that found in patients with RSV infection (10.5%), with statistical significance (p=0.006).13 In the series evaluated by Bayhan et al., 39.3% of patients with pertussis had inspiratory stridor and 87.1% had cyanosis.14
Pneumonia was the main complication found in the patients studied herein. This complication is the most common cause of death related to pertussis. In the United States and Canada, pneumonia was observed in 5.2% and 9.4% of cases, respectively.15,16B. pertussis triggers a cascade of inflammatory events that includes acute pulmonary vasoconstriction and increased leukocyte circulation, which affects pulmonary blood circulation, exacerbates hypoxemia, and creates a vicious cycle of pulmonary hypertension.17
In this study, most deaths occurred during the last three years of the study, and in children younger than 2 months of age. According to SINAN data, a total of 15,428 cases of pertussis were reported in Brazil in 2011, of which 2,248 were confirmed, with 56 deaths and mortality of 2.5%.
The number of cases continued to increase in 2012, with 5,416 confirmed cases, 84 deaths associated with the disease, and mortality of 1.6%. In 2013, the state of Paraná accounted for 10% of deaths recorded in Brazil.12 In England, between 2001 and 2011, a total of 48 deaths were recorded due to the disease in children younger than 1 year of age; of these, 41 (85.41%) were younger than 66 days of life.18 In the US in 2012, a total of 48,277 cases were reported and there were 18 (0.04%) deaths, which is the highest recorded number of cases of the disease in the last 60 years in that country.19 It is observed that the disease lethality in Brazil exceeded 40 times that found in the USA.
The currently available vaccines are considered safe and immunogenic; however, they provide protection with an approximate effectiveness of 46% after the 1st dose, 79.6% after the 2nd dose, 91.7% after the 3rd dose, and 96.4% after the 4th dose, lasting about 10 years.20,21 In the present study, 59 (5%) children had completed the vaccination schedule (three doses + two boosters), but still developed the disease.
The rep-PCR technique allowed the identification of eight B. pertussis clones (P1 - P8) circulating in the state of Paraná. It is possible that the current clones have different antigenic variations than those found in the clones circulating in the pre-immunization period,22,23 or they might even be different from the strain used in the vaccine production. Therefore, the vaccine would not show the expected effectiveness against all circulating strains. It has been suggested that B. pertussis adapted due to selective pressure exerted by the vaccine during the last 60 years, expressing pertussis toxin, pertactin, and fimbria differently from those found in the vaccine strain. These modifications may explain in part the decrease in vaccine efficacy, prompting the hypothesis that vaccine-induced reduced immunity may be responsible for the increased number of cases of the disease worldwide.24
Another issue is that the current vaccination strategies, focusing only on children, appear insufficient to prevent death in young infants. Thus, new strategies must be analyzed to include vaccination in newborns, adolescents, the elderly, health care workers, professionals working with children in nurseries and kindergartens, and pregnant women.20,25,26 While it is not possible to provide booster doses for the entire population, it is a priority to vaccinate individuals who have direct contact with young infants, especially mothers, as well as to maintain high vaccine coverage in children.25
This study has some limitations. It is possible that the number of pertussis cases was underreported. The lack of familiarity with the pertussis diagnosis, explained by the long period during which the disease remained under epidemiological control, may have influenced the disease incidence rates. Another factor that might have contributed is that some patients have milder symptoms, which are similar to viral infections, making it difficult to suspect and diagnose the disease. In these cases, it is important to identify the infection etiology; however, the confirmation of viral etiology does not rule out the possibility of coinfection with B. pertussis.27
The authors conclude that pertussis is a re-emerging disease in this state, with non-immunized infants representing the most often-affected age range. However, it was observed that some children developed the disease despite having been fully vaccinated. Advanced studies on cell and molecular biology should be encouraged to recognize antigenic changes in different clones of B. pertussis currently circulating and offer new, more effective, and more protective vaccines to the population. The resurgence of the disease also requires urgent changes in the planning of new vaccination strategies aiming to reduce the risk of acquiring the disease and infant morbimortality.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Torres RS, Santos TZ, Torres RA, Pereira VV, Fávero LA, Filho OR, et al. Resurgence of pertussis at the age of vaccination: clinical, epidemiological, and molecular aspects. J Pediatr (Rio J). 2015;91:333–8.