this study aimed to review the literature regarding late preterm births (34 weeks to 36 weeks and 6 days of gestation) in its several aspects.
Sourcesthe MEDLINE, LILACS, and Cochrane Library databases were searched, and the references of the articles retrieved were also used, with no limit of time.
Data synthesisnumerous studies showed a recent increase in late preterm births. In all series, late preterm comprised the majority of preterm births. Studies including millions of births showed a strong association between late preterm birth and neonatal mortality. A higher mortality in childhood and among young adults was also observed. Many studies found an association with several neonatal complications, and also with long-term disorders and sequelae: breastfeeding problems, cerebral palsy, asthma in childhood, poor school performance, schizophrenia, and young adult diabetes. Some authors propose strategies to reduce late preterm birth, or to improve neonatal outcome: use of antenatal corticosteroids, changes in some of the guidelines for early delivery in high-risk pregnancies, and changes in neonatal care for this group.
Conclusionsnumerous studies show greater mortality and morbidity in late preterm infants compared with term infants, in addition to long-term disorders. More recent studies evaluated strategies to improve the outcomes of these neonates. Further studies on these strategies are needed.
revisar a literatura sobre prematuridade tardia (nascimentos de 34 semanas a 36 semanas e seis dias) em seus vários aspectos.
Fonte dos dadosbuscas nas bases MEDLINE, LILACS e Biblioteca Cochrane, sem limite de tempo, e nas referências bibliográficas dos artigos encontrados.
Síntese dos dadosmuitos estudos mostram aumento na taxa de prematuridade tardia nos últimos anos. Em todas as séries, os prematuros tardios correspondem à maioria dos nascimentos prematuros. Estudos envolvendo análises de milhões de nascimentos comprovam a forte associação entre prematuridade tardia e mortalidade neonatal. Também se observou associação com maior mortalidade infantil e no adulto jovem. Muitos estudos encontraram associação com várias complicações neonatais e com problemas e sequelas de longo prazo, tais como: dificuldades na amamentação, paralisia cerebral, asma na infância, pior desempenho escolar, esquizofrenia e diabetes no adulto jovem. Alguns autores propõem estratégias para reduzir a incidência desses nascimentos ou para melhorar seus resultados: utilização de corticosteroides antenatais; mudança em rotinas de interrupção de gestações de alto risco; mudanças nos cuidados neonatais.
Conclusõesmuitos estudos mostram maior morbidade e mortalidade nos prematuros tardios comparados aos recém-nascidos a termo, além de sequelas e complicações de longo prazo. Estudos mais recentes avaliam estratégias para melhorar o prognóstico destes recém-nascidos. Novos estudos com este objetivo são bem-vindos.
Preterm birth, defined as birth before 37 full weeks, remains the leading cause of death and complications in the neonatal period and a major cause of these outcomes in childhood. However, clinical investigations have traditionally focused on premature infants born at a gestational age (GA) of 32 weeks or less, which are obviously at greatest risk. Only recently preterm infants with GA > 33 or 34 weeks have been evaluated more carefully. In practice, newborns with GA of 34 to 36 weeks and six days tend to be considered, both by obstetricians and neonatologists, as having a very similar risk to those born at term.
This attitude is reflected in the obstetrician's routine, for instance, regarding the greater tolerance toward interrupting the pregnancy when there are maternal and/or fetal complications from 34 weeks on,1 as well as in the neonatologist's routine, regarding the tendency to keep these newborns in low-risk nurseries or rooming-in care2 and provide early discharge.3 These practices are due, at least in part, to results of studies by Goldenberg et al.4 and by De Palma et al.5 These authors evaluated the gain for each additional week of gestation between 22 and 37 weeks in increased survival and decreased risk of complications and/or sequelae. They observed that the benefits become less important and more difficult to detect from 33 to 34 weeks on. However, these authors did not compare these results with those of children born at term.
More recent studies have shown, however, that despite having a lower risk than premature infants with lower GA, preterm infants born between 34 and 36 weeks have a much higher risk of death and complications than those born at term. Moreover, as the number of births at this GA is greater than at younger ages, the absolute number of deaths and complications may also be higher. The concern regarding these findings led the National Institute of Child Health and Human Development of the United States to organize a working group to study this theme.6 At this meeting, it was decided that infants born at 34 to 36 weeks and six days of GA would be called late preterm infants.
ObjectivesThis review aims to investigate studies about late prematurity, regardless of the issues addressed, also including studies that evaluated strategies to reduce the incidence and the unfavorable outcomes caused by this condition.
MethodsSearches were performed until December of 2012 in the following databases using the keywords listed below:
MEDLINE – late preterm mortality, late preterm morbidity, late prematurity mortality, late prematurity morbidity, late preterm (always using the conjunction “and”).
LILACS – prematuridade tardia mortalidade, prematuridade tardia morbidade, prematuro tardio mortalidade, prematuro tardio morbidade, prematuro tardio, prematuridade tardia, as well as those in English used in MEDLINE (always using the conjunction “and”).
Cochrane Library: late and preterm. The references of the articles retrieved were also searched.
To choose the studies for discussion, preference was given to studies with the following characteristics:
- a)
meta-analysis studies;
- b)
studies that excluded malformations and made adjustments for confounding variables;
- c)
studies with larger sample sizes;
- d)
studies addressing specific aspects of the subject, not evaluated by others, including review articles with this characteristic;
- e)
studies conducted in Brazil;
Case series studies, i.e., non-analytical studies, as well as studies in languages other than English, Portuguese, or Spanish were excluded.
ResultsA total of 307 articles addressing the topic were retrieved, with 213 original studies (206 in MEDLINE and seven in LILACS) and 94 review articles, letters, or editorials (all in MEDLINE). The results of the 65 selected studies are shown below, grouped into topics.
a) General aspects: There were no articles published before 2000, and most were published after 2005.
There were few Brazilian studies. Almeida et al.7 assessed neonatal resuscitation in several regions of Brazil and observed the need for resuscitative measures in 43.5% of late preterm infants (LPTIs). Ortigosa et al.8 compared the group of LPTI with restricted growth, who were born by scheduled cesarean section, indicated by maternal and/or fetal diseases, with another group of preterm infants with normal growth, without risk factors and born after spontaneous labor. A higher rate of complications was found in the first group, with the following means: phototherapy (5.78 versus 3.19 days, p = 0.005), admission to neonatal intensive care unit (NICU) (5.92 versus 1.28 days; p < 0.0001), hospital length of stay (16.36 versus 4.58 days, p = 0.0001), hypoglycemia (24% versus 6%, p = 0.047), and intraventricular hemorrhage (12% versus 0%, p = 0.037). They concluded that preterm infants with restricted growth are at higher risk than those without this condition. The authors, however, did not control for confounding variables.
Barros et al.9 found a higher frequency of abnormal results in a neurobehavioral examination performed in the first 24 to 72hours of life. Kao et al.10 studied aspects of LPTI behavior related to the capacity to breastfeed, observing a significantly lower capacity when compared to infants born at term. Santos et al.,11 studying a cohort of children born in 2004 in the city of Pelotas, state of Rio Grande do Sul, Brazil, performed two studies.
Porto et al.12 performed a clinical trial to evaluate the effect of antenatal corticosteroid use on LPTI. Araújo et al.13 studied mortality and morbidity. These latter four studies are described elsewhere in this text. The remaining studies were conducted in other countries.
Teune et al.14 carried out the only meta-analysis found in this review, encompassing more than 29 million births. Their results are discussed in subsequent sections of this article. Suzuki et al.15 found no difference in neonatal outcome in LPTI resulting from dichorionic twin pregnancies compared with singleton pregnancies. Refuerzo et al.16 studied neonatal complications in multiple pregnancies only, comparing LPTI with those born at term. They studied an outcome consisting of one or more of the following events: neonatal death, hyaline membrane disease, sepsis, necrotizing enterocolitis, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, retinopathy, and pneumonia. They found a relative risk (RR) of 24.9 (95% CI: 4.8-732.2) for LPTI.
A traditional concept in obstetrics is that once lung maturity is detected by tests performed in the amniotic fluid, the possibility of significant problems in the newborn is unlikely. Some recent studies, however, have relativized this concept. Kamath et al.17 showed a higher frequency of supplemental oxygen with an odds ratio (OR) of 19.14 (95% CI: 1.62-226), phototherapy (OR: 6.67; 95% CI: 1.52-29), and hypoglycemia (OR: 3.95; 95% CI: 1.76 to 8.85) in LPTI infants with confirmatory lung maturity tests, in comparison to those born at term. The authors evaluated three different tests: lecithin/sphingomyelin, phosphatidylglycerol, and lamellar body count, and the assumed maturity criterion was concomitant positivity in the three tests.
Bates et al.18 compared 459 newborns with 36 to 38 weeks and six days of GA, all with positive maturity tests (lecithin/sphingomyelin ratio ≥ 2 and ≥ 3.5 for diabetic mothers), with 13,339 newborns with 39 or 40 weeks of GA whose mothers were not submitted to these tests. They studied a composite outcome consisting of neonatal death, respiratory morbidity, hypoglycemia, jaundice requiring treatment, seizures, necrotizing enterocolitis, hypoxic-ischemic encephalopathy, periventricular leukomalacia, and sepsis, and observed an adjusted OR of 1.7 (95% CI: 1.1-3.5) for pregnancies lasting less than 39 weeks. For hyaline membrane, they found an OR of 7.6 (95% CI: 2.2-26.6).
Tennant et al.19 assessed the frequency of hyaline membrane and transient tachypnea from 34 to 39 weeks using a sequence of lung maturity tests. At their institution, in the event of a negative or inconclusive result (surfactant/albumin ratio), a second test is conducted, which can be phosphatidylglycerol or lecithin/sphingomyelin ratio. Respiratory complications were observed in 38.9% cases in which lung maturity was confirmed by the lecithin/sphingomyelin ratio as the second test. These studies demonstrate that laboratory confirmation of lung maturity does not guarantee the absence of respiratory problems, and that the immaturity of other systems can also cause significant neonatal complications.
Lisinkova et al.20 performed a large, ecological-type study including national data for the year 2004 in the United States, Canada, and 26 European countries. They showed that in countries where the rate of LPTI was higher, there was a lower rate of stillbirths and neonatal deaths. They observed that for a 1% increase in the rate of births between 32 and 37 weeks, there was decrease in intrauterine deaths over 32 weeks, measured by an adjusted OR of 0.94 (95% CI: 0.92-0.96). The same increase was associated with a reduction in neonatal deaths over 32 weeks (adjusted OR: 0.88, 95% CI: 0.85-0.91), of intrauterine deaths at 37 or more weeks (adjusted OR: 0.88, 95% CI: 0.85-0.91), and neonatal deaths at 37 or more weeks (adjusted OR: 0.82, 95% CI: 0.78-0.86). The authors’ argument was that the births resulting from medical interruption in this range (32 to 37 weeks) are usually beneficial, because they were generally performed in fetuses or newborns who would have otherwise died.
Therefore, despite the large number of studies demonstrating the greater risks of late preterm births when compared to full term births, they argue that children born after pregnancy interruption at this stage could not be compared to those born at full term, as they would be, both in utero and in neonatal life, at greater risk, and that the intervention would have a protective role. There would be an indication bias, and an ecological study would be an alternative to overcome this bias.21
Similarly, Joseph et al.22 argued that the evaluation of neonatal outcome of pregnancy interruptions should be performed within the specific risk group. In that study, they present U.S. national data comparing the years 1996-1997 with 2004-2005, in the population of children born to women with hypertension. There was an increase in births at 34 to 36 weeks, which was concomitant with a decrease in neonatal mortality at this same range. They also showed data from other countries, disclosing similar results. Therefore, they argued that increases in interruptions in this range that have been reported recently have been generally beneficial. Their arguments, similar to those of Lisinkova et al.,20 are based on ecological analysis, that is, analysis comparing populations, rather than individuals.
- b)
Frequency and temporal trend: the frequency of LPTI in relation to total births depends on the type of institution where the study is performed, and it is higher in tertiary care centers. In all series, however, LPTI correspond to the majority of preterm infants. Furzán and Sanchez,23 McIntire et al.,24 and Guasch et al.25 observed that, of the population of premature infants, 63.2%, 76%, and 79% were LPTI, respectively. Carter et al.26 found a prevalence of 9% of preterm births for the entire United States from 2000 to 2006, and the LPTI accounted for the vast majority of preterm infants.
Several reports indicate an increase in the LPTI over time. Davidoff et al.27 showed a change in the mean GA at birth from 40 weeks in 1992 to 39 in 2002, among all spontaneous deliveries the U.S., and this was attributed to the increase in the rate of LPTI. Yoder et al.28 found a 37% increase in the proportion of LPTI at a tertiary center in the U.S., from 1990-1998.
Part of this increase was due to the rise in multiple pregnancies resulting from in vitro fertilization, but a large part of this increase was probably caused by an increase in medical interruption of pregnancy, and also possibly by other factors that are yet to be well identified.
There are no data on the frequency of LPTI in Brazil, and there is even difficulty to accurately estimate the frequency of preterm infants as a whole, due to the low reliability of data on GA.29 A recent review found rates for overall prematurity up to 15% in the South and Southeast (1978-2004) and up to 10.2% in the Northeast (1984-1998), but failed to obtain data from the North and Midwest regions.30
- c)
Causal and associated conditions: the causes of spontaneous birth in LPTI probably do not differ very much from causes in lower GAs, including the fact that, in most cases, they are not known. Hiltunen et al.31 demonstrated that when the mother is a carrier of factor V Leiden, the risk of late prematurity increases, but not that of early prematurity. However, this condition is probably associated with a small part of these births. Santos et al.,11 in Brazil, observed an association between late prematurity and maternal age younger than 20 years, with a prevalence ratio (PR) of 1.3 (95% CI: 1.1-1.6), and also with lack of prenatal care, PR 2.4, (95% CI: 1.4-4.2).
An important aspect is to know the proportion of LPTI births resulting from medical interruption of pregnancy and the specific indications for such interruptions. Laughon et al.32 reported 29.8% of cases resulted from spontaneous deliveries, 32.3% from premature rupture of membranes (PROM), 31.8% from medical interruptions, and 6.1% from undocumented causes. Gyamfi-Bannerman et al.33 found 32.3% of births due to medical interruption. In this group, 56.7% of the interruptions were decided due to reasons not based on scientific evidence. Of these, 80.3% had private health insurance versus 59% in the group with well-based interruptions (p < 0.001). Reddy et al.34 reported a 49% rate of spontaneous births (excluding PROM) and 23.2% of interruptions “with no recorded indications”. Holland et al.35 found 36% of spontaneous births (also without PROM) and 17% of interruptions they classified as “potentially avoidable”, including, in the latter group, 8.2% of “elective interruptions”, that is to say, interruptions “with no medical indication”.
In the last two studies, interruptions “with no recorded indications” or “potentially avoidable” were associated with private medical care. In the majority of Brazilian public hospitals there is no pregnancy interruption upon request by the patient or at the physician's convenience, or, at least, there is no provision for these situations. However, there is a high frequency of pregnancy interruptions in the Brazilian private sector, often by cesarean section.36 It is possible that part of these interruptions occur in the range of late preterm infants.
- d)
Neonatal morbidity: Many studies have addressed complications in preterm infants when compared with those born at term. All studies observed higher morbidity in the preterm group regarding one or more aspects, with statistical significance. Most of these studies performed adjustment for confounding variables. Many of them excluded multiple and high risk pregnancies, as well as malformations.
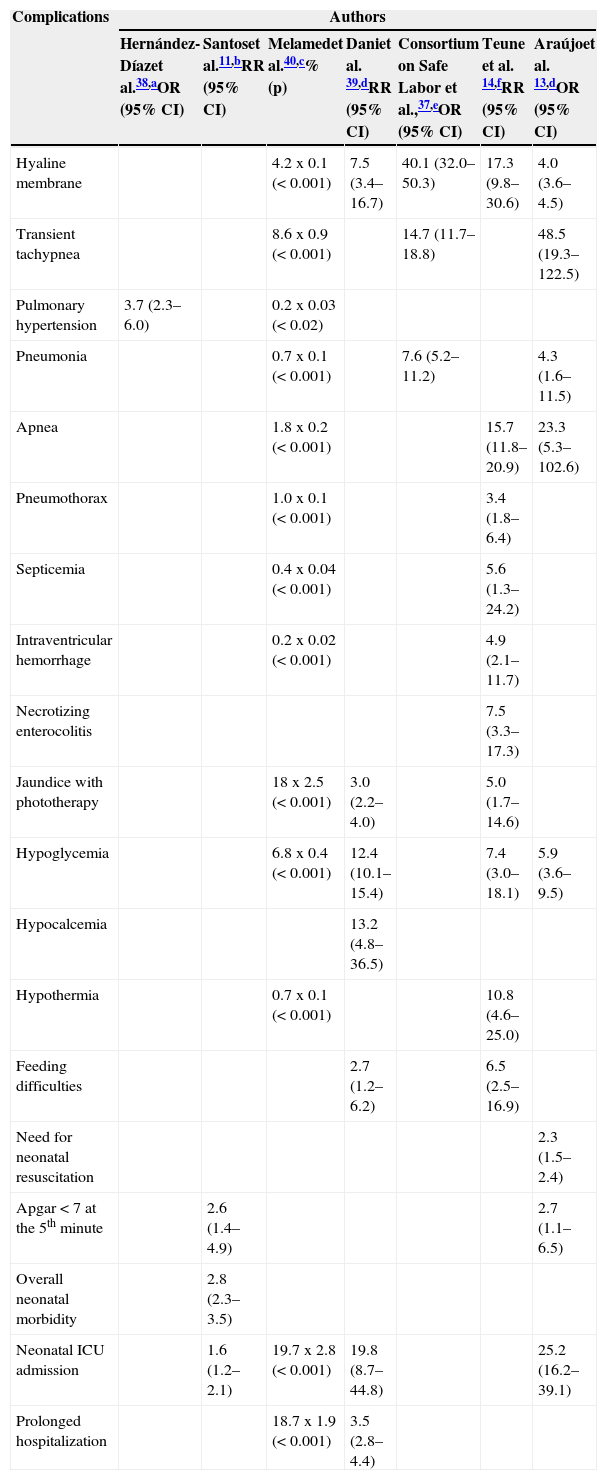
The main morbidity conditions studied were: respiratory, metabolic, sepsis, intraventricular hemorrhage, necrotizing enterocolitis, susceptibility to infection by respiratory syncytial virus (RSV), admission to NICU, and prolonged hospitalization. Table 1 summarizes the findings of the selected studies on morbidity11,13,14,37–40 and describes the most important aspects of their design.
Table 1.Neonatal complications in late preterm infants compared with those born at term: effect or association measures and 95% confidence intervals.
Complications Authors Hernández-Díazet al.38,aOR (95% CI) Santoset al.11,bRR (95% CI) Melamedet al.40,c% (p) Daniet al. 39,dRR (95% CI) Consortium on Safe Labor et al.,37,eOR (95% CI) Teune et al. 14,fRR (95% CI) Araújoet al. 13,dOR (95% CI) Hyaline membrane 4.2 x 0.1 (< 0.001) 7.5 (3.4–16.7) 40.1 (32.0–50.3) 17.3 (9.8–30.6) 4.0 (3.6–4.5) Transient tachypnea 8.6 x 0.9 (< 0.001) 14.7 (11.7–18.8) 48.5 (19.3–122.5) Pulmonary hypertension 3.7 (2.3–6.0) 0.2 x 0.03 (< 0.02) Pneumonia 0.7 x 0.1 (< 0.001) 7.6 (5.2–11.2) 4.3 (1.6–11.5) Apnea 1.8 x 0.2 (< 0.001) 15.7 (11.8–20.9) 23.3 (5.3–102.6) Pneumothorax 1.0 x 0.1 (< 0.001) 3.4 (1.8–6.4) Septicemia 0.4 x 0.04 (< 0.001) 5.6 (1.3–24.2) Intraventricular hemorrhage 0.2 x 0.02 (< 0.001) 4.9 (2.1–11.7) Necrotizing enterocolitis 7.5 (3.3–17.3) Jaundice with phototherapy 18 x 2.5 (< 0.001) 3.0 (2.2–4.0) 5.0 (1.7–14.6) Hypoglycemia 6.8 x 0.4 (< 0.001) 12.4 (10.1–15.4) 7.4 (3.0–18.1) 5.9 (3.6–9.5) Hypocalcemia 13.2 (4.8–36.5) Hypothermia 0.7 x 0.1 (< 0.001) 10.8 (4.6–25.0) Feeding difficulties 2.7 (1.2–6.2) 6.5 (2.5–16.9) Need for neonatal resuscitation 2.3 (1.5–2.4) Apgar < 7 at the 5th minute 2.6 (1.4–4.9) 2.7 (1.1–6.5) Overall neonatal morbidity 2.8 (2.3–3.5) Neonatal ICU admission 1.6 (1.2–2.1) 19.7 x 2.8 (< 0.001) 19.8 (8.7–44.8) 25.2 (16.2–39.1) Prolonged hospitalization 18.7 x 1.9 (< 0.001) 3.5 (2.8–4.4) CI, confidence interval; ICU, intensive care unit; OR, odds ratio; RR, relative risk.
Obs.: all studies shown in the table are observational.
amalformations were excluded, but risk pregnancies or multiple gestations were not excluded; control for confounding variables was performed, including multiple gestations.
bmalformations or risk pregnancies were not excluded, but multiple gestations were excluded and control for confounding variables was performed.
cmalformations and risk pregnancies, multiple gestations, several indications for cesarean section and all forceps births were excluded; control for confounding variables was performed.
dmalformations, risk pregnancies or multiple gestations were not excluded and control for confounding variables was not performed.
Boyce et al., 41 observed a rate of hospitalization for RSV infection in the first year of life of 66:1,000 for children between 29 and 32 weeks, 57:1,000 for those between 33 and 37 weeks, and 30:1,000 for those born at term. Horn et al.42 studied the evolution during hospitalization for RSV infection in the first year of life, stratified by GA at birth. They found an increased frequency of orotracheal intubation (p = 0.002) and longer duration of hospitalization (p < 0.0001) in infants born between 33 and 35 weeks versus 36 or more.
- e)
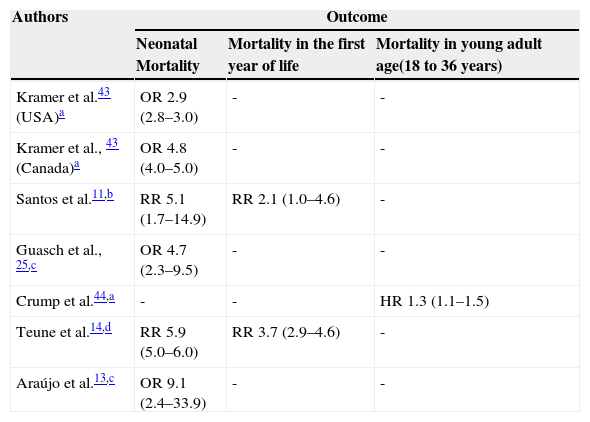
Mortality: all studies that addressed this topic observed higher neonatal and infant mortality in LPTI when compared to those born at term. The studies by Guasch et al.,25 Teune et al.,14 Kramer et al.,43 Crump et al.,44 Santos et al.,11 and Araújo et al.13 are summarized in Table 2, which also summarizes the most important aspects of their design. Crump et al.44 demonstrated that this higher mortality is maintained in the first five years of life, disappears during childhood and adolescence, and reappears in young adults.
Table 2.Mortality among late preterm infants when compared with infants born at term: effect measures and 95% confidence intervals.
Authors Outcome Neonatal Mortality Mortality in the first year of life Mortality in young adult age(18 to 36 years) Kramer et al.43 (USA)a OR 2.9 (2.8–3.0) - - Kramer et al., 43 (Canada)a OR 4.8 (4.0–5.0) - - Santos et al.11,b RR 5.1 (1.7–14.9) RR 2.1 (1.0–4.6) - Guasch et al., 25,c OR 4.7 (2.3–9.5) - - Crump et al.44,a - - HR 1.3 (1.1–1.5) Teune et al.14,d RR 5.9 (5.0–6.0) RR 3.7 (2.9–4.6) - Araújo et al.13,c OR 9.1 (2.4–33.9) - - HR, hazard ratio; OR, odds ratio; RR, relative risk.
Obs.: all studies shown in the table are observational.
amalformations or risk pregnancies were not excluded, but multiple gestations were excluded; control for confounding variables was performed, including malformations.
bmalformations or risk pregnancies were not excluded, but multiple gestations were excluded; control for confounding variables was performed.
Pulver et al.45 compared neonatal and infant mortality in LPTI versus those born at term (39 to 42 weeks), considering the weight/GA ratio. After exclusion of congenital diseases, they observed a RR of 14.2 (95% CI: 4.1-49.1) for neonatal death in female LPTIs who were small for GA when compared to full-term female infants appropriate for GA. The LPTIs that were appropriate for GA also had high risk in the same comparison (RR: 4.1; 95% CI: 1.7-9.6). They found similar results for infant mortality. They concluded that LPTIs that are small for GA constitute a group with particularly increased risk for neonatal and infant death. McIntire et al.24 compared the neonatal mortality of 34 to 42 weeks of GA, using mortality at 39 weeks as reference, which was the lowest in the study population. They found significantly higher mortality that had a trend of decrease compared to that of the reference up to the 37 week. These authors included only low-risk pregnancies, excluded malformations, and performed adjustments for confounding variables.
- f)
Long-term effects: Raby et al.46 studied a cohort in Boston, USA. They compared children born between 36 and 38.5 weeks who had no malformations, were not admitted to NICU, and had no respiratory infection in the first year of life, with controls born between 38.6 and 40 weeks. At 6 years, the first group had a higher risk of asthma, compared to the second (adjusted OR: 5.6; 95% CI: 2.5-12.38). Woythaler et al.47 observed a higher frequency of delayed mental development at 2 years (OR: 1.52; 95%CI: 1.26-1.82) and delayed psychomotor development (OR 1.56; 95% CI: 1.30-1.89) in LPTIs compared to infants born at term.
In Brazil, Santos et al.48 showed higher frequency of inadequate growth at 2 years (OR: 2.30; 95% CI: 1.40-3.77). Peacock et al.49 studied performance in regular preschool tests, comparing infants born between 32 and 37 weeks to full-term infants. They found a lower frequency of good performance among preterm infants (adjusted OR: 0.74; 95% CI: 0.59-0.92). Teune et al.14 found greater risk for cerebral palsy (RR 3.1; 95% CI: 2.3-4.2) and mental retardation (RR: 1.5; 95% CI: 1.2-1.9). Moster et al.50 reported an increased risk of schizophrenia (RR: 1.4; 95% CI: 1.1-1.8) and a lower proportion of young individuals who finished college/university (OR: 0.87; 95% CI: 0.84-0.89). Teune et al.14 found a lower chance of finishing high school (RR: 0.96; 95% CI: 0.95-0.97). Crump et al.,51 in a study of nearly 650,000 newborns in Sweden, found an association between prematurity and diabetes in young adults (25 to 37 years), including those born from 35 to 36 weeks. For this range, they found a risk for diabetes of any type (adjusted OR: 1.18; 95% CI: 1.04-1.33) and type I diabetes (adjusted OR: 1.22; 95% CI: 1.05-1.42).
- g)
Re-hospitalizations and costs: Bird et al.52 found higher costs for the healthcare system for LPTIs in the first year of life. The mean difference (adjusted values) was U$ 108 for outpatient care (95% CI: 58-158) and U$ 597 for hospital care (95% CI: 528-666) for each child, when compared with infants born at 37 to 42 weeks. McIntire et al.24 found mean hospital costs of US$ 6,094 for each neonate at 34 weeks and US$ 2,019 for children born at 36 weeks, versus US$ 1,258 for those born at 39 weeks (p < 0.001). Jain et al.53 reported that 17.7% of emergency room visits in the first 31 days of life consisted of LPTI, when compared with the national mean of 8.8% in the United States for births at this GA. Escobar et al.54 found more re-hospitalizations between 15 and 182 days in neonates born at 36 weeks when compared with those born at 38 to 40 weeks (RR: 1.67; 95% CI: 1.23-2.25).
Murthy et al.,55 in Illinois, United States, observed a historical increase in the frequency of labor inductions between 34 and 37 weeks: 5.4:1,000 in 1991 to 15.2:1,000 in 2003 (p < 0.001). They also observed an increase in legal suits for medical malpractice, which they considered to be associated with increased induction (p = 0.004).
- h)
Breastfeeding: a higher frequency of difficulties in LPTI breastfeeding has been documented, such as early weaning, poor growth, and dehydration, as well as breastfeeding jaundice.56
- i)
Strategies to address the problem: the relevance of the increased risks for LPTI, as demonstrated above, is widely documented and studies have been performed to evaluate possible strategies to address the matter.
The following should be considered:
- 1)
Attempted tocolysis: Most obstetrics services perform tocolysis up to 33 weeks and six days of gestation.57 There is evidence of little benefit in neonatal outcomes with tocolysis.58 Part of this benefit is attributed to the extra time of gestation obtained for the action of corticosteroids given to the mother to accelerate fetal lung maturity. It is unclear, however, whether these benefits extend to the age range of the LPTI. It is possible that the non-performance of tocolysis after 34 weeks is partly due to the fact that corticosteroids are usually not used during this period. New studies on tocolysis in this group are needed.
- 2)
Review pregnancy interruption routines: the decision to perform the delivery before spontaneous labor onset, either by labor induction or cesarean section, is a frequent situation in obstetrics. For each condition, the physician attempts to assess the risk of maintaining the pregnancy versus the risks associated with induction and with cesarean section, when induction fails or when it is contraindicated. Moreover, and more importantly, when it is decided to interrupt the pregnancy before term, the maternal and/or fetal risk of maintaining the pregnancy versus the risk of prematurity is evaluated. This assessment is complex and is subject to errors, as any risk assessment. The knowledge that the risks of birth between 34 and 37 weeks are higher than previously understood could change the traditionally standardized conduct in some clinical situations, such as:
- •
Preterm premature rupture of membranes (PPROM): it is quite common in the obstetrics services to interrupt pregnancy in the presence of PPROM from 34 weeks on, with or without lung maturity assessment.59 Recent studies, however, have questioned this practice. A Cochrane Library review60 focused on the expectant management versus interruption in pregnancies lasting less than 37 weeks. They found no significant differences in both the neonatal and maternal outcomes. Mateus et al.61 observed a higher frequency of hyaline membrane disease in women with PPROM who had the pregnancy interrupted at 34 weeks, when compared to those interrupted at 35 weeks (RR: 3.4; 95% CI: 1.5-7.7) and 36 weeks (RR: 8.6; 95% CI: 2.7-27.5). They also observed significant differences in the same comparisons for NICU admission and length of hospitalization. The interruptions were performed electively, i.e., in the absence of infection or other events rather than PPROM. There were no cases of sepsis in this series of 192 cases.
A recent clinical trial:62 PPROM Expectant Management versus Induction of Labor (PPROMEXIL) of 536 pregnant women between 34 and 37 weeks of GA compared interruption by induction or cesarean section (due to obstetric indications) 24hours after the rupture versus expectant management while monitoring for infection, up to 37 weeks of GA. They found no significant differences for neonatal sepsis. However, a higher incidence of hypoglycemia (RR: 2.16; 95% CI: 1.36-3.43) and jaundice (RR: 1.47; 95% CI: 1.13-1.90) was observed in the interruption group. An extension of this study63 with 200 pregnant women showed similar results, even when the data were analyzed together with those from the previous study.
There is currently another ongoing clinical trial64 with the same goal: Preterm Prelabour Rupture Of the Membranes close to Term (PPROMT), comprising approximately 1,800 pregnant women. The authors of the PPROMEXIL62 study believe that, even if the PPROMPT study or a meta-analysis including this and other clinical trials show a significant difference in relation to neonatal sepsis, this difference would not be clinically relevant, as the incidence of this complication was very small (1.1% in the expectant group versus 0.4% in the interruption group in the PPROMEXIL study). The authors attribute this low incidence to the most frequent contemporary use of prophylactic antibiotics in cases of PPROM. They also attribute to this use the difference in the results of current studies when compared to previous studies performed with the same objective.
- •
Restricted fetal growth and oligohydramnios: Galan65 proposes for cases of restricted fetal growth, when there is no change in the assessments of fetal well-being or other changes that justify early interruption, that the latter take place between 37 and 38 weeks. This author proposes the same conduct in cases of isolated oligohydramnios without PPROM. Baschat66 performed an extensive review of restricted fetal growth. He emphasizes that currently, two types of presentation of this condition can be identified: one of early onset, detectable before 34 weeks, and another that is detected after this age. According to this author, in cases of late onset, neurological and cognitive sequelae detected in the long term are associated mainly with compensatory circulatory changes of hypoxemia, usually detected by Doppler velocimetry, especially in the middle cerebral artery. It can then be assumed that, in the absence of these changes, under careful surveillance, it would be acceptable to wait until the pregnancy aproaches or reaches term.
- •
Diabetes in pregnancy: Catalano and Sacks67 state that the best time to interrupt the pregnancy in several clinical diabetes situations remains a controversial matter and that clinical trials are needed in order to define these practices on a more robust basis. Vignoles et al.68 studied cases of severe respiratory failure in newborn infants of diabetic mothers who delivered after 34 weeks. In the multivariate analysis, they found prematurity (OR: 6.13; 95% CI: 1.8-21.2) and gestational diabetes (OR: 11.55; 95% CI: 3.9-33.9) as independent risk factors for this complication.
- •
Preeclampsia: there is a reasonable consensus that it is not advisable to wait for the end of pregnancy in the presence of preeclampsia. It remains unclear, however, what is the best time to interrupt the pregnancy in cases with mild preeclampsia. It is necessary to assess the risk of maternal and fetal worsening versus the risks of prematurity. There is an ongoing clinical trial comparing outcomes of expectant management versus interruption in LPTI.69
- 3)
Consider the use of corticosteroids: There is evidence of benefits regarding the use of corticosteroids for induction of fetal lung maturity up to 34 weeks, with no clear benefits above this age. Similarly to tocolysis, it is common practice to restrict the use of corticosteroids up to 34 weeks.70
Some observational studies suggest benefits of this practice in LPTI, with reduced respiratory morbidity71 (OR: 0.39) and overall morbidity72 (OR: 0.24). A clinical trial performed in Turkey showed decreased hyaline membrane disease and need for neonatal resuscitation.73 A clinical trial in Brazil showed no benefits for respiratory morbidity, but observed a lower proportion, although not significant, of overall morbidity in cases who received corticosteroids: 62% in treated cases versus 72% in the control group (p = 0.08).12
Another clinical trial observed fewer NICU admissions in neonates born at term from cesarean sections without labor and whose mothers received this medication.74 Further clinical trials are needed for this purpose.
- 4)
Changes in neonatal routines: for instance, more restrictiveness regarding the choice of rooming-in or low risk nursery,2 as well as in early discharge.3 Based on their study of neonates with kernicterus, Bhutani & Johnson75 emphasize that a large number of preterm infants who developed this complication were discharged early, and in most cases, the risk factors for this complication were not taken into account, among these, prematurity.
Many studies show higher mortality and higher frequency of several complications in preterm infants when compared to full-term infants. This difference is statistically significant and clinically relevant in most of the comparisons. It is noteworthy that some studies observed an association not only with death and neonatal problems, but also with diseases and sequelae that manifest in the long-term. The argument that these associations are confounded by the higher frequency of conditions found in LPTI that worsen the prognosis themselves, such as maternal illnesses, PPROM, malformations, etc., is weak, as most studies excluded or performed adjustments for these conditions.
The findings of the studies by Goldenberg et al.4 and De Palma et al.5 are probably valid. Their particularity is that comparisons were made only within the group of premature infants. When comparing the preterm neonates with infants born at term, which was performed in more recent studies, it was observed that the former have a risk of death and complications that is higher and great for contemporary standards.
It appears that the need for confirmation of the consistency and magnitude of these associations (late preterm birth and unwanted outcomes) has lessened, and that it is necessary to shift resources to the evaluation of the proposed strategies for addressing this problem. As discussed above, both clinical trials and observational studies are needed with this group of patients, covering aspects such as use of antenatal corticosteroids, attempted tocolysis, and reassessment of routines for the interruption of high-risk pregnancies. Proposals for increased neonatal surveillance for these infants are also expected.
The recently published studies by Lisinkova et al.20 and Joseph et al.22 may be the subject of considerable controversy. However, the points of view presented by the authors would apply primarily to deliveries resulting from medical interruption. The increased risk of preterm infants compared to those born at term, however, is not limited to high-risk pregnancies or medical interruptions. Many of the studies included only low-risk pregnancies,24,40 and even these showed a major association with complications and deaths. Furthermore, in most series, the majority of late preterm newborns were the result of spontaneous deliveries.
Of the abovementioned strategies to address the issue of late preterm birth, only the revaluation of medical interruption could be questioned, if these authors’ arguments are considered. However, even the arguments on interruption can be questioned. The limitations of ecological studies are well known,76 as they compare different populations and, therefore, the analyses are not performed on an individual basis. It is uncertain, for instance, whether the children who did not die during intrauterine life or in the neonatal period in populations with higher rates of late preterm birth are precisely those whose pregnancy was interrupted between 32 and 37 weeks.
One possibility that cannot be ignored is that the lower number of stillbirths and neonatal deaths is due to overall better quality of obstetric and neonatal care in these populations, and that the higher availability of maternal and fetal monitoring methods leads, in parallel, to a higher rate of interruption before term; this higher rate would result in a relative worsening of the outcomes.
Another possibility is that the results are due in part to the higher rate of interruptions and, in part, to better overall care; the result attributed by authors to the first component would be then “contaminated” by the performance of the second component. Nevertheless, the proposal of not interrupting any gestation before 37 weeks has never been suggested. The emphasis, taking into account the knowledge added by the studies discussed in this study, is that, when comparing the risks of maintaining the pregnancy with those of prematurity, pregnancies between 34 and 37 weeks should not be considered as “virtually at term” (and consequently, that there is no benefit in extending them), and each clinical case should be analyzed on an individual basis.
A modality of late preterm birth that is probably important in Brazil, although it also occurs in other countries, is that resulting from personal (whether from the patient or physician), non-medical reasons, which lead to pregnancy interruption. It is possible that this type of interruption occurs more often at the full 37 weeks, which is, by definition, a term pregnancy, but still with higher morbidity and mortality when compared with 39 weeks.24
As discussed33–36 in the “causal and associated conditions” section, this type of situation occurs more often in the private healthcare sector. It is difficult to estimate the exact frequency of this type of interruption, as it is common for the motivation not to be explicitly documented, but rather justified under other diagnoses or indications. Similarly, a policy of hospitals to reduce this practice would have limited results; first, due to the difficulty of identifying cases, and second, due to the difficulty of standardization of private medical activity. The authors believe, however, that part of the trend for this practice among some professionals is due to the assumption that it does not have major consequences. It can therefore be expected that the disclosure of the results of more recent studies, such as those discussed above, may change certain practices, at least in part.
Conflict of interestsThe authors declare no conflicts of interest.