This paper aims to review data on the association of obesity and iron deficiency in children and adolescents, exposing the possible involvement of hepcidin and interleukin-6 (IL-6), obesity's inflammation biomarkers.
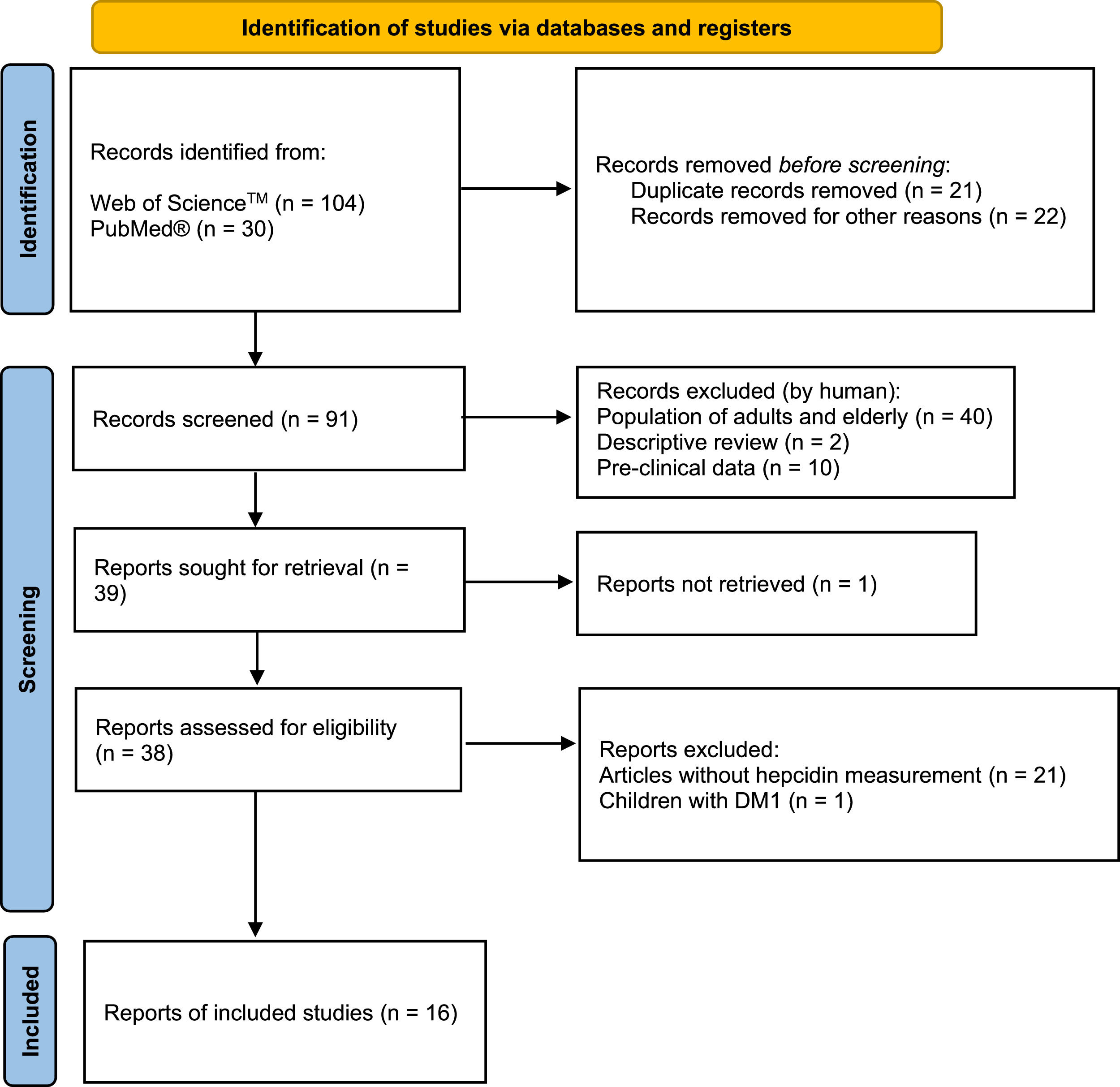
Data sourceArticles from PUBMED and WEB OF SCIENCE database with no chronological limit were reviewed to write this systematic review. Keywords such as children, obesity, iron deficiency, and hepcidin were used. After deleting duplicated and review articles, 91 were screened, and 39 were selected as eligible. Sixteen articles were included because they involved serum hepcidin levels in obese children and adolescents as outcomes.
Summary of findingsFinally, those 16 articles were organized in two tables: one includes therapeutic interventions, and the other does not. As hepcidin was discovered in 2000, the first articles that presented serum hepcidin's quantification in obese children and adolescents, homeostasis iron markers, and their possible association with obesity's inflammatory environment began to be published in 2008.
ConclusionsObesity's chronic inflammation state leads to the production of IL-6, which acts as a signaling molecule for hepcidin synthesis, resulting in iron deficiency, which is common in obese children and adolescents who respond inadequately to iron supplementation. On the other hand, that population responds adequately to therapeutic intervention programs that lead to weight loss, guaranteeing iron homeostasis improvement. Therefore, perhaps it is time to discuss serum hepcidin level quantification as part of evaluating children and adolescents with iron deficiency, which could guide clinical choices that might lead to better therapeutic outcomes.
The prevalence of obesity in children worldwide has risen. The World Health Organization data indicate that, in 2016, 18% of children and adolescents aged 5-19 were overweight or obese, while the data collected in 1975 showed that this percentage was 4%.1 Obesity is a chronic disease and one of the world's most significant health problems leading to risk factors for premature death. Iron deficiency (ID) is a coexisting condition with obesity in children and adolescents, and controlling it is also a global health priority.2 Childhood ID, even in the absence of anemia, has a negative impact on cognition, behavior, and motor skills that can persist for a long time. Children with ID have lower scores on language, motor scales, and environmental sound perception than children with normal iron nutritional status.3 This article aims to review evidence on the link between obesity and iron deficiency in children and adolescents. Thus, data from the scientific literature were interpreted, evaluating the role of obesity-associated inflammation and excessive hepcidin production.
MethodsThis systematic review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines.4 The literature research was conducted in the following online bibliographic databases: PUBMED and WEB OF SCIENCE. No chronological limit was used. The search terms consisted of “children,” “obesity,” “iron deficiency,” and “hepcidin.” After deleting duplicated and review articles, 93 articles were manually screened. Considering PICOS criteria, this review includes articles with: children and adolescents diagnosed with obesity as population (P); intervention (I) was not an inclusion criterion; lean or metabolic healthy children and/or adolescents as a comparison ©; and measurement of serum hepcidin as an outcome (O). Only original full-length articles were included in this systematic review (S) (Figure 1). Articles exclusion criteria also followed PICOS criteria: a) Population of adults and elderly, b) Pre-clinical data, c) Letter and author's reply, d) Articles without hepcidin measurement, and e) Non-obese children.
Contextualization and resultsIron homeostasisIron is one of the main nutrients in the human body, necessary to produce hemoglobin and synthesized compounds responsible for intracellular oxidative processes, among other functions. The total amount of iron in the body is 4 to 5 grams, most of which is in the form of hemoglobin or stored in the form of ferritin; therefore, ID's main consequence is the development of iron deficiency anemia (IDA).5,6
The iron supply for body use is done from the intestinal absorption of inorganic or heme form from dietary sources and by recycling erythrocytes by macrophages. The small intestine is capable of absorbing the inorganic iron for luminal uptake done by the divalent metal transport protein (DMT-1) that requires the conversion of ferric iron (Fe+3) to ferrous iron (Fe+2). In contrast, iron from the heme group is internalized to the cytoplasm of the enterocytes by the heme-1 transport protein (HCP1). The iron absorbed in heme form is released from protoporphyrin by heme oxygenase. It will be part of the pool of non-heme iron, stored in ferritin or released into the blood. Iron is released into the bloodstream by ferroportin, a protein that, like DMT-1, is selective for Fe+2. After reaching circulation, iron must be transported to the tissues by transferrin; however, this protein is selective for Fe+3, and the iron exported by ferroportin needs to be oxidized by the enzyme hephaestin.5 Aside from dietary absorption, the most crucial iron source is the recycling of erythrocytes carried out by the spleen and bone marrow macrophages and the liver's Kupffer cells. After red blood cell lysis, the phagocytic cells release iron from hemoglobin to blood to be transported by transferrin to the bone marrow to synthesize new red blood cells or to the liver and other storage tissues.5,6
Obesity, inflammation, and iron deficiencySeltzer and Mayer raised the first hypothesis for obesity and ID association in 1963, suggesting that it was due to the deficient iron intake by obese children due to an unbalanced diet, rich in carbohydrates and fats, poor in essential nutrients like iron.7 However, this hypothesis was refuted by Menzie and collaborators (2008), that confirmed the non-association between low dietary iron intake and ID in obese children.8 Additionally, a study by Aerbeli and colleagues (2009) showed that obese pediatric patients had the same bioavailability of iron as lean patients, and the heme iron consumption was regular or even increased in the obese patients.9
Therefore, a new hypothesis that ID in obese children could be related to inflammation caused by obesity was postulated. It is currently known that in obesity, adipose tissue expansion is associated with the establishment of local inflammation. In most obese individuals, adipose tissue initiates the production of inflammatory adipokines to the detriment of anti-inflammatory adipokines production.10 Interleukin (IL)-6, IL-1, IL-8, and tumor necrosis factor (TNF)-α production is performed mainly by macrophages infiltrated in adipose tissue, while adipocytes contribute to leptin production, for example. IL-6 and TNF-α contribute significantly to the production of acute-phase liver proteins such as C-Reactive Protein (CRP), α-1 acid glycoprotein (AGP), ferritin, and hepcidin.11 The unbalanced adipokines production by adipose tissue results in a chronic and low-grade inflammatory process contributing to the establishment of comorbidities associated with obesity.11
Hepcidin is a peptide hormone produced mainly in hepatocytes. However, it has recently been discovered that neutrophils, monocytes, macrophages, lymphocytes, adipocytes, pancreatic beta cells, and renal cells also produce hepcidin.12,13 The most discussed hepcidin function is its ability to regulate iron homeostasis. An increase in hepcidin expression results in a decrease in serum iron concentration, while a reduction in its synthesis increases the concentration of iron in the bloodstream.12 The mechanism of action of hepcidin occurs by binding to ferroportin, preventing the release of iron to plasma and promoting an iron trapping inside the cells that contain membrane ferroportin, such as macrophages, enterocytes, hepatocytes, and placental syncytiotrophoblasts. In addition, hepcidin can also inhibit the DMT-1 expression in enterocytes, reducing inorganic iron absorption in the intestinal lumen. Several physiological and pathological factors regulate hepcidin synthesis. A state of iron sufficiency acts on hepatocytes inducing hepcidin production to avoid excess of this nutrient in the blood, while the decrease in plasma iron inhibits hepcidin synthesis. Hypoxia signaling inhibits hepcidin synthesis since iron availability is essential for hemoglobin synthesis. The inflammatory process is an important inducer of hepcidin expression, and IL-6 is the main inflammatory cytokine pointed in this process. Therefore, the chronic low-grade inflammation associated with obesity induces hepcidin synthesis and explains hypoferremia.12
Hepcidin is usually detected in biological fluids like plasma and urine by mass spectrometry (MS) and immunochemistry (IC) based measurement procedures. However, researchers demonstrated that hepcidin levels vary with those measurement procedures, exposing the need to standardize their use in patient care.14,15 Efforts are being carried out to establish standard hepcidin reference ranges. This action might help uniformize clinical decisions, which could be useful not only for iron-restrictive anemias such as those associated with inflammatory diseases, neoplasia, and chronic kidney disease but also for iron-overload disorders such as β-thalassemia and hereditary hemochromatosis.16,17 Perhaps, in the near future hepcidin might also be used as a biomarker to diagnose different diseases.
Iron deficiency in childhood: the presence of hepcidin and IL-6Several studies have demonstrated the occurrence of ID in obese children, and more recently, some studies that correlated ID with hepcidin levels and inflammatory markers in these patients were published. The pioneering study was carried out in the 1960s by the Jean Mayer research group.7 In this study, the serum iron levels found in obese boys and girls (70.8 µg/100mL and 75.6 µg/100mL, respectively) were significantly lower than those found in normal-weight boys and girls (96.9 µg/100mL and 89.2 µg/100mL, respectively), demonstrating that the serum iron values are lower in the obese group compared to a normal weight group of children and adolescents.18 Another important study showed that the response to iron supplementation did not produce satisfactory results in children and obese women by evaluating different iron supplementation programs to combat anemia in developing countries.19
After the knowledge about hepcidin's role in controlling iron homeostasis, del Giudice and collaborators (2009) published the results of a study with 60 obese and 50 non-obese children showing a positive link between leptin, IL-6, hepcidin levels in obese children and decreases in serum iron and transferrin saturation levels. The data from this study supported the idea that leptin and IL-6 are important inflammatory mediators that stimulate hepcidin production in obese children and, consequently, result in iron deficiency (Table 1).20 In the same year, Aeberli and collaborators conducted a study with children aged 6-14 years, confirming a higher prevalence of ID in obese children in comparison with normal-weight children (20% of obese individuals had ID, while only 6% of normal-weight had ID) associated with high levels of hepcidin, IL-6, CRP, and leptin.9 They add data about dietary iron intake that did not differ between groups, reinforcing the theory that chronic inflammation induced by obesity, with increased serum hepcidin levels, would be responsible for ID. Subsequent studies also confirmed that dietary iron intake was not reduced in obese children and adolescents (Table 2).21
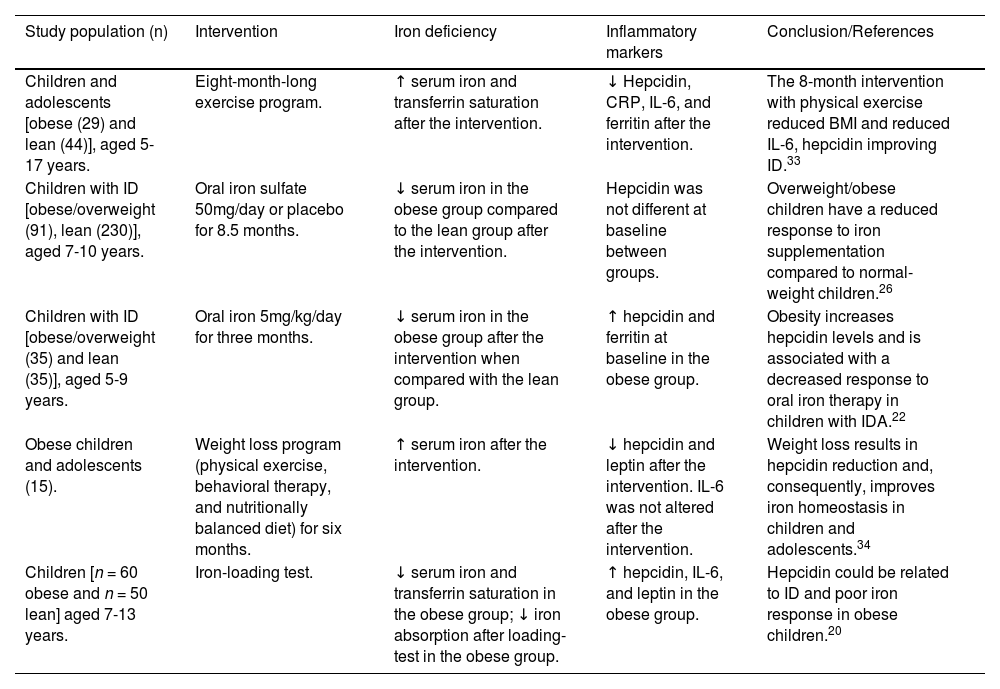
Iron deficiency, inflammation and obesity in children and adolescents. Studies with interventions.
| Study population (n) | Intervention | Iron deficiency | Inflammatory markers | Conclusion/References |
|---|---|---|---|---|
| Children and adolescents [obese (29) and lean (44)], aged 5-17 years. | Eight-month-long exercise program. | ↑ serum iron and transferrin saturation after the intervention. | ↓ Hepcidin, CRP, IL-6, and ferritin after the intervention. | The 8-month intervention with physical exercise reduced BMI and reduced IL-6, hepcidin improving ID.33 |
| Children with ID [obese/overweight (91), lean (230)], aged 7-10 years. | Oral iron sulfate 50mg/day or placebo for 8.5 months. | ↓ serum iron in the obese group compared to the lean group after the intervention. | Hepcidin was not different at baseline between groups. | Overweight/obese children have a reduced response to iron supplementation compared to normal-weight children.26 |
| Children with ID [obese/overweight (35) and lean (35)], aged 5-9 years. | Oral iron 5mg/kg/day for three months. | ↓ serum iron in the obese group after the intervention when compared with the lean group. | ↑ hepcidin and ferritin at baseline in the obese group. | Obesity increases hepcidin levels and is associated with a decreased response to oral iron therapy in children with IDA.22 |
| Obese children and adolescents (15). | Weight loss program (physical exercise, behavioral therapy, and nutritionally balanced diet) for six months. | ↑ serum iron after the intervention. | ↓ hepcidin and leptin after the intervention. IL-6 was not altered after the intervention. | Weight loss results in hepcidin reduction and, consequently, improves iron homeostasis in children and adolescents.34 |
| Children [n = 60 obese and n = 50 lean] aged 7-13 years. | Iron-loading test. | ↓ serum iron and transferrin saturation in the obese group; ↓ iron absorption after loading-test in the obese group. | ↑ hepcidin, IL-6, and leptin in the obese group. | Hepcidin could be related to ID and poor iron response in obese children.20 |
Iron deficiency, inflammation and obesity in children and adolescents. Studies without interventions.
| Study population (n) | Iron deficiency/Dietary iron intake | Inflammatory markers | Conclusion/References |
|---|---|---|---|
| Children and adolescents [obese (63) and lean (27)], aged 5-15 years. | ↑ serum iron and ferritin in the obese group. ↓ transferrin saturation in the obese group. | ↑ hepcidin in the obese group. In a subgroup of obese with ID, there was no difference in hepcidin-25 between obese without ID. | Children and adolescents with obesity have higher ID prevalence and serum hepcidin levels.28 |
| Children and adolescents [obese (50) and lean (60)], aged 6-16 years. | ↓ serum iron and transferrin saturation in the obese group. | ↑ Pro-hepcidin and ferritin in the obese group. | Obese children are at increased risk of iron deficiency.25 |
| Children [obese (29) and lean (29)], aged 6.5–8.7 years. | Serum iron was not different between groups; ↓ ferroportin in the obese group. /↑ Iron and vitamin C in the obese group. | ↑ Leptin, insulin, hepcidin, and CRP in the obese group. | In obese children with sufficient iron intake, the altered ferroportin-hepcidin axis can occur without ID signs. The role of other micronutrients, such as C-vitamin, should be considered in the iron status of these children.21 |
| Obese children and adolescents [type 2 diabetes (10), impaired glucose tolerance (8), and normal glucose tolerance (20)] aged 6–21 years. | sTfR was not different between groups. | Hepcidin and IL-6 were not different between groups; | Metabolic status (diabetes mellito or glucose tolerance/intolerance) did not alter hepcidin or sTfR in obese children and adolescents.31 |
| Children and adolescents [obese (180) and lean (57)], aged 5-18 years. | Serum iron and ferritin were not different between groups. | ↑ Leptin, hepcidin, and CRP in the obese group; IL-6 was not different between groups. | Elevated hepcidin values did not result in ID in obese children and adolescents.29 |
| Adolescents [obese (40) and lean (40)], aged 12-14. | ↓ serum iron in the obese group. | ↑ hepcidin and CRP in the obese group. | ID in obese adolescents may result from reduced absorption and/or increased hepcidin-mediated iron sequestration.24 |
| Children and adolescents [obese (159), overweight (113), and lean (377)], aged 7-15 years. | Serum iron was not different between groups. | ↓ hepcidin and IL-10 in obese/overweight group; ↑ ferritin, IL-1β, and nitric oxide in obese/overweight group. | Decreased circulating IL-10 concentration could protect young overweight/obese adolescents girls against the development of ID.32 |
| Children [obese/overweight (52) and lean (312)], aged 3-6 years. | ↑ ID and ↓ sTfR in the obese/overweight group./ The median daily iron supply was 13.5 mg/day for both groups. | ↑ hepcidin, CRP, leptin, ferritin, and α-1-acid glycoprotein in the obese/overweight group; IL-6 was no different. | The role of obesity-related inflammation in ID should be considered even when the prevalence of overweight/obesity is low, as observed in children from very low-income families.30 |
| Children and adolescents [obese (50 without NAFLD and 30 with NAFLD) and lean (30)], aged 7-18 years. | Serum iron and transferrin saturation were not different between groups. | ↑ hepcidin only in obese with NAFLD. | Obese children with NAFLD are at greater risk of iron deficiency and iron metabolism disorders, and hepcidin level can be used as a diagnostic tool and follow-up marker.27 |
| Children and adolescents [obese (50) and lean (50)], aged 3-16 years. | ↓ serum iron, hemoglobin, transferrin saturation, and sTfR in the obese group. | ↑ hepcidin and ferritin in the obese group. | ID and elevated hepcidin were prevalent in obese children.23 |
| Children and adolescents [obese (85) and lean (33)], aged 7-12 years. | ↓ serum iron and sTfR in the obese group./Dietary iron intake and bioavailability were not different between groups. | ↑ hepcidin, CRP, IL-6, and leptin in the obese group. | The reduction in the iron available for erythropoiesis in overweight children is not associated with a low diet iron supply but with hepcidin-mediated iron disturbance.9 |
Sanad et al. conducted a study with obese and lean children with IDA and three months of oral iron therapy. Iron status (serum iron, ferritin, transferrin, and transferrin saturation) and serum hepcidin levels were measured before and after iron therapy. The serum hepcidin levels found in the obese group were significantly higher than that of the healthy group. In contrast, in the lean group with IDA, the hepcidin levels were lower than that of the healthy group, confirming the link between obesity and increased serum levels of hepcidin. After three months of oral iron therapy, hepcidin levels in the non-obese group with IDA increased significantly, indicating that hepcidin responds to iron changes. But in the obese group with IDA, hepcidin levels did not change after three months of iron supplementation, reinforcing the idea that its production is stimulated by the chronic inflammation resulting from obesity.22 There were studies published by Hamza and collaborators (2013), Nazif and collaborators (2015), and Dogan and collaborators (2020) detailed in Table 2, confirming the connection between hepcidin, obesity, and ID.23-25 However, the study published by Baumgartner and collaborators (2011) with 321 South African children with ID, 28% overweight and obese, found no difference in baseline hepcidin levels. Nonetheless, the authors confirmed that overweight and obese children had reduced responses to oral iron supplementation compared to normal weight.26 The study published by Demircioglu and collaborators (2014) observed that, in obese patients, hemoglobin and iron concentration values were lower compared to the control group values, but the differences were not statistically significant, as well as the serum hepcidin level, which was also higher in the obese group, but with no statistical significance. However, when examined in subgroups of obese patients with non-alcoholic fatty liver disease (NAFLD), they observed statistically significant increased levels of hepcidin in that group.27 In recent research, higher levels of hepcidin were only observed in obese children and adolescents when sub-grouped to obese with ID.28
Studies have also tried to confirm the IL-6 role in increasing hepcidin expression and in ID in obese children. Although reports have shown the association between IL-6 and hepcidin levels in obese children, subsequent studies have not confirmed this relationship by recording high serum levels of hepcidin in obese children compared to lean children, but similar IL-6 serum levels.9,21,29,30 Shalitin and collaborators (2018) analyzed 38 obese children and adolescents divided into a group with type 2 diabetes mellitus (n = 10), a group with glucose intolerance (n = 8), and a group with normal glucose tolerance (n = 20) to investigate the values of IL-6, hepcidin, soluble transferrin receptor, and the presence of obstructive sleep apnea. They found no significant differences between the levels of IL-6 and hepcidin in the groups studied, except for patients with obstructive sleep apnea, a condition characterized by chronic inflammation and increased levels of IL-6.31 Finally, a study by Chang and collaborators (2014) evaluated the participation of other cytokines and suggested a protective role to a combination of high nitric oxide and low IL-10 levels in the establishment of inflammation and ID in obese children.32
To study the effects of physical exercise on hepcidin levels, inflammatory markers, and iron status in obese children and adolescents, Coimbra and collaborators (2017) carried out an analysis with an eight-month intervention. In this context, 73 obese children and adolescents were divided into a group without physical activities (n = 29) and another with physical activities (n = 44). Initially, IL-6 levels correlated positively with hepcidin values and negatively with iron status. However, after the physical activity program intervention, IL-6, hepcidin, C-reactive protein, and soluble transferrin receptor levels decreased in the obese group that performed physical activities, while the iron concentration increased. The authors showed that the reduction of adipose tissue stocks and, consequently, the decrease in the chronic state of inflammation helped control inflammatory markers and increases the concentration of iron in the body.33 This study corroborates a previous report by Amato and collaborators (2010) that demonstrated BMI, leptin, and IL-6 reduction in children and increased iron status after a six-month intervention with physical activity as described in Table 1.34
Of the studies included in this review, ten out of sixteen recorded ID in obese children and adolescents, nine of which found a positive association between obesity, ID, and high levels of hepcidin. Six papers did not record ID in the obese groups and out of those six studies, four reported increased serum hepcidin levels in obese children and adolescents. Of the four studies where hepcidin levels were higher in the obese group and ID was not recorded, lower iron levels without statistical significance,27 a younger control group in comparison to the obese group,21 non-homogeneous distribution between gender and the presence of a small sample27,29 were pointed out by the authors as possible causes of the discrepancy to the other data in the literature. However, Sal and collaborators also suggested that in obese children with sufficient iron intake, the altered ferroportin-hepcidin axis could occur without signs of iron deficiency.29 It is noteworthy that in the other two studies where ID was not observed, one showed no difference between the BMI of the subgroups involved[31] and another one registered low levels of IL-10 in the studied obese population, suggesting that this can inhibit the expression of IL-6 and therefore of hepcidin.32 However, only six studies evaluated IL-6 levels in obese children and adolescents with ID, and only two associated IL-6 with hepcidin, obesity, and ID.
Brazilian scenario of childhood obesity and anemiaA systematic review and meta-analysis reviewed 134 studies to estimate the prevalence of anemia in Brazilian infants and children.35 The study reported that 1/3 (33%) of children aged zero to 3.5 years old suffer from anemia and, therefore, are exposed to iron deficiency consequences. So, anemia is still a substantial public health problem in Brazil, showing the necessity to create programs that diagnose childhood anemia, such as iron status biomarkers measurement, and also that stimulate strategies that promote access to healthier food by infants and children.35
Another systematic review and meta-analysis studied obese Brazilian children aged < 10 years. Fifty-three studies were reviewed, revealing that, in the 2010s decade, childhood obesity's prevalence was 12.0%, being more common in boys from more developed Brazilian regions.36 Despite the low prevalence of overweight and obesity among disadvantaged preschoolers from Brazil's northwest region, reported by Gibson and coworkers, adiposity and ID were associated with that population.30 Additional studies on the relationship between ID and obesity, considering the role of hepcidin and inflammatory markers, need to be carried out involving Brazilian children and adolescents.
ConclusionsThere is a strong association between obesity and ID in children and adolescents. Increased hepcidin levels seem to be the linking point of those factors because this peptide hormone prevents iron release from cellular storage to the bloodstream and reduces intestinal iron absorption. However, there are disagreements regarding IL-6′s participation in hepcidin excessive production in obese patients. Further studies are needed to elucidate this matter.
Since iron storage and absorption depend on hepcidin levels, their measurement should be considered and standardized in obese children and adolescents. Furthermore, interventions against chronic inflammation, such as weight loss and physical activities, may be contemplated since controlling inflammatory factors improves iron homeostasis. Overall, combined actions would probably enhance life quality and health benefits for children and adolescents.
FundingThis review article was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 303625/2019-8). Pedro F. Berton was a Scientific Initiation Fellow from PUC-Campinas.