The aim of this study was to compare two different empiric treatments for late-onset neonatal sepsis, vancomycin and oxacillin, in a neonatal intensive care unit with a high prevalence of coagulase-negative Staphylococcus.
MethodsA cross-sectional study was conducted in an neonatal intensive care unit from 2011 to 2014. Data from the medical records of at-risk newborns were collected daily. Infections were defined according to the National Health Surveillance Agency criteria. Data analysis was performed using an internal program.
ResultsThere was a significant reduction in the number of Staphylococcus aureus infections (p=0.008), without endocarditis, meningitis, or lower respiratory tract infection, as well as a reduction in the frequency of deaths related to S. aureus infection. There were no significant changes in the incidence of Gram-negative bacterial or fungal infections. An increase in coagulase-negative Staphylococcus infections was observed (p=0.022). However, there was no measured increase in related morbidity and mortality. There was a reduction in the median number of days of treatment with oxacillin from 11.5 to 6 days (p<0.001) and an increase of one day in the median number of days of treatment with vancomycin (p=0.046).
ConclusionsModification of the empiric treatment regimen for neonatal late-onset sepsis with use of oxacillin showed a significant reduction in S. aureus infections, as well as a reduction in the frequency of infections with major organ system involvement and mortality due to infection with this microorganism. As a result, oxacillin can be considered as an effective treatment for late-onset sepsis, making it possible to avoid broad-spectrum antibiotics.
Comparar dois períodos com diferentes esquemas empíricos para tratamento de sepse neonatal tardia, incluindo vancomicina ou oxacilina respectivamente, em Unidade Neonatal de referência com alta prevalência de Staphylococcus coagulase negativo.
MétodosEstudo transversal, realizado em Unidade Neonatal de referência, de 2011 a 2014. A coleta de dados foi realizada diariamente por vigilância ativa em prontuário de recém-nascidos de risco. As infecções foram notificadas conforme critérios definidos pela Agência Nacional de Vigilância Sanitária. O banco de dados e a análise foram realizados em programa interno.
ResultadosOcorreu redução significativa da notificação de infecções por Staphylococcus aureus (p=0,008), sem notificações de endocardite, meningite e infecções de vias aéreas inferiores, além de redução na frequência de óbitos pelo micro-organismo e sem alteração significativa nas incidências de infecções por bactérias Gram negativas e fungos. Houve aumento de infecções S. coagulase negativo (p=0,022), mas sem aumento de morbidade e mortalidade. Ocorreu redução na mediana do tempo de uso de oxacilina, de 11,5 para 6 dias (p<0,001), com aumento de mediana de um dia de uso de vancomicina (p=0,046).
ConclusõesA modificação do esquema empírico com utilização de oxacilina revelou redução significativa das infecções por S. aureus, além da redução na frequência de infecção de foco profundo e mortalidade pelo micro-organismo. Considera-se que oxacilina pode ser utilizada como esquema de tratamento de sepse neonatal tardia, evitando-se o uso de antibióticos de largo espectro.
The most prevalent microorganisms in late-onset neonatal sepsis described in international literature are coagulase-negative Staphylococcus (CoNS).1–6 While rates of laboratory-confirmed sepsis due to these microorganisms vary from 30% to 60%,1 higher rates have also been reported. These microorganisms account for 77.9% of late-onset neonatal sepsis in industrialized countries and 46.5% in developing regions.2 However, infections caused by these commensal microorganisms are often questioned due to difficulties in confirmation and differences in notification criteria.1,7
It should also be considered that CoNS are minimally invasive microorganisms that colonize infants after birth and are normally present in the microbiomes of different body sites. They have the beneficial effect of stimulating the innate immune response and improving the defense against other pathogens.2 However, defense mechanisms can be inadequate in newborn infants, increasing their susceptibility to infection by these microorganisms.3 On the other hand, although they are considered to be microorganisms responsible for sepsis in newborn infants, they present insidious evolution and low morbidity and mortality.2,5,8–11
It is known that the resistance profile of CoNS can exceed 90% for isoxazolyl penicillin.3 As a result, vancomycin has been considered the standard treatment.1,12 However, vancomycin restriction as empiric therapy for late-onset neonatal sepsis has been indicated in literature.9–11,13
The objective of this study was to compare, epidemiologically, two different empiric treatments for late-onset sepsis in a neonatal intensive care unit (NICU) with a high prevalence of CoNS and oxacillin-sensitive Staphylococcus aureus.
MethodsThis observational prospective study was conducted at Hospital das Clínicas, at the Federal University of Minas Gerais (HC/UFMG), from January 2011 to December 2014. HC/UFMG, a university hospital, is a resource for managing high obstetric risk in Belo Horizonte and in the state of Minas Gerais.
The target population consisted of all newborn infants in the NICU considered at risk for late-onset sepsis. Risk factors included weight under 1500g, presence of a central venous catheter (CVC), use of mechanical ventilation (MV), surgery, and treatment with antimicrobial agents. These at-risk infants were followed daily by specialized Hospital Infection Control Commission (HICC) staff. Data were collected by active surveillance, review of medical records, and discussion with the care team. Infections were reported according to the national legislation,14 with infection criteria defined by the Agência Nacional de Vigilância Sanitária (ANVISA),15 based on the National Healthcare Safety Network (NHSN).7,16
The inclusion criteria considered all newborn infants treated with oxacillin or vancomycin as empiric treatment for late-onset sepsis, reported after 48h of life, as defined by ANVISA.15 “Sepsis” and “bloodstream infection” were used synonymously.
A sample size was calculated considering the prevalence of laboratory-confirmed CoNS and S. aureus infections at a rate of almost 30% and 15% respectively, based on a population of 300 patients at risk per year.
An internal program of HICC was used for statistical analysis. Descriptive analysis included frequency of at-risk patients, frequency of patients with hospital-associated infections (HAI), frequency of HAI, cumulative incidence of HAI (number of HAI per 100 patients at risk) and HAI incidence density (number of HAI per 1000 patient-days). The incidence density of HAI was also stratified according to weight range and topography of infection. Density of infection of device-associated infections was measured, including infections associated to CVC, MV, and indwelling urethral catheter (IUC).
The frequency of microorganisms has been described in groups (CoNS, S. aureus, gram-negative bacteria and fungi) and by frequency of antimicrobial agents used for empiric or specific Staphylococcus spp. coverage (oxacillin and vancomycin). In general, these antimicrobial agents are empirically or specifically used for treatment of infection with Staphylococcus spp. Other antimicrobial agents were not described, as they are not relevant to this study.
Microbiological isolation in patient samples was performed by automated method (VITEK®2, BioMérieux Inc, USA) and susceptibility testing by disk diffusion agar (Kirby–Bauer). The sensitivity profile of microorganisms was defined according to the hospital's HICC and based on the Clinical and Laboratory Standards Institute (CLSI).
For comparative analysis, two periods were defined: January 2011 to December 2012 (Period 1) and January 2013 to December 2014 (Period 2). During Period 1, vancomycin was used in the empiric treatment regimen for late-onset sepsis, while oxacillin was used during Period 2.
Mortality (deaths over total number of patients at risk) and lethality (deaths over total number of patients with HAI), considering CoNS or S. aureus, were compared in both periods. Death was considered associated with infection if it occurred during or within 15 days of treatment with the study antibiotic.
Morbidity was defined as infection with major organ system involvement such as endocarditis, lower respiratory tract infection (pneumonia or tracheal or bronchial infection), or central nervous system infection with isolation of these microorganisms, as well as number of days of antimicrobial treatment with oxacillin or vancomycin.
Statistical analysis was performed by a statistician using SPSS® (SPSS Inc., version 15.0, USA) and EpiInfo® v. 7.0 (CDC, USA). Descriptive analysis included frequency, percentage, mean, standard deviation, median and range. Comparative analysis was performed using X2 or Fisher test for categorical variables and Student's t-test or the Mann–Whitney test for quantitative variables, according to variance analysis by Levine's test. Odds ratio was used to calculate the relative measure of events between the two periods of study and 95% confidence interval (95% CI) as a measure of the precision of the event estimated. Statistical significance was considered when p<0.05.
Other preventive and infection-reducing strategies have been continuously performed in the NICU by HICC staff. This study was approved by the Institutional Review Board of Research Ethics Committee of UFMG.
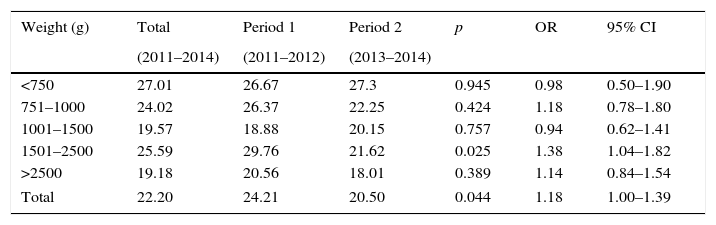
ResultsThroughout the study period, 1229 at-risk patients were followed, totaling 26,260 patient days. A total of 367 patients had 583 episodes of HAI, with an incidence density of 22.20 HAI episodes per 1000 patient days. Table 1 shows HAI incidence density per birthweight range, with a significant reduction in infections among patients ranging from 2500g to 1501g during Period 2. There was significant reduction in the total number of reported infections (p=0.044; OR: 1.18; 95% CI 1–1.39) (Table 1).
Incidence densitya of healthcare-associated infections by birthweight range, Neonatal Intensive Care Unit, HC/UFMG, 2011–2014.
| Weight (g) | Total | Period 1 | Period 2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| (2011–2014) | (2011–2012) | (2013–2014) | ||||
| <750 | 27.01 | 26.67 | 27.3 | 0.945 | 0.98 | 0.50–1.90 |
| 751–1000 | 24.02 | 26.37 | 22.25 | 0.424 | 1.18 | 0.78–1.80 |
| 1001–1500 | 19.57 | 18.88 | 20.15 | 0.757 | 0.94 | 0.62–1.41 |
| 1501–2500 | 25.59 | 29.76 | 21.62 | 0.025 | 1.38 | 1.04–1.82 |
| >2500 | 19.18 | 20.56 | 18.01 | 0.389 | 1.14 | 0.84–1.54 |
| Total | 22.20 | 24.21 | 20.50 | 0.044 | 1.18 | 1.00–1.39 |
A total of 296 (54.81%) and 355 (52.98%) of patients at risk during Period 1 and Period 2 were male, respectively. There was no statistical difference in regards to gender (p=0.564; OR: 1.08; 95% CI 0.85–1.36), but 19 newborns had no defined gender.
The cumulative incidence of HAI was 47.44% of at-risk patients, with a significant reduction from the first to the second study period: from 53.8% to 42.4% (p<0.001).
Considering device-associated infections, there was no significant difference in the incidence density of infections by device-day between the two periods of study. Density of CVC-associated bloodstream infection was 17.29 infections per 1000 CVC days and varied from 15.39 to 19.09 (p=0.117; OR: 0.81; 95% CI 0.62–1.06). Density of ventilator-associated pneumonia was 1.93 infections per 1000 MV days and varied from 2.09 to 1.79 (p=0.789; OR: 1.17; 95% CI 0.38–3.62). Density of IUC-associated urinary tract infection was 6.05 per 1000 IUC days and varied from 8.46 to 4.33 (p=0.243; OR: 1.96; 95% CI 0.62–6.16).
Frequency of episodes of HAI treated with both antibiotics was compared between the two periods and the implementation of the new protocol was considered effective. There was a significant decrease in the use of vancomycin, from 175 to 97 episodes of HAI treated with vancomycin (p<0.001; OR: 1.98; 95% CI 1.52–2.60), and there was a significant increase from 30 to 132 episodes of late HAI treated with oxacillin (p<0.001; OR: 4.68; 95% CI 3.07–7.17).
Considering the frequency of microorganisms isolated in cases of HAI in at-risk patients, there was a significant reduction in HAI due to S. aureus when oxacillin was part of the initial empiric regimen (p=0.012), and an increase in HAI due to CoNS (p=0.028), but no significant changes were observed in the ratio of gram-negative bacteria or fungi (Table 2). All S. aureus isolated from patient samples with HAI associated infections in Period 1 were oxacillin sensitive. During Period 2, only one sample presented an oxacillin resistance profile at sensitivity test. This reveals that the prevalence of oxacillin-resistant S. aureus at this NICU was calculated as 2.4% (n=1/42).
Frequency of microorganisms isolated in healthcare-associated infections by patient at-risk, Neonatal Intensive Care Unit, HC/UFMG, 2011–2014.
| Microorganism | Total | Period 1 | Period 2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| 2011–2014 | 2011–2012 | 2013–2014 | ||||
| n (%) | n (%) | n (%) | ||||
| Coagulase negative Staphylococcus | 117 (9.52) | 40 (7.37) | 77 (11.22) | 0.028 | 0.63 | 0.41–0.95 |
| Staphylococcus aureus | 42 (3.42) | 27 (4.97) | 15 (2.19) | 0.012 | 2.34 | 1.18–4.67 |
| Gram negative bacillus | 91 (7.40) | 47 (8.66) | 44 (6.41) | 0.167 | 1.38 | 0.88–2.17 |
| Fungi | 14 (1.14) | 6 (1.10) | 8 (1.17) | 0.865 | 0.95 | 0.27–3.13 |
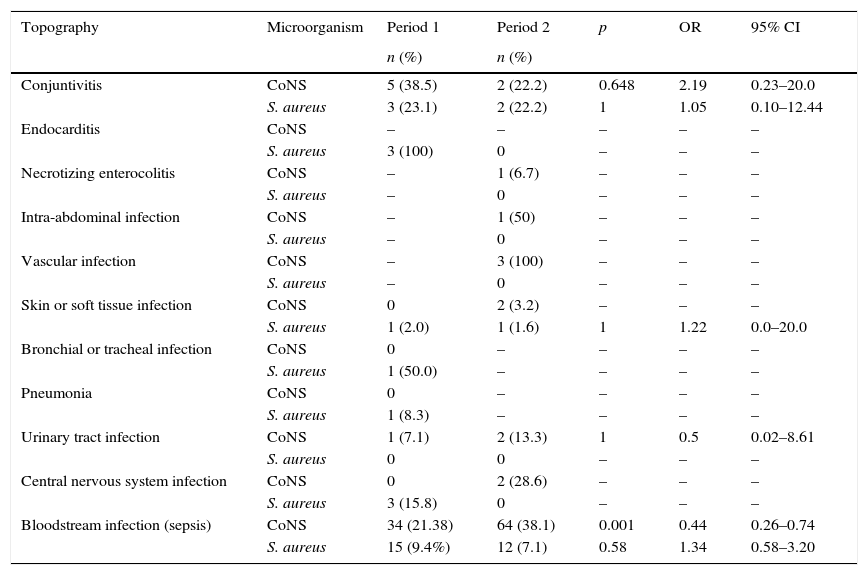
Table 3 shows HAI by topography, considering S. aureus and CoNS associated infections. No infections with major organ system involvement, such as endocarditis, lower respiratory tract infection, or central nervous system infection with isolation of S. aureus, were noted in Period 2; only conjunctivitis, skin infection, and bloodstream infection by this microorganism were reported.
Healthcare-associated infections by S. aureus and coagulase negative Staphylococcus (CoNS) by topography, Neonatal Intensive Care Unit, HC/UFMG, 2011–2014.
| Topography | Microorganism | Period 1 | Period 2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Conjuntivitis | CoNS | 5 (38.5) | 2 (22.2) | 0.648 | 2.19 | 0.23–20.0 |
| S. aureus | 3 (23.1) | 2 (22.2) | 1 | 1.05 | 0.10–12.44 | |
| Endocarditis | CoNS | – | – | – | – | – |
| S. aureus | 3 (100) | 0 | – | – | – | |
| Necrotizing enterocolitis | CoNS | – | 1 (6.7) | – | – | – |
| S. aureus | – | 0 | – | – | – | |
| Intra-abdominal infection | CoNS | – | 1 (50) | – | – | – |
| S. aureus | – | 0 | – | – | – | |
| Vascular infection | CoNS | – | 3 (100) | – | – | – |
| S. aureus | – | 0 | – | – | – | |
| Skin or soft tissue infection | CoNS | 0 | 2 (3.2) | – | – | – |
| S. aureus | 1 (2.0) | 1 (1.6) | 1 | 1.22 | 0.0–20.0 | |
| Bronchial or tracheal infection | CoNS | 0 | – | – | – | – |
| S. aureus | 1 (50.0) | – | – | – | – | |
| Pneumonia | CoNS | 0 | – | – | – | – |
| S. aureus | 1 (8.3) | – | – | – | – | |
| Urinary tract infection | CoNS | 1 (7.1) | 2 (13.3) | 1 | 0.5 | 0.02–8.61 |
| S. aureus | 0 | 0 | – | – | – | |
| Central nervous system infection | CoNS | 0 | 2 (28.6) | – | – | – |
| S. aureus | 3 (15.8) | 0 | – | – | – | |
| Bloodstream infection (sepsis) | CoNS | 34 (21.38) | 64 (38.1) | 0.001 | 0.44 | 0.26–0.74 |
| S. aureus | 15 (9.4%) | 12 (7.1) | 0.58 | 1.34 | 0.58–3.20 |
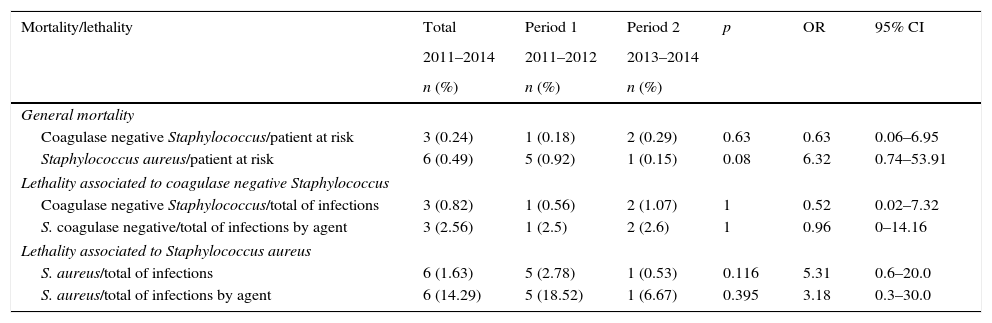
Mortality and lethality were calculated by comparing cases of death due to HAI caused by S. aureus or CoNS between the two periods (Table 4). Mortality was considered to be the number of deaths associated with these infections per total number of deaths. Lethality was considered to be the number of deaths associated with these infections per total number of cases of HAI by each microorganism. There were no significant changes, but there was only one death associated with S. aureus during Period 2 (when empiric therapy included oxacillin), compared to five deaths in Period 1. No statistical difference was observed in mortality associated with CoNS infection, with fewer associated deaths.
Mortality and lethality of patients with Staphylococcus spp. hospital-associated infections, Neonatal Intensive Care Unit, HC/UFMG, 2011–2014.
| Mortality/lethality | Total | Period 1 | Period 2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| 2011–2014 | 2011–2012 | 2013–2014 | ||||
| n (%) | n (%) | n (%) | ||||
| General mortality | ||||||
| Coagulase negative Staphylococcus/patient at risk | 3 (0.24) | 1 (0.18) | 2 (0.29) | 0.63 | 0.63 | 0.06–6.95 |
| Staphylococcus aureus/patient at risk | 6 (0.49) | 5 (0.92) | 1 (0.15) | 0.08 | 6.32 | 0.74–53.91 |
| Lethality associated to coagulase negative Staphylococcus | ||||||
| Coagulase negative Staphylococcus/total of infections | 3 (0.82) | 1 (0.56) | 2 (1.07) | 1 | 0.52 | 0.02–7.32 |
| S. coagulase negative/total of infections by agent | 3 (2.56) | 1 (2.5) | 2 (2.6) | 1 | 0.96 | 0–14.16 |
| Lethality associated to Staphylococcus aureus | ||||||
| S. aureus/total of infections | 6 (1.63) | 5 (2.78) | 1 (0.53) | 0.116 | 5.31 | 0.6–20.0 |
| S. aureus/total of infections by agent | 6 (14.29) | 5 (18.52) | 1 (6.67) | 0.395 | 3.18 | 0.3–30.0 |
Morbidity was also evaluated considering the duration of antimicrobial treatment. There was a significant reduction in duration of treatment with oxacillin in Period 2 with the median time reducing from 11.5 to 6 days (p<0.001). Duration of treatment with vancomycin increased by just one day, ranging from eight days in Period 1 to nine days in Period 2 (p=0.046).
DiscussionAfter the introduction of empiric treatment with oxacillin in the therapeutic regimen for late-onset sepsis, a significant reduction in cases of HAI caused by S. aureus sensitive to oxacillin (p=0.012) was observed. The incidence density of infections also significantly decreased, and it was mainly evident in patients with weight ranging from 1501 to 2500g. A previous study17 already showed a greater number of patients in this weight range at risk for laboratory-confirmed bloodstream infections, probably due to the profile of this NICU, as it is a center of fetal medicine. These patients have prolonged hospitalizations and they are at risk of requiring surgery, CVC, and MV that may increase risk of infection.
Despite the increase in cases of infection associated with CoNS, no increase in mortality and evaluated morbidity was observed, indicating that this microorganism can be considered less pathogenic and presents insidious progression when compared to S. aureus as reported in literature.1,2,12
It also should be pointed out that CoNS infection may be questioned and diagnostic criteria require at least two blood cultures with the same microorganism.7,15 In addition, other studies demonstrate that it is possible to follow clinical outcomes, wait for culture results, and also postpone administration of antimicrobial agents in patients with suspected CoNS infection.9–11,18
After modification of the empiric treatment regimen to include the use of oxacillin, there was an increase in CoNS-associated infections. However, no statistical differences in percentages of infection associated with gram-negative bacteria and fungi were detected. It was considered that there was no negative interference in flora profile and, consequently, to patient exposure profile. Furthermore, an overall statistical reduction of HAI was achieved. It should be emphasized that a smaller number of infections is directly related to lower use of antimicrobial agents. As reported by Pinto et al.,6 criteria to reduce sepsis included significantly decreasing the use of vancomycin and carbapenem as well as the number of antimicrobial agents used, although those authors did not present a flora profile.
As published in other reports,9,11 the present study revealed that empiric treatment with oxacillin even significantly reduced the duration of treatment (p<0.001), in more than 50% of days, with increase in only one day of vancomycin in Period 2. The reduction in days of oxacillin use can likely be associated with the appropriate empiric or specific use, since oxacillin has better antimicrobial activity and therapeutic efficacy in sensitive S. aureus infections. It is important to highlight that S. aureus has an oxacillin-sensitive profile in the present NICU, supporting the change of empiric therapy.
In a national study by Bentlin et al.,19 which investigated practices related to prevalence of late-onset sepsis in premature infants, the authors noted that centers using empiric therapy with oxacillin and aminoglycosides have a lower incidence of late-onset sepsis, probably due to more effective treatment.
Considering the recommendation of isoxazolyl penicillins associated with gentamicin as empiric treatment for late-onset neonatal sepsis, a study by Chiu et al.9 also showed a significant reduction in the use of vancomycin and increased use of oxacillin without significant difference in neonatal morbidity and mortality as assessed by incidence of late-onset sepsis, meningitis, and deaths. However, those authors did not compare patients with laboratory-confirmed S. aureus and CoNS-associated HAI as in this study, which showed a significant reduction in episodes S. aureus-associated infections, with as well as fewer cases of infections with major organ system involvement such as endocarditis, lower respiratory tract infection, and central nervous system infection.
In addition to a statistical reduction in the overall number of HAI cases as well as HAI due to susceptible S. aureus, fewer infections with major organ system involvement were also observed, reduced mortality associated with this microorganism was noted, and a significant reduction in days of treatment duration and hospitalization was demonstrated. These findings can be considered adequate to avoid morbidity associated with sepsis and exposure to other adverse events. As reported by Kaufman,20 accuracy in diagnosis and reduction of the use of antimicrobial agents are important to prevent infections in general and, specifically, neonatal mortality related to sepsis.
A multicenter study with 348 NICUs11 evaluated immediate use of vancomycin (<1 day) and delayed use (1–3 days) in the first episode of late-onset sepsis associated with CoNS. The authors reported that there was no difference in the rates of mortality at 7 or 30 days after the initiation of treatment or at discharge. Furthermore, patients who started the use of vancomycin immediately had a significantly longer duration of treatment, with a median of two additional days. In another multicenter study conducted by the same group,18 there was no difference in mortality when comparing the newborns with possible, probable or confirmed infection by CoNS. Additionally, the authors reported that patients with infections associated with CoNS had significantly lower mortality than patients with negative blood cultures, considering that higher mortality could be attributed to other microorganisms not isolated in blood cultures. It must be considered that an antimicrobial agent may be used unnecessarily, as possible or probable infections by CoNS are overestimated.
Cotten et al.,21 in a multicenter cohort, found in a multivariate analysis that prolonged duration of initial therapy in days was associated with death of extremely low birth weight infants, especially when treatment with antimicrobial agents exceeded five days, with increased odds per day of antimicrobial use. It must also be considered that mortality increases when empiric therapy is inadequate, a factor which can increase treatment duration.
A previous study in the same NICU revealed high mortality of patients with sepsis with reported laboratory-confirmed S. aureus.5 In this study, a reduction in the frequency of deaths due to S. aureus-associated HAI from Period 1 (five deaths) to Period 2 (one death) was noted. This reduction tended toward significance (p=0.08). The only death associated with S. aureus in Period 2 was that of a patient with several malformations and potential trisomy 13. Due to the clinical severity of the patient's condition, tests were performed to screen for infection. However, treatment with oxacillin was initiated when the blood culture results were available, two days after testing. The death may be associated with the underlying disease and delay in the use of an appropriate antimicrobial agent.
The present study identified just one and two cases of death due to CoNS-associated HAI, respectively, in Periods 1 and 2, without statistical significance. Literature also discloses that mortality associated with infection by CoNS is related to 1% of cases.5,10 Karlowicz et al.10 investigated causes of fulminant late-onset sepsis and observed that even when cases of CoNS infection were associated with death, patients had other comorbidities or cultures grew more than one microorganism, suggesting contamination. Makhoul et al.8 assessed risk factors for early mortality after late-onset neonatal sepsis and they reported that patients with infections caused by CoNS had a lower risk of death when compared to patients with infections caused by other microorganisms.
In a study by Hemels et al.,13 cefazolin associated with gentamicin was used as empiric treatment for late-onset neonatal sepsis due to CoNS. Clinical response was observed in 87% of treated patients and sensitivity to cefazolin was observed in 88% of the strains analyzed. No difference in length of stay (0.77 days) or mortality (p=0.33) was observed among treated patients with sepsis due to sensitive or resistant strains of CoNS. Those authors suggest that other beta-lactam antibiotics may be useful in the treatment of HAI in newborn infants, decreasing the need for the use of vancomycin.
Considering rational use of antibiotics, vancomycin use should be restricted in units with high prevalence of S. aureus resistant to vancomycin, in cases without clinical or laboratory response within 48–72h despite use of a beta-lactam such as oxacillin, and defined cases of CoNS resistant to oxacillin without clinical response and which is not considered to be commensal.1,22 These recommendations follow the guidelines of the Centers for Disease Control and Prevention, in order to avoid significant increase in strains of Enterococcus spp. and other bacteria resistant to vancomycin.23 The restriction of utilization of antimicrobial agents based on stewardship is important to reduce interference in microbiome, selection of resistant microorganisms and atopic manifestations, and other complications in premature infants, such as enterocolitis and death.24
In conclusion, the present study showed that there was no worsening of outcomes in newborns when using an anti-staphylococcal beta lactam to treat late-onset sepsis, considering that rates of mortality and morbidity associated with CoNS infections did not increase and that there was also an improvement of rates of S. aureus associated infections. Thus, antimicrobial stewardship with use of oxacillin may be recommended in late-onset neonatal sepsis, according to the epidemiological profile of each NICU, which must be associated with other effective infection prevention practices, in order to avoid the use of broad-spectrum antimicrobial agents.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Romanelli RM, Anchieta LM, Silva AC, de Jesus LA, Rosado V, Clemente WT. Empirical antimicrobial therapy for late-onset sepsis in a neonatal unit with high prevalence of coagulase-negative Staphylococcus. J Pediatr (Rio J). 2016;92:472–8.
Study conducted at Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil.