Evaluate biomarkers capable of safely guiding Yellow fever vaccine (YFV) vaccination among individuals suspicious of hen's egg allergy, and identify factors associated with a higher risk for adverse events after immunization (AEAI).
MethodsPatients underwent skin prick test (SPT) for standardized allergens: whole egg, egg white, egg yolk; YFV (1:10 dilution; Biomanguinhos-Fiocruz), and intradermal test (IDT; YFV 0.02 mL, 1:100 dilution) and positive and negative controls. Serum levels of specific IgE (sIgE) for a whole egg, egg white, egg yolk, egg albumin, ovomucoid, lysozyme, and conalbumin (ImmunoCap®; ThermoFisher®) were obtained. Patients sensitized to YFV were submitted to YFV desensitization, and those negatives received YFV (0.5mL) and remained under surveillance for at least one hour.
Results103 patients were enrolled, 95% under 12 years old. 71% (81/103) of patients had reactions: 80% immediate, 11% mixed, and 9% delayed. There was an association between positive skin test results with YFV and the severity of the reaction (OR:7.64; 95%CI:1.61-36.32; p = 0,011). Only the presence of sIgE to ovomucoid was associated with clinical symptoms (p = 0,025). Thirty patients underwent the YFV desensitization protocol.
ConclusionThere is a relationship between the positivity of the egg's components and the severity of the clinical reaction. Furthermore, the relationship between the positivity of the tests with the YFV and egg's components may show a tendency to look at ovomucoid and conalbumin, but it is not a certainty. Therefore, further studies are needed to confirm these associations, and for now, the authors still recommend using the vaccine for testing when necessary.
In the last few decades, the prevalence of food allergy has increased considerably worldwide, and hen's egg allergy has ranged between 0.5% and 9.3%.1-7 Patients with hen's egg allergy have restrictions on the use of some vaccines due to the possibility of adverse events after immunization (AEAI) resulting from the presence of hen's egg allergens in the vaccine, generated during the manufacturing process.8-23
In addition to the composition of the vaccine, the technique used in its administration of vaccinated individuals (genetic predisposition) or the concomitance with other diseases may justify the AEAI.12 The possible causal agents of these reactions are infectious components, adjuvants (e.g.: aluminum), stabilizers (e.g.: gelatin), preservatives (e.g.: thimerosal), antibiotics (e.g.: neomycin), and the biological culture medium (e.g.: hen embryo cells).8-15 Regarding hen's egg proteins, the maximum allowed and accepted concentration at 2μg/mL is established as safe for patients with a previous history of anaphylaxis to hen's egg.8
Yellow fever (YF) is a viral hemorrhagic disease caused by a virus in the family Flaviviridae and transmitted by vectors such as Aedes aegypti or Haemagogus spp.YF occurs in several countries around the world, mainly in Africa, Central and South America.9,24 In Brazil, YF has always been endemic in the Amazon region and, after 2017, it reemerged with sudden dissemination in urban areas, affecting a high number of the population, in addition to presenting greater clinical severity and a lethality rate of 33.6%. Therewith, the yellow fever vaccine (YFV) has become mandatory.24
YFV is grown on embryonated hen eggs and since it is not heated during its production, it presents residual amounts of egg proteins.8-11,13,14,15-22 This amount is variable according to the origin of the vaccine, often above what is allowed, and other times not informed by the manufacturer.18,19 In Brazil, there are two types of YFV available, one produced by Biomanguinhos-Fiocruz and distributed by the public network, and another produced by Sanofi Pasteur (Stamaril®) which is used in the private network.14 The YFV produced by Biomanguinhos-Fiocruz has in its composition: live attenuated virus of 17DD substrain, derived from an African sample of wild YF virus, grown in hen embryo cells, and sucrose, sodium glutamate, sorbitol, hydrolyzed bovine gelatin, erythromycin, kanamycin as excipients.25 The concentration of ovalbumin is not described in the package insert, however, levels of up to 4.42 µg/mL have been identified, depending on the manufacturing batch.18,19
For the final diagnosis of food allergies, an oral challenge test with the trigger food is required. Due to the yellow fever epidemic, it was not possible to delay vaccination, and patients with a history of egg allergies were vaccinated after skin tests with YFV.8-10,13,14,16-23
In the present study, among individuals with a clinical suspicion of hen's egg allergy, the authors studied markers (skin and serological tests) capable of safely guiding the vaccination mode (usual versus desensitization) and identifying potential factors associated with a higher risk for the occurrence AEAI to YFV in these individuals.
MethodsPatientsIt's a retrospective study that analyzed 113 individuals (aged 9.96 months to 54 years) that were referred to the study by doctors or by the Reference Center for Special Immunobiological (CRIE) at Escola Paulista de Medicina, Federal University of São Paulo (EPM-UNIFESP), a convenience sampling, with a history of allergy to hen egg (raw and/or processed) and that has to be immunized with YFV, between February and November 2018.
These patients were referred to the Allergy, Clinical Immunology, and Rheumatology outpatient department at the Department of Pediatrics, EPM-UNIFESP, obtaining personal data, previous history of reaction to egg (age of the first reaction, clinical manifestations, time between ingestion and appearance of symptoms, treatment received and recurrence), personal and family history. The time elapsed between exposure to egg/egg-containing food and the appearance of reactions categorized them as immediate (up to 2 hours), mixed (when there were immediate and late symptoms at the same exposure) and late (between 6 and 48 hours after contact).26 Those using corticosteroids, immunosuppressants, H1 antihistamines in the week before the study or had a history of immunodeficiency or comorbidity and/or acute febrile illness that contraindicated YFV, were not admitted.
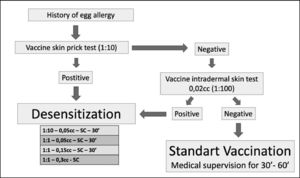
Study protocolSkin testsAll participants underwent a skin prick test (SPT) using standardized allergens: whole egg, egg white, egg yolk (IPI ASAC Brasil®, concentration 2 mg/mL, variation of up to 20%), YFV (1:10 dilution; Biomanguinhos-Fiocruz) in addition to positive (histamine: 1 mg/mL) and negative (saline) controls. The appearance of a wheel with an average diameter equal to or greater than 3 mm above the negative control, 15 min later, characterized the SPT as positive.14
The patients were also submitted to an intradermal test (IDT) using YFV 0.02 mL in a 1:100 dilution and reading after 15 min. The appearance of a wheal twice the initial size characterized the test as positive.14 The dilution of the YFV used was obtained after previous tests in 10 individuals not allergic to the hen egg and, therefore, evaluated as the lowest possible dilution unable to induce the appearance of wheal in an irritating way.
Determination of specific serum IgEA peripheral blood sample (4 mL) was obtained to determine the serum levels of specific IgE (sIgE) for whole egg, egg white, egg yolk, ovalbumin, ovomucoid, lysozyme and conalbumin using the ImmunoCap® technique (ThermoFisher®) and the result equal to or greater than 0.35 kUA/L was considered positive.27
Desensitization protocolPatients characterized as sensitized to YFV (SPT and/or IDT positive with YFV) were submitted to the YFV desensitization protocol proposed by Marinho et al.,14 as a form of vaccination. Those with negative tests received YFV (0.5 mL) and remained under surveillance for at least one hour.
Desensitization was performed in patients admitted to the day hospital bed at Hospital São Paulo (HSP) EPM-UNIFESP. The patients were pretreated with a double dose of second-generation oral H1 antihistamine and peripheral venous access with saline solution (0.9%) was installed. After 30 min, the protocol was started with the administration of 0.05 mL of YFV (diluted 1:10) subcutaneously (SC) and the patients were kept under observation for the next 30 min. In the absence of any symptoms, new doses of pure YFV (without dilution) were administered (0.05 mL, 0.15 mL and 0.30 mL) at 30 min intervals always in the absence of symptoms. At the end of the protocol, all patients received the recommended dose of YFV (0.5 mL) and remained under observation for more than two hours after the end of the procedure. In the presence of a reaction, patients were treated appropriately14 (Figure 1).
Statistical analysisThe data obtained were transported to an Excel spreadsheet and analyzed by software R version 3.6.3. Quantitative and qualitative variables were assessed by parametric and non-parametric tests. Pearson's Chi-square test, Fisher's exact test, and logistic regression models were used to study the association between clinical factors and sensitization to hen egg fractions with the YFV skin test. For this purpose, the following variables were used as predictors: classification of symptoms (categorized as mild, moderate and severe) and the presence of sensitization to hen egg and its fractions: whole egg, egg white, egg yolk, egg albumin, ovomucoid, lysozyme and conalbumin. All IgE were categorized as positive or negative according to the pre-established cutoff value (ImmunoCap ≥ 0.35 KUA / L). The outcome variables were positive or negative skin tests with YFV (SPT and/or IDT).
The study was approved by the Research Ethics Committee of EPM-UNIFESP under registration number 2,880,147, and all participants signed the informed consent form and the consent form when pertinent.
ResultsOut of the 113 patients, 103 subjects completed the study (72 or 69.9% men) aged from 9.96 months to 54 years (mean age 4.72 years; standard deviation 6.11 years) and distributed by age group as follows 0 to 5 years: 78 patients; 6 to 12 years: 20 patients; 12 to 18 years: 3 patients; over 18 years: 2 patients. The 10 patients excluded from the study did not complete the entire protocol.
The clinical manifestations described in contact with hen egg and fractions were predominantly: cutaneous (urticaria, isolated or associated with angioedema in more than 70%), followed by gastrointestinal (nausea, vomiting, abdominal pain), respiratory (cough, dyspnea, wheezing), worsening of the symptoms of atopic dermatitis, and anaphylactic reactions. The FPIES (Food protein-induced enterocolitis syndrome)28 was reported by a patient admitted to the study caught the authors’ attention. Thereby, 71% (81/103) of patients manifested reactions: 80% (65/81) immediate reactions, 11% (9/81) mixed, and 9% (7/81) delayed. 21% (22/103) of the participants were unable to report this time.
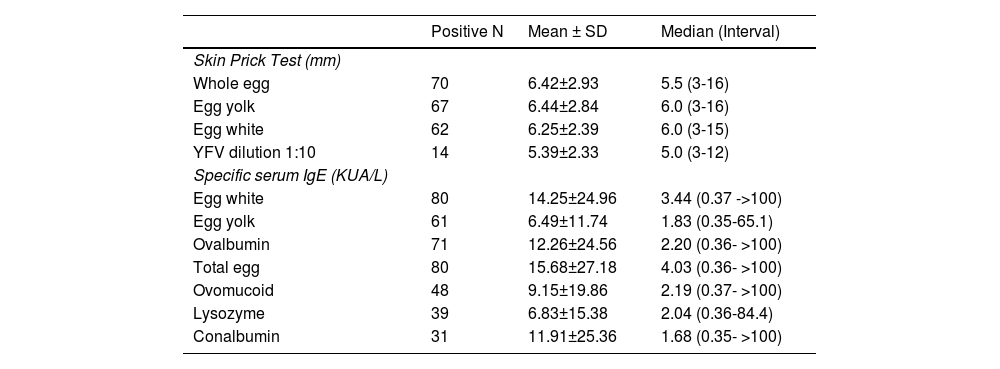
The laboratory parameters referring to SPT and the determination of sIgE levels to the whole egg and its fractions are shown in Table 1. The highest percentage of sensitization (positive sIgE) was to the whole egg, to the white egg, to ovalbumin, and to the yolk.
Distribution of participants with positive results according to: mean values, standard deviation (SD), median, minimum and maximum values, of the wheal induced by allergen and by the yellow fever vaccine (YFV), and specific serum IgE (sIgE) to the egg and fractions to the initial evaluation.
There was a higher frequency of association between positive skin test results (SPT or IDT) and the severity of the reaction and sensitization to whole eggs and fractions (Table 2), confirming the relationship between clinical and laboratory variables and skin tests with YFV. When using multivariate logistic regression analysis, the authors found the reporting of severe symptoms and the positivity of skin tests (SPT and/or IDT) with YFV to be significant (OR:7.64; 95%CI:1.61-36.32; p = 0,011). However, when evaluating them separately, the authors found a significant risk between the association of positive sIgE to ovomucoid and positive YFV SPT, whereas, regarding positive ITD, this association was found with the variables of having severe symptoms or having sIgE to conalbumin (Table 3). The analysis of the possible relation between the presence of sIgE and clinical symptoms, when exposed to the whole egg and fractions, revealed a significant association only with the ovomucoid (p = 0,025).
Distribution of patients according to the result of SPT, IDT or both (positive or negative) with YFV and the intensity of the reaction and presence or absence of sIgE to the egg and fractions - frequencies (absolute and relative) and analysis with Chi-square test and Fisher's exact test.
Specific serum IgE: absent - values below 0.35 kUA / L; present - values greater than or equal to 0.35kUA / L
*The p values were omitted from the table, but all had statistically significant results (p < 0.05).
Factors associated with positive skin prick test (SPT) and / or intradermal (IDT) positive for yellow fever vaccine: multivariate logistic regression (Backward).
OR, Odds ratio; 95% CI, 95% confidence interval; IgE, specific serum IgE.
Thirty patients underwent the desensitization protocol, and the rest received a single dose of YFV. The majority of those desensitized to YFV were male and under 10 years of age. None of them presented any AEAI. Among those vaccinated with only one dose, three had AEAI: a patient with local reaction and angioedema who was treated with oral H1 antihistamine presenting complete improvement of symptoms; one with erythema at the local of injection, and who did not need any treatment, and one with hand angioedema that was treated with oral H1 antihistamine with improvement of the symptoms.
DiscussionIn the present study, the authors found that the history of allergy to hen's egg occurred predominantly in the pediatric age group, since more than 70% of the sample studied was under 5 years of age, like that observed by others.4-6
Although the gold standard for the diagnosis of allergy to whole eggs and its fractions is the oral challenge test (OCT), there are many limitations to its performance (allergen preparation, hospital environment, material for cardiovascular resuscitation and trained staff), even more so in epidemic situations in which vaccination is the only proven measure to control the disease, such as yellow fever.2,29,30 Therefore, the stigma of having an egg allergy can delay YFV in many patients.
The individuals evaluated here were referred because they had a history of allergy to hen eggs and must be immunized with YFV. Thereat, obtaining a complete and standardized clinical history was the quick solution for vaccinating this population with the guarantee of clarifying food allergy in a second step. Most of the previous reactions described by parents and/or patients were of the immediate type, predominantly of isolated or concomitant urticaria and/or angioedema forms. This creates anguish for both parents and doctors, especially non-specialists since the administration of vaccines that contain eggs in their composition increases the risk of AEAI.
The vaccines that contain egg in their composition are triple viral (MMR), Influenza, and YF. However, it is known that MMR and Influenza, due to their concentrations below 2 μg/dose, are safe and can be administered to egg-allergic individuals, making previous skin tests unnecessary.13,14 However, in YFV the variable concentrations of ovalbumin are dependent on the batch and origin of the vaccine and with concentrations higher than those allowed to be safely administered to individuals who are allergic to eggs.18,20
So far, the performance of YFV in patients with a severe reaction to whole egg and its fractions must be performed after an adequate investigation using a protocol with YFV by a trained specialist.14 However, in Brazil, a country of continental dimensions, the difficulty of carrying out these tests and even having a doctor trained for such procedure demonstrates the urgent need to identify and implement other markers that enable the best choice of vaccine administration in these patients, always safely and without delay in vaccination.
Moreover, the authors are going through a complicated time due to the increase in the number of people who do not want to be vaccinated and/or allow their children to be vaccinated. It is a very serious fact that does not only put them at risk of serious diseases but the community as well. Therefore, the egg-allergy obstacle to an indication of vaccination can no longer be a reason that undermines vaccine measures.
There are several protocols described and not universally standardized, on how to administer YFV in patients who are allergic to eggs.8,13,14,16,17,19-23 The most recent suggests that egg-allergic patients with mild clinical forms could be immunized, without performing skin tests with YFV, in two stages under medical supervision (10% of the dose at 0 minutes and 90% at 30 minutes).19,23
The protocol used by us was followed by a low rate of adverse reactions compared to those who received the vaccination in the usual way: 4.1% (3/73) of the total number of patients, lower than that described by Pacheco et al.,12 who reported an accumulated total of AEAI reported in Brazil of 6.6% in the evaluated period. No patient submitted to the desensitization protocol presented any AEAI.
Moreover, YFV is not always available to perform skin tests, therefore the authors looked for a biomarker to help us choose the method for performing YFV. The present study revealed a higher association between positive skin test results and the severity of the reaction and sensitization to whole eggs and fractions.6 To find biomarkers that facilitate safe vaccination, the authors found an association of positive sIgE to ovomucoid and positive SPT to YFV, whereas, regarding positive ITD, this association was found with the variables of having severe symptoms or having sIgE to conalbumin. The ovomucoid component is related to severe hypersensitivity reactions and due to the small sample size, the analysis may have bias related to the clinical severity of the patients.19
On the other hand, conalbumin is contained in some pharmacological presentations and even if not actively measured in YFV, it is encouraging as a possible marker. More clinical trials are needed for this correlation to be robust. The authors can envision that in the future, ovomucoid and conalbumin will be able to help as biomarkers, however, at the moment, tests with YFV are necessary for the decision on how to vaccinate.
The present study has limitations such as being retrospective, using YFV from a single source (Biomanguinhos-Fiocruz), and which contains in its composition: of gelatin, erythromycin, and kanamycin, possible allergenic substances. In addition, the number of patients was limited for some statistical analyzes. Furthermore, to previous studies on YFV always analyzed only the present amount of ovalbumin, there is a lack of evaluation on the presence of ovomucoid and conalbumin, which are likely to be present, but not yet evaluated in this vaccine. Furthermore, there are just a few studies in the literature about the subject.
In conclusion, there is a relationship between the positivity of the egg's components and the severity of the clinical reaction. Furthermore, the relationship between the positivity of the tests with the YFV and egg's components may show a tendency to look at ovomucoid and conalbumin, but it is not a certainty. Therefore, further studies are needed to confirm these associations, and for now, the authors still recommend using the vaccine for testing when necessary.
The vaccine was given by the Reference Center for Special Immunobiological (CRIE) of the Escola Paulista de Medicina, Federal University of São Paulo (EPM-UNIFESP); and the other resources were from the Division of Allergy, Clinical Immunology and Rheumatology of the EPM-UNIFESP.