To perform a systematic review of the literature for scientific evidence of possible differences in cortisol concentrations in adolescents with eating disorders.
Source of dataElectronic searches were conducting in the PubMed, Scientific Electronic Library Online, Virtual Health Library, and Science Direct databases for articles published between 2007 and 2017 using the keywords, cortisol, hydrocortisone; eating disorders, bulimia, bulimia nervosa, anorexia, anorexia nervosa; adolescence, adolescent, adolescents.
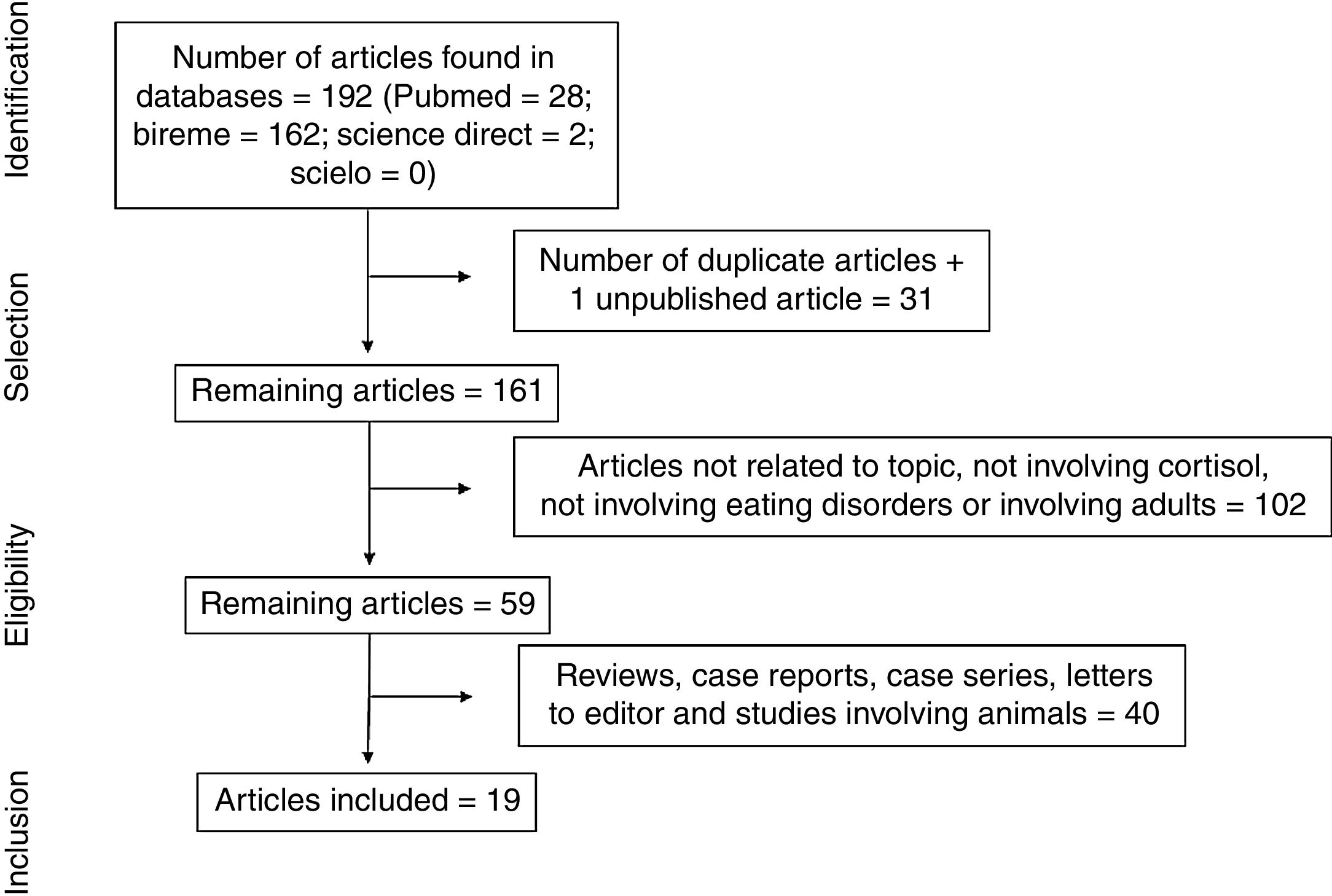
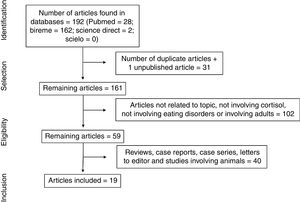
Synthesis of dataA total of 192 articles were found. After the analysis of the eligibility criteria using the PRISMA method, 19 articles were selected for the present review. Most studies were conducted in Europe. Adolescents diagnosed with anorexia nervosa were evaluated in all studies, except one, when other eating disorders were investigated. Blood was the means used for the determination of cortisol. In ten studies, cortisol levels were higher in the group with anorexia than the control group and a reduction in cortisol levels occurred in the adolescents after being submitted to nutritional recovery.
ConclusionsPatients with eating disorders may have several clinical consequences, such as changes in body fat distribution, changes in bone mineral density, worsening of neurocognitive ability, and endocrine changes (e.g., hypercortisolemia), which in turn can lead to hyperglycemia, insulin resistance, hypertension, and increased risk of infections. The findings demonstrate that adolescents with eating disorders, especially anorexia nervosa, have increased cortisol levels, which are reduced after the treatment period. Further studies on differences in cortisol concentrations in adolescents with other eating disorders are needed, using different methods.
Realizar uma análise sistemática da literatura em busca de evidências científicas de possíveis diferenças nas concentrações de cortisol em adolescentes com transtornos alimentares.
Fonte de dadosPesquisas eletrônicas foram realizadas nas bases de dados do Pubmed, da Scientific Electronic Library Online, da Biblioteca Virtual da Saúde e do Science Direct em busca de artigos publicados entre 2007 e 2017 que utilizaram as palavras-chave: cortisol, hidrocortisona, transtornos alimentares, bulimia, bulimia nervosa, anorexia, anorexia nervosa, adolescência, adolescente e adolescentes.
Síntese dos dadosForam encontrados 192 artigos. Após a análise dos critérios de elegibilidade utilizando o método PRISMA, 19 artigos foram selecionados para esta análise. A maioria dos estudos foi realizada na Europa. Os adolescentes diagnosticados com anorexia nervosa foram avaliados em todos os estudos, com exceção de um, em que outros transtornos alimentares foram investigados. A coleta de sangue foi o meio utilizado para a determinação do cortisol. Em dez estudos, os níveis de cortisol estavam mais elevados no grupo com anorexia do que no grupo de controle e ocorreu uma redução nos níveis de cortisol nos adolescentes após serem submetidos a uma recuperação nutricional.
ConclusõesOs pacientes com transtornos alimentares podem apresentar diversas consequências clínicas, como alterações na distribuição de gordura corporal, alterações na densidade mineral óssea, piora da capacidade neurocognitiva e alterações endócrinas, como a hipercortisolemia que, por sua vez, pode levar à hiperglicemia, resistência à insulina, hipertensão e ao aumento do risco de infecções. Os achados demonstraram que os adolescentes com transtornos alimentares, principalmente a anorexia nervosa, apresentaram níveis mais elevados de cortisol, que são reduzidos após o período de tratamento. São necessários estudos adicionais sobre as diferenças nas concentrações de cortisol em adolescentes com outros transtornos alimentares, utilizando meios diferentes.
According to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), eating disorders are characterized by severe disturbances in eating behavior and have a multifactor etiology involving genetic predisposition as well as socio-cultural, biological, and psychological influences.1 Behaviors related to eating disorders, such as perpetual dieting, the desire to be thinner, or compensatory behavior (self-induced vomiting and excessive exercise) are frequently observed in young women.2
The World Health Organization (WHO) defines adolescence as the second decade of life (10–19 years).3 Mäkinen et al.4 describe adolescence as a phase of life marked by profound physical, cognitive, emotional, and social changes that require adaptations to the adoption of new practices, behaviors, and autonomy for adult life. Therefore, adolescence is a period in which individuals are highly susceptible to various behavioral disorders, including eating.
Stress has been identified as a potential risk factor for the development of eating disorders.5 There is evidence that patients often experience severe chronic stress stemming from life events prior to the onset of said disorders.6 Cortisol is one of the steroidal hormones directly related to high levels of stress. Preliminary studies offer evidence that patients with anorexia nervosa present high levels of cortisol due to the association between these disorders and behavioral characteristics (high levels of depression and stress). In addition, hypercortisolemia, elevation of urinary free cortisol, and alteration of the circadian rhythm of cortisol may be present in patients with normal weight who present with bulimia nervosa7; moreover, patients with unspecified eating disorders also have higher cortisol levels than their corresponding controls.8–10 Cortisol is one of the most abundant androgenic hormones in the body and is increased in the presence of physical and/or psychological stress. Elevated cortisol can trigger psycho-physiological reactions that result in hyperfunctioning of the sympathetic nervous system and endocrine system, specifically the adrenal glands.11,12 It is produced by the adrenal gland, which is involved in the response to stress and may play an important role in eating behavior, in addition to being associated with increased energy intake in healthy individuals. The production of cortisol has a circadian rhythm that depends on the stimulation of the adrenocorticotropic hormone (ACTH). Its level is higher near the beginning of daily activities, decreasing over the 24h.13
Although the clinical manifestations of hypercortisolemia in patients with anorexia nervosa may appear inconsistent with those observed in patients with Cushing's syndrome, upon further examination several parallels can be observed.14 Blood cortisol levels at night are inversely associated with bone mineral density and positively associated with the severity of depression and anxiety symptoms in women with anorexia nervosa.15 As cortisol stimulates gluconeogenesis, an increase in cortisol concentrations, in addition to high levels of GH, may be another adaptive mechanism to maintain euglycemia in this condition of severe malnutrition. In addition, glucocorticoids are endogenous antagonists of leptin and insulin.16
Usually, there is an excellent association between cortisol production and the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis, being easily detected in saliva, blood, and urine. Cortisol can be considered as an excellent biomarker of HPA axis function and, consequently, of the study of the effects of stress in humans.17 Repeated and prolonged activation of certain systems, including HPA axis, will put individuals at greater risk of developing physical (e.g., myocardial infarction, multiple sclerosis, abdominal pain, menstrual disorders, viral infections, diabetes, arthritis rheumatoid, and cancer) and psychological disorders (e.g., depression, schizophrenia, anxiety, and cancer).18
Considering the importance of hormonal changes that occur as clinical complications of patients with eating disorders as well as the relationship of such changes with the maintenance of these disorders, the aim of the present study was perform a systematic review of the literature on differences in cortisol concentrations in adolescents with eating disorders.
MethodsConfiguration and registry of studyThe methods employed for the present study were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol for this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), under reference number CRD42017067630. The articles selected for review were submitted to an interpretative analysis directed by the guiding question.
Search strategyThree reviewers searched for articles published in journals indexed in the PubMed, Scientific Electronic Library Online (Scielo), Biblioteca Virtual de Saúde (Bireme) and ScienceDirect databases. The following key words were employed: hydrocortisone OR cortisol AND “eating disorders” OR anorexia OR “anorexia nervosa” OR bulimia OR “bulimia nervosa” AND adolescents OR adolescent OR adolescence (Mesh).
Selection criteriaStudies were selected for the present systematic review based on the following inclusion criteria: epidemiological studies (cross-sectional, case–control, cohort, and clinical trial) that evaluated the concentration of cortisol in adolescents with eating disorders published between 2007 and 2017. Epidemiological studies not related to the topic of interest, experimental studies, letters to the editor, case studies, literature reviews, and studies involving animals were excluded.
ResultsThe electronic search led to the retrieval of 192 articles (Fig. 1). One of the articles had been accepted for publication, but the full text had not yet been published. After the removal of duplicates, the articles were read and analyzed by three independent reviews, who had undergone a training and calibration exercise. A total of 102 articles were excluded for not addressing the topic of interest, not analyzing cortisol, not addressing eating disorders, or involving adults. After the exclusion of review articles, case reports, case series, letters to the editor, and studies involving animals, 19 articles were selected for review (Table 1) and analyzed based on previously established categories. Seven categories were considered: location in which the study was conducted; year of publication; sample selected; type of eating disorder; methods for diagnosing eating disorder; methods for collecting and analyzing cortisol; and cortisol results with comparison between group with eating disorder and healthy control group.
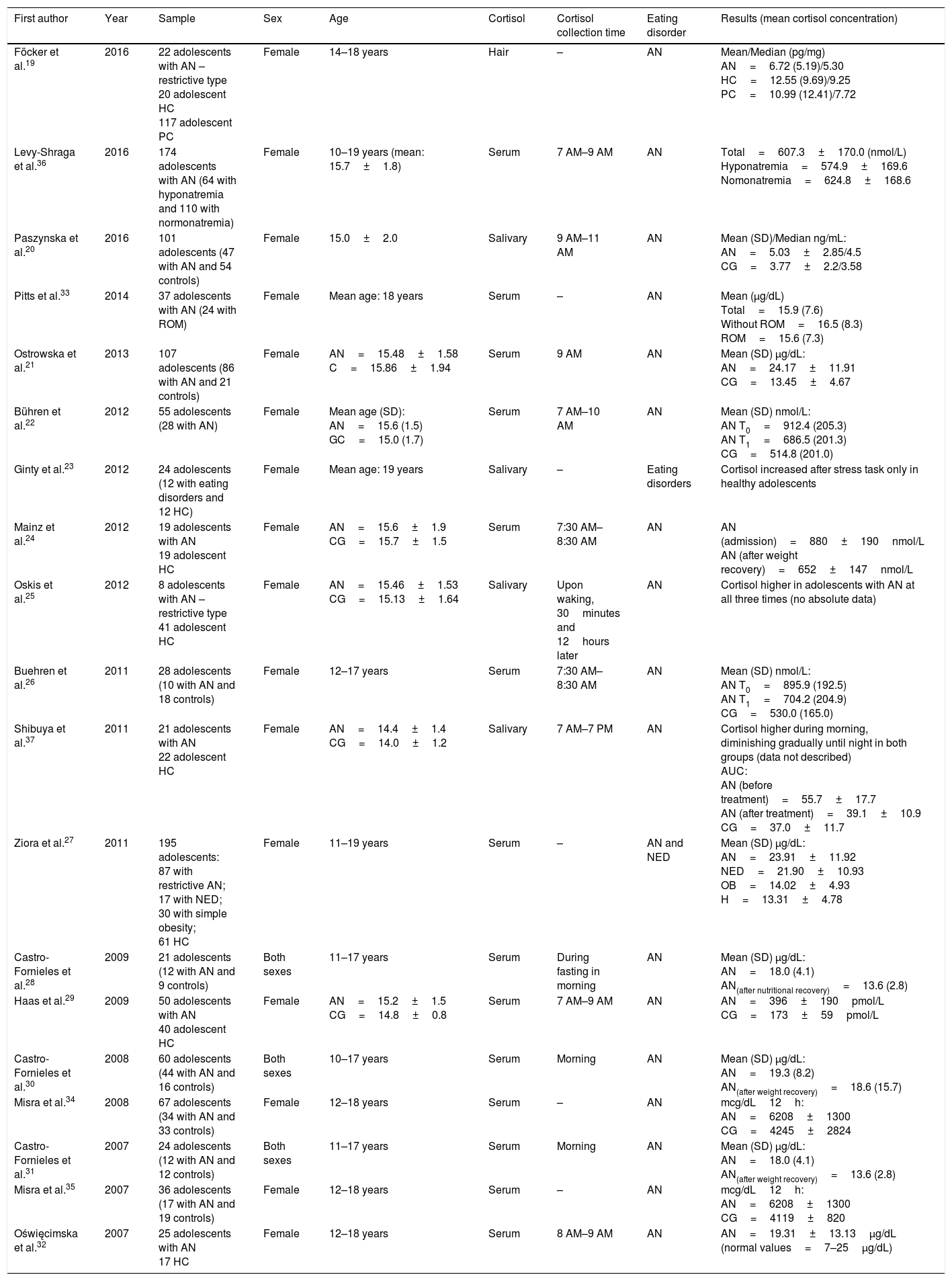
Characteristics of the 19 studies on the changes in cortisol in adolescents with eating disorders included in this review.
| First author | Year | Sample | Sex | Age | Cortisol | Cortisol collection time | Eating disorder | Results (mean cortisol concentration) |
|---|---|---|---|---|---|---|---|---|
| Föcker et al.19 | 2016 | 22 adolescents with AN – restrictive type 20 adolescent HC 117 adolescent PC | Female | 14–18 years | Hair | – | AN | Mean/Median (pg/mg) AN=6.72 (5.19)/5.30 HC=12.55 (9.69)/9.25 PC=10.99 (12.41)/7.72 |
| Levy-Shraga et al.36 | 2016 | 174 adolescents with AN (64 with hyponatremia and 110 with normonatremia) | Female | 10–19 years (mean: 15.7±1.8) | Serum | 7 AM–9 AM | AN | Total=607.3±170.0 (nmol/L) Hyponatremia=574.9±169.6 Nomonatremia=624.8±168.6 |
| Paszynska et al.20 | 2016 | 101 adolescents (47 with AN and 54 controls) | Female | 15.0±2.0 | Salivary | 9 AM–11 AM | AN | Mean (SD)/Median ng/mL: AN=5.03±2.85/4.5 CG=3.77±2.2/3.58 |
| Pitts et al.33 | 2014 | 37 adolescents with AN (24 with ROM) | Female | Mean age: 18 years | Serum | – | AN | Mean (μg/dL) Total=15.9 (7.6) Without ROM=16.5 (8.3) ROM=15.6 (7.3) |
| Ostrowska et al.21 | 2013 | 107 adolescents (86 with AN and 21 controls) | Female | AN=15.48±1.58 C=15.86±1.94 | Serum | 9 AM | AN | Mean (SD) μg/dL: AN=24.17±11.91 CG=13.45±4.67 |
| Bühren et al.22 | 2012 | 55 adolescents (28 with AN) | Female | Mean age (SD): AN=15.6 (1.5) GC=15.0 (1.7) | Serum | 7 AM–10 AM | AN | Mean (SD) nmol/L: AN T0=912.4 (205.3) AN T1=686.5 (201.3) CG=514.8 (201.0) |
| Ginty et al.23 | 2012 | 24 adolescents (12 with eating disorders and 12 HC) | Female | Mean age: 19 years | Salivary | – | Eating disorders | Cortisol increased after stress task only in healthy adolescents |
| Mainz et al.24 | 2012 | 19 adolescents with AN 19 adolescent HC | Female | AN=15.6±1.9 CG=15.7±1.5 | Serum | 7:30 AM–8:30 AM | AN | AN (admission)=880±190nmol/L AN (after weight recovery)=652±147nmol/L |
| Oskis et al.25 | 2012 | 8 adolescents with AN – restrictive type 41 adolescent HC | Female | AN=15.46±1.53 CG=15.13±1.64 | Salivary | Upon waking, 30minutes and 12hours later | AN | Cortisol higher in adolescents with AN at all three times (no absolute data) |
| Buehren et al.26 | 2011 | 28 adolescents (10 with AN and 18 controls) | Female | 12–17 years | Serum | 7:30 AM–8:30 AM | AN | Mean (SD) nmol/L: AN T0=895.9 (192.5) AN T1=704.2 (204.9) CG=530.0 (165.0) |
| Shibuya et al.37 | 2011 | 21 adolescents with AN 22 adolescent HC | Female | AN=14.4±1.4 CG=14.0±1.2 | Salivary | 7 AM–7 PM | AN | Cortisol higher during morning, diminishing gradually until night in both groups (data not described) AUC: AN (before treatment)=55.7±17.7 AN (after treatment)=39.1±10.9 CG=37.0±11.7 |
| Ziora et al.27 | 2011 | 195 adolescents: 87 with restrictive AN; 17 with NED; 30 with simple obesity; 61 HC | Female | 11–19 years | Serum | – | AN and NED | Mean (SD) μg/dL: AN=23.91±11.92 NED=21.90±10.93 OB=14.02±4.93 H=13.31±4.78 |
| Castro-Fornieles et al.28 | 2009 | 21 adolescents (12 with AN and 9 controls) | Both sexes | 11–17 years | Serum | During fasting in morning | AN | Mean (SD) μg/dL: AN=18.0 (4.1) AN(after nutritional recovery)=13.6 (2.8) |
| Haas et al.29 | 2009 | 50 adolescents with AN 40 adolescent HC | Female | AN=15.2±1.5 CG=14.8±0.8 | Serum | 7 AM–9 AM | AN | AN=396±190pmol/L CG=173±59pmol/L |
| Castro-Fornieles et al.30 | 2008 | 60 adolescents (44 with AN and 16 controls) | Both sexes | 10–17 years | Serum | Morning | AN | Mean (SD) μg/dL: AN=19.3 (8.2) AN(after weight recovery)=18.6 (15.7) |
| Misra et al.34 | 2008 | 67 adolescents (34 with AN and 33 controls) | Female | 12–18 years | Serum | – | AN | mcg/dL12h: AN=6208±1300 CG=4245±2824 |
| Castro-Fornieles et al.31 | 2007 | 24 adolescents (12 with AN and 12 controls) | Both sexes | 11–17 years | Serum | Morning | AN | Mean (SD) μg/dL: AN=18.0 (4.1) AN(after weight recovery)=13.6 (2.8) |
| Misra et al.35 | 2007 | 36 adolescents (17 with AN and 19 controls) | Female | 12–18 years | Serum | – | AN | mcg/dL12h: AN=6208±1300 CG=4119±820 |
| Oświęcimska et al.32 | 2007 | 25 adolescents with AN 17 HC | Female | 12–18 years | Serum | 8 AM–9 AM | AN | AN=19.31±13.13μg/dL (normal values=7–25μg/dL) |
AN, anorexia nervosa; HC, healthy controls; PC, psychiatric controls; ROM, return of menstruation; CG, control group; NED, non-specified eating disorder; AUC, area under curve.
Most studies (n=14) were conducted in Europe.19–32 Three studies were conducted in the Americas,33–35 and two studies were conducted in Asia.36,37 The year with the largest number of publications (n=4) was 2012.22–25 All studies involved female adolescents and three also involved male adolescents.28,30,31
Regarding the collection method, cortisol levels were evaluated in blood samples in most studies (n=14).21,22,24,26–36 Cortisol was measured in saliva samples in four studies20,23,25,37 and in hair follicles in one study.16 Regarding the time of sampling, most studies (n=11) collected the hormone exclusively in the morning period.20–22,24,26,28–32,36 Only one study did not evaluate adolescents with a diagnosis of anorexia nervosa.23
In ten of the studies, cortisol levels were higher in the group of adolescents with anorexia when compared with the control group.20–22,25–27,29,34,35,37 In one study, cortisol levels were lower in the group of adolescents with anorexia than in the control group.16 In three studies, cortisol was measured in a group of adolescents with anorexia, and no comparison with a control group was made.32,33,36 In four studies, the levels of this hormone were compared before and after treatment involving weight gain, and a reduction was found in the post-treatment evaluation.24,28,30,31
DiscussionThe aim of the present study was to perform a systematic review of the literature regarding differences in cortisol concentrations in adolescents with eating disorders. The analysis of the articles confirms anorexia nervosa as the most widely studied eating disorder, especially in Europe. The main findings were the higher level of cortisol in adolescents with anorexia nervosa in comparison with healthy controls and the reduction in the levels of this hormone following weight recovery.
Anorexia nervosa is a condition of severe malnutrition that is prevalent among adolescent girls and young women, affecting 0.2–1% of this population.38–42 Studies addressing this condition are more common than those addressing other eating disorders, which may be explained by the fact that the signs and symptoms are easier to detect. Moreover, the clinical complications of this condition are more severe and the mortality rate is higher. Anorexia nervosa is characterized by a distorted body image and very low body weight associated with the inability to gain or maintain weight; in women, the DSM-IV included amenorrhea for at least three menstrual cycles in the diagnostic criteria.43 However, the revised criteria on weight are less strict in the DSM-5, and amenorrhea is no longer necessary for the diagnosis.1 Although most of the studies analyzed were conducted with adolescents with anorexia nervosa, the research attempted to include eating disorders in general.
Three articles analyzed in the present review also included males. Data on the prevalence of eating disorders in the male population are somewhat scarce. For many years, it was believed that eating disorders only affected the female population, and many evaluation tests have a gender bias, as they were created for women; therefore the prevalence of this problem is underestimated the male population.44 The most widely cited study estimates prevalence rates of 0.3% for anorexia nervosa, 0.5% for bulimia nervosa, and 2% for binge eating disorder among males using the DSM-IV criteria.40 Thus, males account for 25% of cases of anorexia and bulimia and 36% of cases of binge eating disorder. Regarding differences in cortisol levels between the sexes, some studies indicate that differences in the response to neurobiological stress favor men,45,46 but there have been many reports demonstrating the opposite effect,47,48 while some do not report differences between the sexes.49,50 In addition, other factors could influence this expression, among which neuropsychiatric disorders, such as anxiety, depression, and post-traumatic stress disorder, which are related to stress and are influenced by sex hormones and gonadal.51–54 Cortisol was measured in blood and saliva samples, and, in one study, hair follicles. Regardless of the type of collection, significant differences were found between the groups with eating disorders and healthy controls. However, the evaluation of hormone concentrations in saliva is more reliable, as the blood collection process in itself causes stress and can raise cortisol levels. Moreover, strong correlations have been found between salivary and serum cortisol in normal individuals at all ages, and samples remain stable when frozen for long periods of time. Hormone concentrations in saliva reflect the fraction not linked to carrier protein (free hormone fraction).55 One of the studies in the present review validated the use of salivary cortisol to investigate the activity of the HPA axis in adolescents with anorexia nervosa.37 According to a recent study, patients suffering from eating disorders show a blunted HPA axis reactivity to stress exposure and a generally reduced sympathetic/exaggerated parasympathetic nervous system activity, that is, the HPA axis interferes with the circadian cycle.56
In ten studies, cortisol levels were compared between adolescent with anorexia and healthy controls, and higher levels were observed in the group with the eating disorder. Other studies have reported that anorexia nervosa is associated with a state of relative hypercortisolemia in adults and adolescents.15,57,58 Elevated cortisol has multiple effects, such as reduction in bone mineral density59 and poorer cognitive performance.60 Elevated cortisol also promotes an increase in the release of free fatty acids due to lipolysis and lower activity of lipoprotein lipase, thereby causing an increase in insulin resistance and hyperglycemia.61 Shibuya et al.37 concluded that a higher basal cortisol concentration may be an indicator of anorexia severity. Likewise, Estour et al.57 suggests that cortisol could be used as an important prognostic predictor. However, levels rarely exceed double the upper limit of normality. Individuals with a low body mass index and low levels of fat mass, fasting glucose, and insulin have higher levels of cortisol, which is consistent with the theory that an increase in cortisol is an adaptation mechanism to maintain normoglycemia in a state of low energy availability.62
The increase in cortisol levels in adolescents with anorexia is due to hyperactivity of the HPA axis,63 which results in the release of cortisol from the adrenal glands. Studies have demonstrated an increase in salivary or serum levels of cortisol in the acute phase of anorexia,64–66 and it has been suggested that this situation is a biological adaptation to hunger as a consequence of chronic food restriction.37 The reduction in cortisol levels following weight recovery lends support to this theory.
In the present study, only changes in cortisol in adolescents with eating disorders were considered. It is possible that an analysis involving adults could offer conflicting results regarding cortisol levels.
ConclusionsAdolescents with anorexia nervosa have higher levels of cortisol (serum or salivary) than healthy controls. Elevated cortisol has many consequences, including low bone mineral density and a poorer cognitive performance. A very low frequency of other eating disorders, such as bulimia nervosa, was found in the studies analyzed, the results of which could be conflicting when compared with patients with anorexia nervosa. The main difficulty in diagnosing bulimia is the lack of significant weight loss, which differs from anorexia.
The increase in cortisol in adolescents with anorexia nervosa raises questions regarding the extent to which this population may suffer the consequences of this condition in the long term and whether such changes (i.e., hyperglycemia and hyperinsulinemia) persist even after weight recovery and the normalization of the levels of this hormone.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to the Council for the Advancement of Higher Education Personnel (CAPES) for funding this study and the researchers of the Eating Disorders and Behaviors Research Group of the Federal University of Pernambuco (UFPE).
Please cite this article as: Luz Neto LM, Vasconcelos FM, Silva JE, Pinto TC, Sougey ÉB, Ximenes RC. Differences in cortisol concentrations in adolescents with eating disorders: a systematic review. J Pediatr (Rio J). 2019;95:18–26.