To analyze the effects of exposure to hyperoxia (100% oxygen) on the lung histoarchitecture of neonatal mice.
MethodsNeonatal Balb/c mice were exposed to hyperoxia (HG) (100% oxygen) (n= 10) in a chamber (15 x 20 x 30cm) for 24hours with a flow of 2 L/min. The control group (CG) (n=10) was exposed to normoxia in the same type of chamber and for the same time. After exposure, the animals were euthanized by decapitation; the lungs were removed and processed for histological examination according to the laboratory routine. Three-mm thick sections were stained with hematoxylin and eosin (H&E). The morphometric analysis was performed with in order to analyze the macrophages present in the alveolar lumen, surface density (Sv) of gas exchange, volume density (Vv) of lung parenchyma, and areas of atelectasis.
ResultsA decrease in the number of alveolar macrophages (MØ) was observed in the HG (HG=0.08±0.01 MØ/mm2, CG=0.18±0.03 MØ/mm2, p=0.0475), Sv of gas exchange in HG (HG=8.08±0.12mm2/mm3, CG=8.65±0.20mm2/mm3, p=0.0233), Vv of lung parenchyma in HG (HG=54.7/33.5/83.5%/mm2; CG=75/56.7/107.9%/mm2, p<0.0001) when compared with the CG. However, there was an increase in areas of atelectasis in HG (HG=17.5/11.3/38.4 atelectasis/mm2, CG=14/6.1/24.4 atelectasis/mm2, p=0.0166) when compared with the CG.
ConclusionThe present results indicate that hyperoxia caused alterations in lung histoarchitecture, increasing areas of atelectasis and diffuse alveolar hemorrhage.
Analisar os efeitos da exposição à hiperóxia (100% de oxigênio) sobre a histoarquitetura pulmonar de camundongos neonatos.
MétodosCamundongos neonatos da linhagem Balb/c foram expostos à hiperóxia (GH) (100% de oxigênio) (n=10) em uma câmara (15 x 20 x 30cm) por 24h, com fluxo de 2 L/min. O grupo controle (GC) (n=10) foi exposto a normóxia em um mesmo tipo de câmara e pelo mesmo tempo. Após a exposição, os animais foram sacrificados por decapitação, os pulmões foram removidos para análise histológica e processados de acordo com a rotina do laboratório. Cortes de 3μm de espessura foram corados com hematoxilina e eosina (H&E). A análise morfométrica foi realizada com o objetivo de analisar macrófagos presentes na luz alveolar, densidade de superfície (Sv) de trocas gasosas, densidade de volume (Vv) de parênquima pulmonar e áreas de atelectasias.
ResultadosFoi verificada diminuição do número de macrófagos alveolares (MØ) no GH (GH=0,08±0,01 MØ/mm2; GC=0,18±0,03 MØ/mm2; p=0,0475), Sv de troca gasosa no GH (GH=8,08±0,12mm2/mm3; GC=8,65±0,20mm2/mm3; p=0,0233), Vv de parênquima pulmonar no GH (GH=54,7/33,5/83,5%/mm2; GC=75/56,7/107,9%/mm2; p<0.0001) quando comparado com o GC. Entretanto, houve aumento de áreas de atelectasias no GH (GH=17,5/11,3/38,4 atelectasia/mm2; GC=14/6,1/24,4 atelectasia/mm2; p=0,0166) quando comparado com o GC.
ConclusãoNossos resultados indicam que a hiperóxia promoveu alterações na histoarquitetura pulmonar, aumentando áreas de atelectasia e hemorragia alveolar difusa.
It is estimated that 3.9 of the 10.8 million child's deaths worldwide occur in the first 28 days of life. Over 96% of these deaths occur in developing countries. Pneumonia may be associated with a low Apgar score (severe respiratory alterations at birth), which is commonly associated with chorioamnionitis, inflammation of the fetal membranes (chorion and amnion) due to a bacterial infection, usually related to prolonged vaginal deliveries and also to inhalation of infected amniotic fluid. In most cases, this leads to fetal asphyxia. One of the most obvious manifestations is hypoxemia, followed by chest indrawing and cyanosis.
As treatment, studies have shown that drug therapies using antibiotics are effective, as well as the use of oxygen in order to reverse the initial hypoxemia and reduce the risk of mortality.1 The use of oxygen is among the first lines of therapy for hypoxemia caused by pulmonary and heart disease,2–4 which involves treating hypoxia by oxygen inhalation at a pressure greater than that of ambient air, which facilitates hematosis and reduces ventilatory work, in order to maintain adequate oxygenation with PaO2>50mmHg and<70mmHg.5
Although O2 therapy is considered essential for life, studies show an association between oxygen toxicity and retinopathy of prematurity, chronic lung disease, bronchopulmonary dysplasia, atelectasis by resorption, tracheobronchitis, depression of mucociliary activity, nausea, anorexia, headache, lung epithelial damage, damage to the blood-air barrier, and pulmonary edema. Moreover, the injury can be intensified when combined with mechanical ventilation.6 Recent studies suggest that the pulmonary epithelial damage induced by exposure to high concentrations of oxygen, specifically, has been associated with oxidative stress,7 based on the hypothesis that hyperoxia induces an increase in the number of oxygen free radicals, reactive species capable of reacting with biomolecules and causing direct damage to membrane proteins and DNA.8
After pulmonary epithelium lesion, in particular, there is activation of macrophages and an inflammatory cascade, followed by pulmonary edema and presence of fibrin, collagen, and neutrophilic aggregate.9 The literature describes animal models exposed to hyperoxia only in adult mice, when their lungs are already fully formed. The effects of high concentrations of oxygen at the time of lung formation, i.e., the lungs of newborns, are yet to be clearly described in Balb/c mice. Therefore, this study aimed to evaluate the histological patterns in lungs of neonatal mice 12hours after birth, exposed to hyperoxia for 24hours.
MethodsEthical aspectsThe experiment was performed in accordance with the provisions of the Brazilian Society of Science in Laboratory Animals, and was approved by the Ethics Committee for Animal Research of the University.
AnimalsTwenty Balb/c neonatal mice, approximately 12hours after birth, with a mean weight of 1.5g (despite the low weight of newborn mice, their anatomical structures are well-defined, allowing for experimental manipulation) were obtained from the Laboratory of Experimental Pathology and Biomorphology of the Centro de Ciências da Saúde (CCS) of the Universidade Severino Sombra, Brazil. The animals’ nutrition in the postnatal period until euthanasia was provided by ad libitum breastfeeding (breastfeeding in mice lasts on average 19 to 21 days after birth).
Experimental designExposure to oxygenThe animals were divided into two groups: control group (CG) - mice exposed to ambient air and to the same conditions of the experimental group and the hyperoxia group (HG) - mice exposed to hyperoxia for 24h.
For the animals exposed to hyperoxia, an acrylic inhalation chamber was used (30cm long, 20cm wide, and 15cm high), as described by Nagato.10 Oxygen 100% was purchased from White Martins® (White Martins Praxair Inc. – São Paulo, Brazil). The oxygen cylinder was coupled to the oxygen inhalation chamber through a silicone conduit. The gas was released into the chamber with a constant flow of 2 L/min, thus ensuring an oxygen flow that would supply and saturate the environment.
After a period of time, when oxygen had filled the chamber space, all mice (except the control group, which inhaled ambient air) were placed in the inhalation chamber and removed after 24h. The oxygen concentration was measured continuously through an oxygen cell (C3 – Middlesbrough, England). The mice received water and food ad libitum, and were kept in individual cages with controlled temperature and humidity (21±2°C, 50±10%, respectively), and submitted to inverted 12-h cycles of light/dark (artificial lights, 7 p.m. to 7 a.m).
This experimental model was designed to mimic the conditions of supplemental oxygen that neonates receive in intensive care units in the first days of life when associated with a pathological picture, during lung formation.
Euthanasia and organ removalAt the end of the O2 exposure time, euthanasia was performed by decapitation. A ventral midline incision was performed to remove the skin, starting from the chest to the abdominal region. Access to the thoracic cavity was made using a subxiphoid incision with removal of the diaphragm and costal osteotomy, to expose the mediastinum. After exposure of the mediastinum, a lung perfusion was performed by sectioning the left atrium, followed by a puncture in the right ventricle, injecting 1mL of saline solution with 0.9% NaCl with constant pressure controlled by a pump (Sykam – Gewerbering, Germany). After perfusion, the lungs were removed by plucking through the mediastinum.
Histological analysisBoth lungs were fixed through a cannula inserted in the trachea by instillation of formalin (Vetec Química Fina – Duque de Caxias, Brazil) buffered (10%) with constant pressure controlled by a pump. After 48h, they were processed through the following steps: dehydrated in increasing concentrations of ethanol (50%, 70%, 92.8%, and 99.3%), diaphonized in xylene, and paraffin-embedded. Three-μm-thick sections were stained with hematoxylin and eosin (H & E).
Morphometric analysisRepresentative and proportional lung samples stained with H & E were randomly studied; 15 random fields were assessed by histological slide, under 40x magnification. The analyzed section came from the lungs equally embedded in paraffin and sectioned in the same direction, aiming to analyze all of the representative parts of the entire lung and proportionally in all analyses. Analyses were performed to determine the number of macrophages present in the alveolar lumen, the volume density (Vv) of parenchyma, surface density (Sv) of gas exchange, and areas of atelectasis and erythrocytes in airspaces. Both analyses were performed by observing the microscope slide on a TV monitor (Sharp – 14″) where a test system was superimposed on the screen, and the analyses were made in relation to tissues. Sv of gas exchange was verified through the test system of cycloid arcs with proportional orientation to the sine of the vertical axis angle. The measurement was performed by counting coincident points on the portion of the gas exchange surface, with the test system superimposed on the lung tissue image.11
The Vv of lung parenchyma was measured through the M42 test system in an irregular arrangement of points. Analysis was conducted by overlapping the test system to an image of lung tissue, and coincident points of the lung parenchyma were counted.11
Statistical analysisTo determine the sample size, a statistical power of 0.9 was adopted. The data used for this calculation were obtained from studies performed by this group,10 taking into account the expected data from larger standard error of the mean. Therefore, a resulting sample size of 10 for each group was obtained. The normality of all data was tested through the Kolmogorov-Smirnov test. Parametric data were expressed as mean±standard error of mean, followed by unpaired Student's t-test. Nonparametric data were expressed as median/minimum/maximum value, and the Mann-Whitney test was used. The difference was considered significant when p-value<0.05.
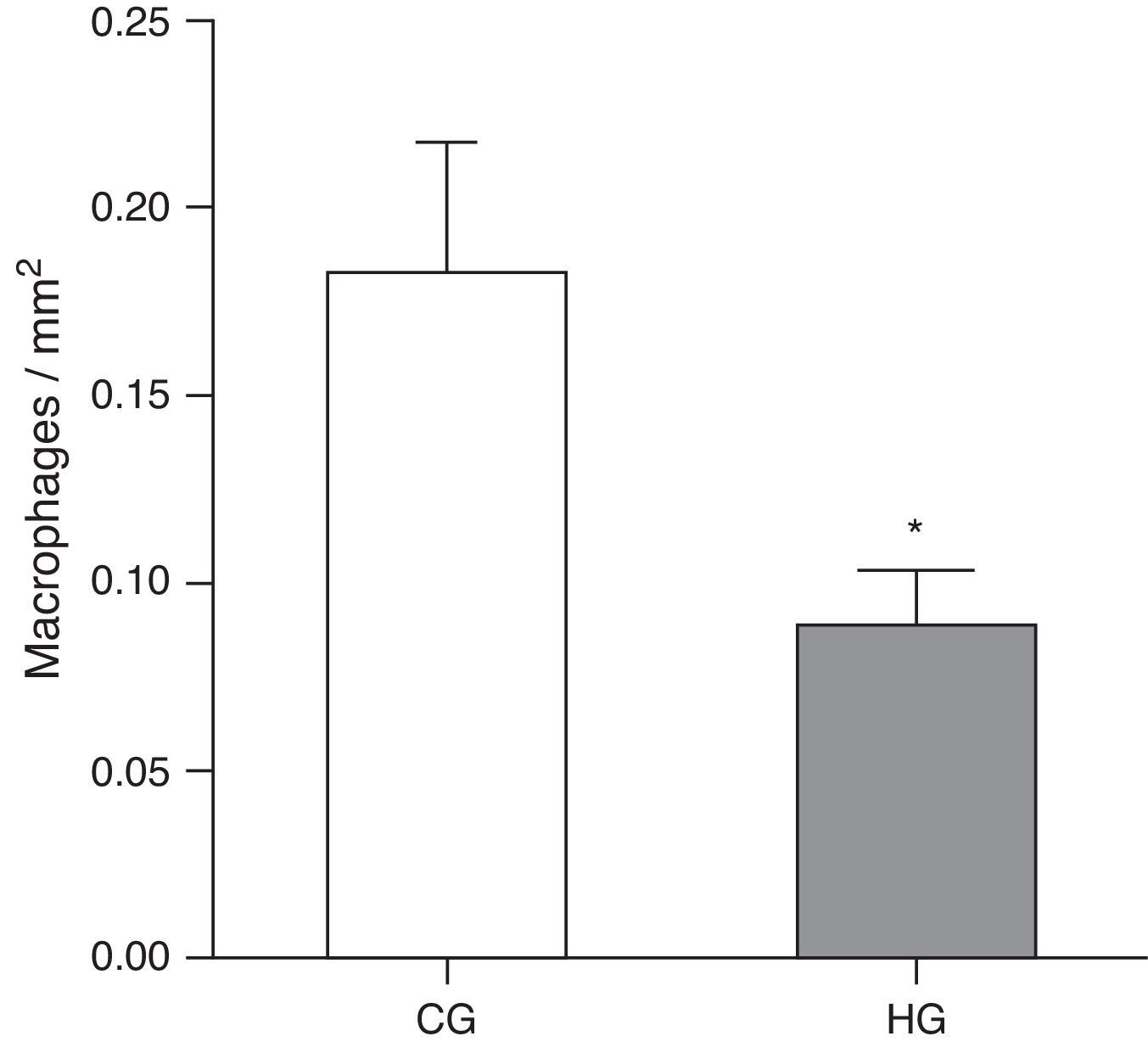
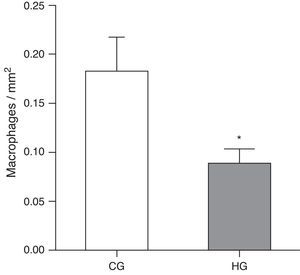
ResultsAfter exposure to hyperoxia for 24h, it was observed that the HG (HG=0.08±0.01 MØ/mm2) showed a decrease in alveolar macrophages in the alveolar lumen (p=0.0475) compared to CG (CG=0.18±0.03 MØ/mm2) (Fig. 1).
Mean number of alveolar macrophages per microscopic field in the lung parenchyma in neonatal mice exposed to ambient air and hyperoxia for 24h.
CG=control group, animals exposed to ambient air; HG=hyperoxia group, animals exposed to 100% oxygen for 24hours.
* Means difference between the CG and HG, with a p-value=0.0475 in the unpaired Student's t-test.
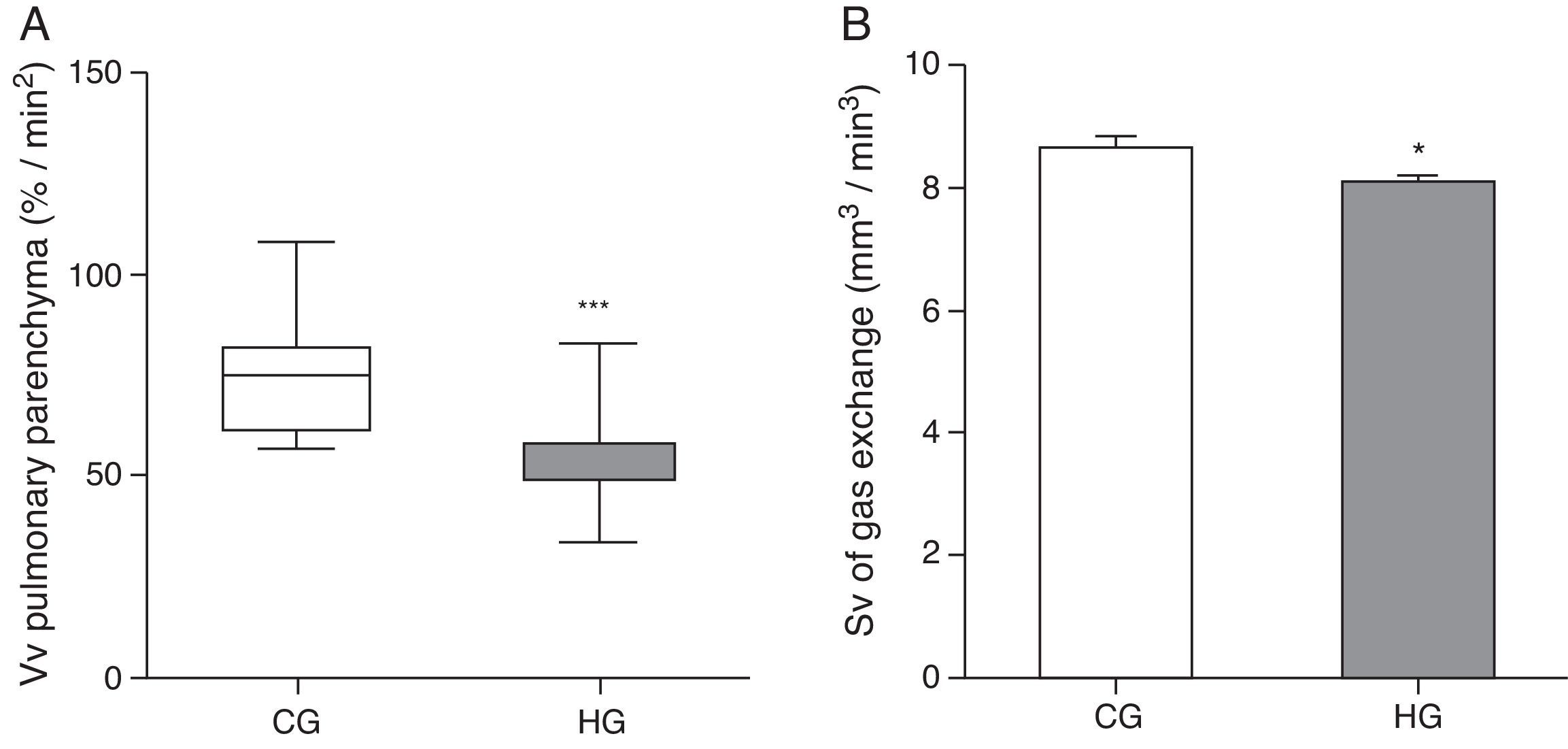
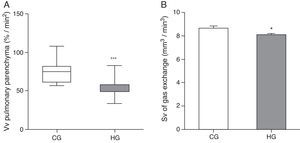
As for morphometric analyses, a decrease in the Vv of lung parenchyma was observed in the HG=54.7/33.5/83.5%/mm2, CG=75/56.7/107.9%/mm2, p<0.0001) (Fig. 2A), and the Sv of gas exchange of HG (HG=8.08±0.12mm2/mm3; CG=8.65±0.20mm2/mm3, p=0.0193 (Fig. 2B).
Volume density (Vv) and surface density (Sv) in the pulmonary parenchyma in neonatal mice exposed to ambient air and hyperoxia for 24h.
CG=control group (animals exposed to ambient air); HG=hyperoxia group, (animals exposed to 100% oxygen for 24hours).
*** Means difference between CG and HG, with p-value<0.0001 at Mann-Whitney test.
* Means difference enters the CG and HG, with p-value=0.0193 at unpaired Student's t-test.
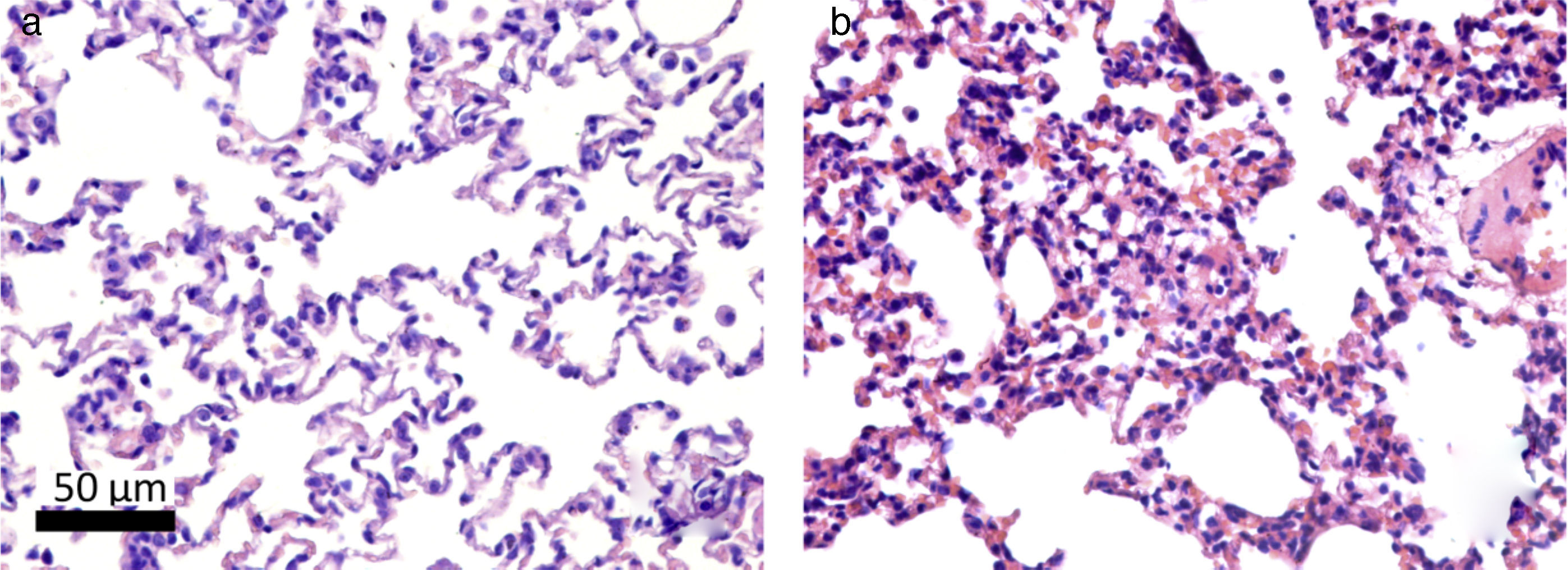
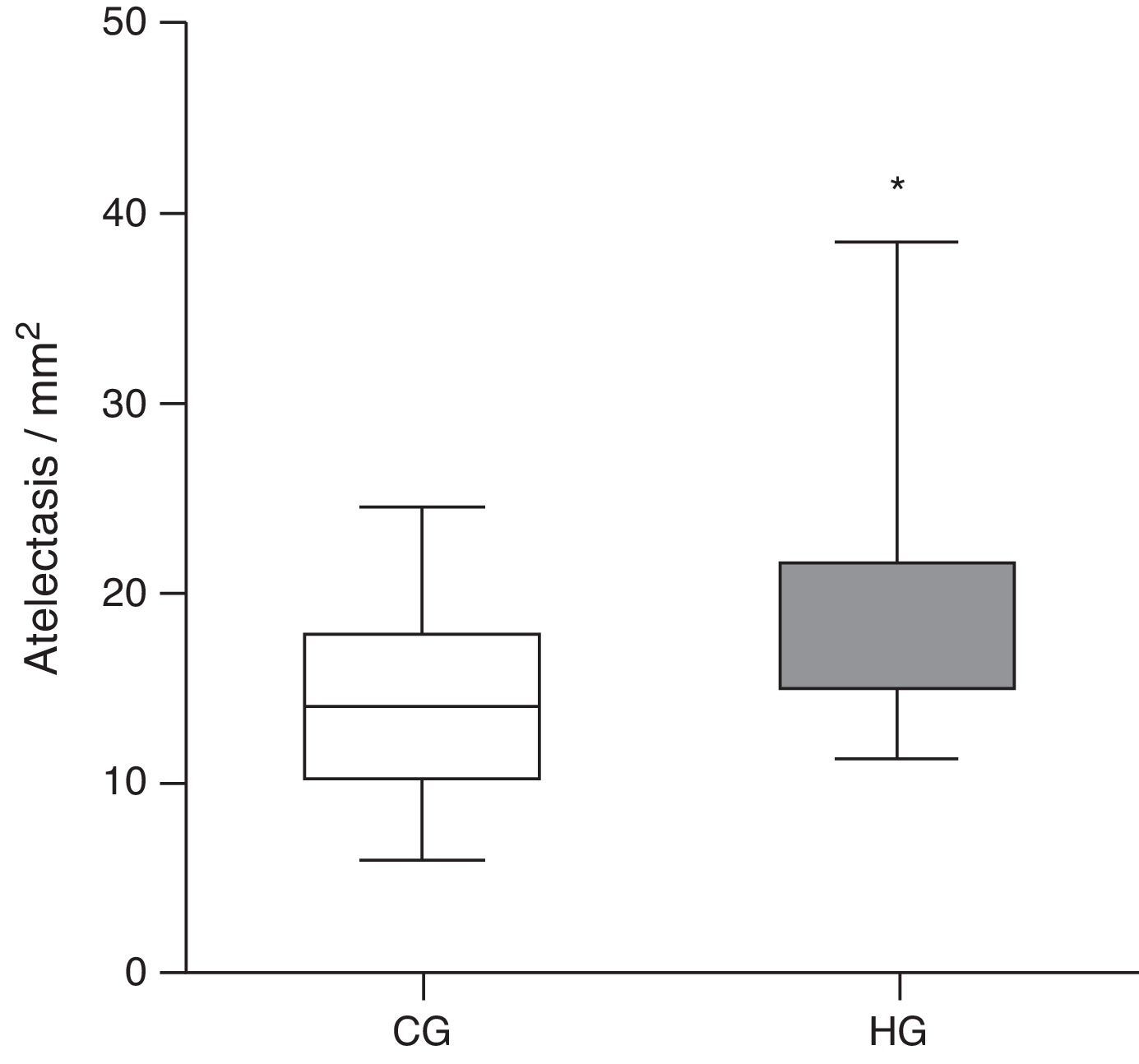
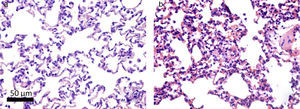
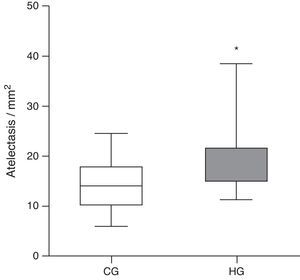
Histologically, the CG was characterized by presence of lung parenchyma of normal aspect (Fig. 3A). The HG showed diffuse parenchymal abnormalities, with varying degrees of intensity (from mild to severe). The presence of areas of atelectasis and the presence of red blood cells in the alveolar lumen, as shown in Fig. 3B, were the most frequent alterations and were significantly increased in HG (HG=17.5/11.3/38.4 atelectasis/mm2, CG=14/6.1/24.4 atelectasis/mm2, p=0.0166) when compared to the CG (Fig. 4).
Photomicrographs representative of histological sections of lung parenchyma of neonatal mice exposed to ambient air and hyperoxia for 24h. A Vv decrease can be observed in the lung parenchyma, gas exchange Sv, and atelectasis increase in HG (b) compared with the CG (a). Hematoxylin and eosin (H & E).
Mean number of alveoli with atelectasis per microscopic field in the lung parenchyma of neonatal mice exposed to ambient air and hyperoxia for 24h.
CG, control group (animals exposed to ambient air); HG, hyperoxia group, (animals exposed to 100% oxygen for 24h).
* Means the difference between CG and HG with p-value=0.0166 in Mann-Whitney test.
The present study analyzed the effects of exposure to high concentrations of oxygen on lung histological patterns of neonatal Balb/c mice.
It was observed that hyperoxia induced a decrease in the number of alveolar macrophages, modified the lung histoarchitecture, and increased the number of red cells in the air spaces.
The HG showed a decrease of macrophages in the alveolar space after 24h of exposure. In their studies, Petrache et al.12 demonstrated in vitro (after 24 hour-exposure to O2>95%) and in vivo (after the mice were exposed for 72h to 100% O2) that alveolar macrophages undergo apoptosis when compared to macrophages in normoxia. It was also demonstrated that, in the first 30minutes of hyperoxia, the increased activity of ERK (extracellular signal-regulated kinase) protected alveolar macrophages, decreasing the rate of apoptosis. However, the same did not occur in the period from 8 to 24h, because ERK activity returned to normal values.
Nyunoya et al.13 observed that the decreased activity of phosphatases during hyperoxia, including PP2A and MKP-3, is related to ERK inhibition, which decreases macrophage survival. Similar results were observed in the present study, as there was a significant decrease in alveolar macrophages in the lung of newborn animals exposed to hyperoxia for 24h. In another study, macrophages cultures exposed to 95% oxygen (hyperoxia) showed reduced proliferation when compared to cultures exposed to 21% oxygen (normoxia). This reduction was probably mediated by actin polymerization induced by oxidative stress, which altered the phagocytic capacity to pathogens.14 Thus, it is suggested that hyperoxia may influence both the increase in apoptosis and the decrease in proliferation of alveolar macrophages.
Another study by Thébaud et al.15 demonstrated that exposure to oxygen therapy at high concentrations interferes with the development of lung parenchyma, as newborn rats had a lower expression of vascular endothelial growth factor (VEGF) and, consequently, a decrease in the number of blood capillaries, which resulted in increased air spaces. Mascaretti et al.16 also reported this decrease in the number of alveoli in an experimental model of exposure to hyperoxia in preterm rabbits of the New Zealand lineage. Animal models have demonstrated structural lung abnormalities resulting from exposure to hyperoxia.17,18
Neonates are subject to alterations caused by oxygen exposure, since their antioxidant system develops later. These alterations make the neonate vulnerable to such lesions, including parenchymatous lesions, which may be irreversible.19 Dauger et al.20 studied mice exposed to hyperoxia at 65% over a period of 28 days after birth, demonstrating a smaller number of alveoli, albeit with increased alveolar lumen. The alterations lasted for seven months after exposure, evidencing that hyperoxia causes permanent alterations in lung structure. Neonatal mice are at the saccular stage of lung development, and decreased alveolarization is a prevalent characteristic.21 This pattern was demonstrated in the present study. However, exposure to hyperoxia exacerbated the decrease in volume density of the lung parenchyma and gas exchange surface area, compared to animals exposed to ambient air.
In clinical practice, atelectasis is often found during general anesthesia, especially in the postoperative period and/or during mechanical ventilation.6 The present results indicate that exposure to hyperoxia for 24h resulted in an increase in areas of pulmonary atelectasis. This can be explained by the induction of atelectasis by resorption, a mechanism responsible for impairment of gas exchange and of structural lung parenchyma.22 Loewen et al.23 studied rabbits of the New Zealand lineage and demonstrated the beneficial effect of supplementation of exogenous surfactant in lungs exposed to hyperoxia. In their study, animals exposed to hyperoxia at 100% associated with surfactant supplementation presented a decrease in areas of atelectasis, when compared to animals exposed to hyperoxia alone. This suggests that reduction in surfactant production induced by high doses of oxygen promotes increased areas of atelectasis, which was also confirmed by Buonocore et al.24
Studies in experimental animals have demonstrated that hyperoxia promotes increased capillary permeability, extravasation of plasma proteins into the interstitium and alveolar space, and later, after a prolonged exposure, fibrosis in the alveolar wall.25 Tokieda et al.26 studied mice with deficiency of surfactant protein B (SP-B) exposed to hyperoxia at 95%, and observed a susceptibility to pulmonary congestion and bleeding. However, Lian et al.27 demonstrated the protective effects against damage caused by exposure to oxygen therapy. In that study, transgenic animals overexpressing signal transducer and activator of transcription 3(Stat3C) and, consequently, with an increased production of SP-B protein, presented resistance to alveolar hemorrhage. Alveolar hemorrhage,20 non-cardiogenic pulmonary edema,28 and damage to type I pneumocytes27 and type II pneumocyte hyperplasia have been mentioned as alterations resulting from high oxygen concentrations in clinical practice.29
The stages of lung structural development are similar in humans and mice. In the mouse, after the ninth day of gestational development, lung formation begins, characterized by embryonic events that depend on the interaction between epithelial and mesenchymal cells. The 12-hour postnatal period, which was chosen for the start of the present intervention, is described as crucial for the development of histological and biochemical alterations that can be evaluated in this experimental model. Moreover, at this time, the animals are at the intermediate saccular period of lung development and lung structures are significantly formed.30 The objective of the present study was to investigate how the developing lung would be able to respond to exposure to oxygen at high concentrations, considering that in clinical practice newborns receive such treatment (supplemental oxygen therapy) in intensive care units.
The limitation to mimic the time and intensity of oxygen administration in experimental models is due to the lack of clinical studies that indicate the mean time and mean fraction of inspired oxygen used by newborns during the hospitalization period. However, this study stimulates the development of other clinical and experimental findings, for example, feasibility studies and specific markers of apoptosis for alveolar macrophages, all with the aim of achieving therapeutic alternatives for the treatment of medical exposure to oxygen at high concentrations.
Conflicts of interestThe authors declare no conflicts of interest.
To FAPERJ for the scientific initiation grant to undergraduate student Renata Reis and to FAPEMIG for the postdoctoral grant to Professor Frank Silva Bezerra DECBI/UFOP through the project approved by Edict 15/2010 “Programa Primeiros Projetos”.
Please cite this article as: Reis RB, Nagato AC, Nardeli CR, Matias IC, de Lima WG, Bezerra FS. Alterations in the pulmonary histoarchitecture of neonatal mice exposed to hyperoxia. J Pediatr (Rio J). 2013;89:300–6.