This study aimed to estimate the performance of single-phase-enhanced computed tomography and ultrasonography examinations in the preoperative evaluation of solid abdominal tumors and their relationship with relevant adjacent structures in children.
MethodsThis retrospective study included 50 pediatric patients with malignant solid abdominal tumors treated with surgical resection between 2009-2017. Preoperative computed tomography and ultrasonography were compared to operative findings (gold standard) in the diagnosis of invasion or encasement of adjacent structures. Accuracy, sensitivity, specificity, and positive and negative predictive values were evaluated.
ResultsRenal (20.4%) and neuroblastic (19.4%) tumors were the most common. Complete surgical resection with negative margins was achieved in 44 (88%) patients. The comparison between single-phase-enhanced computed tomography and ultrasonography findings showed the following results: sensitivity = 90.3% vs 86.6%, specificity = 86.8% vs 94.6%, negative predictive value = 95.3% vs 94.4%, positive predictive value = 75.3% vs 86.9%, and accuracy = 87.9% vs 92.2%. The correlation (kappa index) between computed tomography and ultrasonography examinations was 0.72 (p < 0.001). In 14% (7/50) of the patients, the invasion of adjacent structures was diagnosed by ultrasonography but not by computed tomography (1 patient had 2 invaded structures).
ConclusionUltrasonography can be considered a complementary method to single-phase-enhanced computed tomography in the preoperative evaluation of children with an abdominal tumor. The present study showed that ultrasonography and single-phase-enhanced computed tomography each possess a high accuracy in the preoperative planning of resection of solid abdominal tumors in children. Thus, it seems that the combination of both imaging methods would be enough for the evaluation of most abdominal tumors in the pediatric population.
In the United States, cancer is the second leading cause of death in children and teenagers between 0 and 14 years old;1 neuroblastic and renal tumors are the most prevalent solid abdominal tumors in this age range.1,2 The complete surgical resection of these tumors increases survival rates, which can reach 90% for Wilms’ tumors and 75% for neuroblastomas.3-5
Imaging methods have different roles in the assessment of children with cancer. They are necessary from diagnosis to staging and treatment. Detailed preoperative evaluation is essential for surgical planning and can be performed with ultrasonography imaging (USG), computed tomography imaging (CT), magnetic resonance imaging (MRI), and nuclear medicine.5-8 CT and MRI are currently considered the methods of choice for staging and preoperative planning for the resection of malignant tumors.6-8 However, both examinations require sedation in small children, and CT additionally exposes patients to radiation, which potentially increases future risks of malignancy.9-12 Radiation exposure can be reduced if the CT is limited to the single-phase contrast technique.5,13-15
On the other hand, USG presents advantages for children: it does not require sedation or contrast agents, nor does it expose children to ionizing radiation. It is also a dynamic imaging technique that allows observation of the movement of intra-abdominal structures during breathing, peristalsis, and gentle abdominal compression maneuvers.8 Even though USG is routinely performed as an initial examination when evaluating abdominal pathologies in children, its role in the preoperative evaluation of solid abdominal tumors has not yet been extensively studied.7,16,17 In a literature review, the authors were able to find only a few studies investigating the role of USG in this setting.16,17
Very few prior studies have evaluated the role of CT and USG in the preoperative assessment of solid abdominal tumors[17] and none has performed a comprehensive evaluation of the accuracy of these examinations in preoperative evaluations. This study aimed to estimate the performance of single-phase enhanced computed tomography CT (sph-CT) and USG in the preoperative evaluation of solid abdominal tumors and their relationship with relevant adjacent structures in children. For the first time in the pediatric literature, the authors have estimated the accuracy of sph-CT and USG in the evaluation of curative tumor resection based on the analysis of the contact surface between the tumor and adjacent organs and structures.
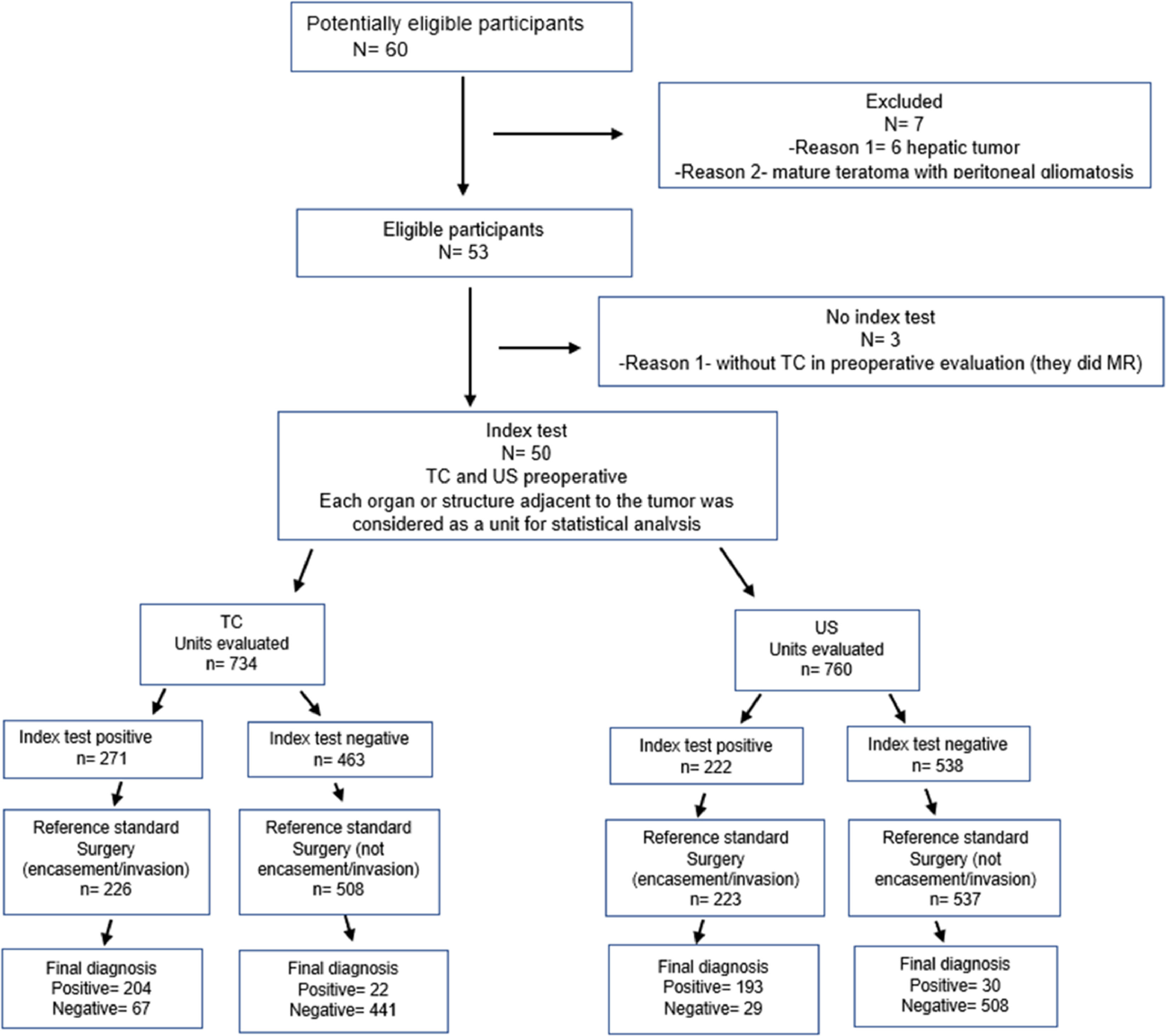
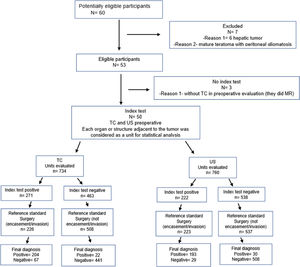
Material and methodsPatientsThis study was designed according to the Standards for Reporting Diagnostic Accuracy (STARD) 2015 statement and was approved by the Institutional Review Board (IRB) of the facility where the work was conducted (opinion No. GPPG-2018-0106) (Figure 1). Due to the completely retrospective design of this investigation and the absence of any intervention, there was no need for written informed consent; accordingly, a waiver was requested and obtained from the IRB. Intraoperative findings were considered the gold standard.
Inclusion criteria were as follows: all patients under 18 years old who were subjected to surgical resection of solid abdominal tumors who had undergone both sph-CT and USG for preoperative evaluation. None of the study patients had any evidence of metastatic spread. Exclusion criteria were the following: patients who underwent USG and MRI but had no CT images; patients who underwent only MRI and CT in the preoperative evaluation, without USG; patients who were not subjected to surgical resection; CT and USG performed for diagnosis, staging, or evaluation of complications; and patients with liver tumors, because the preoperative evaluation was performed by multiphase CT or MRI and their surgical team was not the same. Figure 1 illustrates the participant selection process. Between January 2009 and December 2017, 60 patients were eligible and 50 were included in the study.
USG imageUSG was performed using ALOKA 4000® or PHILIPS HD11® with multi-frequency transducers (2-5 MHz convex and 5-12/5-10 MHz linear). All patients were accompanied by their parents or legal guardian, did not receive sedation or anesthesia, and the examination was performed after the ingestion of liquids.
USG was performed by an experienced pediatric radiologist and followed the institution's protocol, including tumor volume, calculated by the ellipsoid formula (height x width x thickness x 0.523); a grey-scale evaluation of each organ/structure; color Doppler flow of each vessel and circumferential involvement of the main abdominal vessels; the relationship of the tumor with adjacent organs and structures regarding the presence or absence of cleavage planes and signs of invasion; and the dynamic evaluation of the sliding sign between the tumor and adjacent structures during respiratory motion, Valsalva maneuver, gentle abdominal pressure, intestinal peristalsis, and arterial pulsatility. Invasion or encasement of adjacent structures by the tumor was defined as follows:[16] Absent, when the sliding between the tumor and adjacent structures was independent; present, when the tumor and the adjacent structures moved together (en bloc) or when there was no perceived sliding.
CT imageSph-CT was performed using a PHILIPS Brilliance 16 or TOSHIBA Aquillion 64 according to the institution's protocol, which consisted of a contrast-enhanced single-phase image acquired only in the portal venous phase after intravenous administration of iodinated contrast under general anesthesia in small children and without anesthesia in older patients, according to the ALARA (as low as reasonably achievable) principle.18
Consensus for USG and CT image reportsThe authors defined, as a consensus, that the statistical analysis would consider each solid organ, hollow viscus, skeletal muscle structure, and main abdominal vessel in contact or adjacent to the tumor as a study unit.
Image assessment, as well as sph-CT and USG reports of all solid abdominal pediatric tumors included in this study, were based on guidelines for the 2 main types of pediatric abdominal tumors. Neuroblastomas were evaluated according to international guidelines,6 and renal tumors were assessed according to the Umbrella protocol.5 To standardize CT and USG descriptions, the used terms followed the consensus of the neuroblastoma study group:[6] Separation meant there was a visible layer, usually consisting of fat tissue, between the tumor and the adjacent structures; contact meant there was no visible layer between the tumor and the adjacent structures, and for vascular structures, it meant that less than 50% of the circumference was in contact with the tumor; invasion of vascular structures meant that more than 50% of the circumference of the vessel was in contact with the tumor; infiltration meant that the tumor extended to other organs, vessels excluded, or that the margins were not well defined (Supplementary material 1).
Color Doppler USG was performed for all tumors that were in contact with the inferior vena cava, renal veins, superior and inferior mesenteric veins, portal vein, splenic vein, and iliac veins.5
For comparison purposes, abdominal organs and structures were divided into four groups: Group 1– solid organs (liver, spleen, pancreas, kidneys, adrenal glands, uterus, and ovaries); Group 2– major and intermediate abdominal vessels (abdominal aorta, iliac artery and vein, superior mesenteric artery and vein, inferior mesenteric artery and vein, celiac trunk, splenic artery and vein, hepatic artery, renal artery and vein, inferior vena cava, and portal vein); Group 3– gastrointestinal (GI) organs (stomach, small bowel, large bowel, and bladder); and Group 4– neuromuscular structures (diaphragm, abdominal wall, lumbar and sacral nerve roots).
Surgical reportsAll patients underwent surgical resection through an exploratory laparotomy (gold standard). The surgical team had full access to the sph-CT and USG reports. After the surgery, surgeons constructed a surgical report following a checklist based on images of all organs and structures that showed signs of invasion, circumferential involvement, or simply adherence to the tumor. The adjacent structures and organs that had been described as compromised by sph-CT or USG assessments and were found free in surgery (and vice-versa) were also included in the surgical reports. sph-CT and USG findings were correlated to the surgical reports.
Statistical analysisOur primary endpoint was the correlation between sph-CT and USG findings and the surgical findings (gold standard). The analyzed performance metrics included sensitivity (SEN), specificity (SPE), positive predictive value (PPV), negative predictive value (NPV), and accuracy (AC). The Kappa test was used to evaluate sph-CT and USG agreement in the detection of invasion or encasement of organs and adjacent structures by the tumor.19 Statistical analysis was performed using the IBM SPSS Statistics software, version 20.0, and p < 0.05 was considered significant.
ResultsPatient characteristicsFigure 1 depicts the STARD flow diagram for this study. The demographic data and distribution of tumors according to age group are summarized in Tables 1 and 2. This study included 50 patients, of which 28 (56%) were female and 22 (44%) were male. The mean age was 25.5 months. Histologic findings indicated that 19 of the tumors were of neuroblastic origin and 20 were of renal origin; 11 patients presented other types of tumors, of which 5 were adrenal carcinomas. The mean tumor volume was 357cm³ (10-3930cm³). Complete surgical resection with negative microscopic margins (R0) was successfully performed in 44 (88%) patients. In two neuroblastoma patients, only partial tumor resection could be performed (debulking). The remaining 4 patients were considered unresectable (1-adrenal carcinoma, 1-rhabdomyosarcoma of the uterus, and 2-neuroblastic tumors).
Performance measurements are detailed in Table 3. At preoperative planning, sph-CT had an SEN of 90.3%, SPE of 86.8%, PPV of 75.3%, NPP of 95.3%, and AC of 87.9%. USG had an SEN of 86.6%, SPE of 94.6%, PPV of 86.9%, NPP of 94.4%, and AC of 92.2%. The Cohen's kappa coefficient between these two methods in the identification of the invasion/encasement of adjacent structures was 0.72 (p < 0.001). The comparison between sph-CT and USG results by the Chi-squared test with Yates correction showed statistical significance for SPE (p < 0.001), PPV (p = 0.002), and AC (p = 0.006).
Performance of USG and CT examinations in assessing the invasion or encasement adjacent structures in children with solid abdominal tumors.
95%CI, 95% confidence interval; AC, accuracy; CT, computed tomography; NPP, negative predictive value; PPV, positive predictive value; SEN, sensitivity; SPE, specificity; USG, ultrasonography; NPV, negative predictive value.
p- comparison by the Chi-Squared test and Yates correction.
The performance of sph-CT and USG in identifying the invasion/encasement of adjacent structures by the tumor was evaluated by grouping organs as described in the Methods section (Supplementary material 2). In Group 1 (solid organs), 252 units were evaluated by sph-CT and USG. At surgery, 89 units showed signs of invasion/encasement by the adjacent tumor (CT = 108; USG = 87). Sph-CT and USG presented respectively: 93.1% vs 88.7% SEN, 84.1% vs 95% SPE, 75.6% vs 90.8% PPV, 95.8% vs 93.9% NPP, and 87.3% vs 92.8% AC. The kappa correlation coefficient was 0.77 (p < 0.001). The comparison between sph-CT and USG results by the Chi-squared test with Yates correction showed statistical significance for SPE (p = 0.002) and PPV (p = 0.011).
In Group 2 (vessels), 293 units were evaluated by sph-CT and 307 by USG. At surgery, 91 units showed signs of invasion/encasement (CT = 80; USG = 84). Sph-CT and USG, respectively, demonstrated a SEN of 91.2% vs 92.3%, SPE of 91% vs 92.1%, PPV of 82.2% vs 83.2%, NPP of 95.8% vs 96.6%, and AC of 91.1% vs 92.2%. The kappa correlation coefficient was 0.79 (p < 0.001). The comparison between sph-CT and USG by the Chi-squared test with Yates correction did not show statistical significance.
In Group 3 (GI organs), 54 units (20 invasion/encasement) were identified by sph-CT and 59 (8 invasion/encasement) by USG. During surgery, 14 units were detected. Sph-CT and USG provided a SEN of 85.7% vs 63.6%, respectively, a SPE of 80% vs 97.9%, PPV of 60% vs 87.5%, NPP of 94.1% vs 92.2%, and AC of 81.5% vs 91.5%. The kappa correlation coefficient was 0.27 (p < 0.027). The comparison between sph-CT and USG by the Chi-squared test with Yates correction showed statistical significance for SPE (p = 0.016).
In Group 4 (neuromuscular structures), 135 units (42 invasion/encasement) were identified by sph-CT and 142 (29 invasion/encasement) by USG. Surgery detected 33 units with signs of invasion/encasement. Sph-CT and USG demonstrated a SEN of 81.8% vs 74.1%, respectively, a SPE of 85.2% vs 97.3%, PPV of 64.3 vs 88.5%, NPP of 93.6% vs 93.1%, and AC of 84.4% vs 92.3%. The kappa correlation coefficient was 0.57 (p < 0.001). The comparison between sph-CT and USG by the Chi-squared test with Yates correction showed statistical significance for SPE (p = 0.004).
USG allowed the diagnosis of the invasion of vascular structures (aorta, splenic artery, right renal vein, and left renal vein), which was missed by sph-CT in 4 patients (1 for each structure). Similar results were observed in 3 patients with muscular invasion (diaphragm, anterior abdominal wall, and psoas muscle). A large bowel invasion was also detected by USG and missed by the sph-CT. Therefore, in 14% (7/50) of the patients (1 patient had 2 invaded structures), the invasion of adjacent structures was diagnosed by USG but not by sph-CT.
DiscussionThis study estimated the accuracy of abdominal single-phase enhanced CT and USG in the preoperative assessment of solid abdominal tumors in children. The performance of these imaging methods was evaluated in detail by the contact between the tumor and adjacent structures. These 2 methods have been compared by only a few other studies.16,17,20 In the present study (the first ever performed in children), using the detailed methodology of evaluating every contact surface of the tumor with the surrounding structures, sph-CT and USG had accuracies of 88% and 92%, respectively, in the evaluation of organs and structures invaded, adhered to, or encased by the tumor.
In 1986, Reiman et al. compared CT and USG performances in the preoperative evaluation of Wilms tumors in children. Accuracy was 77% for CT and 23% for USG.17 The fact that our results differed so much from those presented by this study is probably due to technical improvements in medical imaging examinations.
More recently, Gupta et al. compared the accuracy of CT and USG in the detection, localization, determination of the extension, and diagnosis of abdominal masses (malignant or benign) in children. The compared accuracy of CT and USG was 100% vs 81% regarding the nature of the tumor, 100% vs 59% considering its extension, 97% vs 64.5% for its localization, and 81% vs 54.5% regarding diagnosis.20
The accuracy of CT and MRI in the preoperative evaluation of abdominal neuroblastoma was recently assessed. CT had an accuracy of 100%, which was similar to our results.21 MRI underestimated tumor extension in 54% of the patients, and CT changed surgical planning in 25% of the patients.21
To the best of our knowledge, this is the first study that compared the accuracy between sph-CT and USG in the preoperative evaluation of solid abdominal tumors in children. The AC of sph-CT was 88%, while that of USG was 92%, with a kappa coefficient of 0.72 (p < 0.001). This correlation was high for adjacent solid organ invasion (0.77) and the invasion of vascular structures (0.79). However, the coefficient was low for detecting the invasion of GI organs (0.27) and moderate for the invasion of muscular structures (0.57).
In the present study, sph-CT underestimated invasion/encasement of adjacent structures in 14% (7/50) of the patients, of which 1 had two undetected invaded structures. Among these patients, 4 presented invasion of vascular structures (8%), and 3 presented invasion of muscular-aponeurotic structures and a large bowel invasion (6%). Additional findings obtained by USG complemented the sph-CT results in 14% of the patients.
Even though it presented a high accuracy (92%), the USG cannot be considered the sole imaging examination for the preoperative assessment of solid abdominal tumors, mainly because it is operator-dependent. Although some authors have described the sliding of organs as a useful tool for determining tumor invasion, no statistical analysis has been performed on this matter.5,7 Apart from the previously cited study,16 no other investigations have been performed with a similar methodology to that used in this work.
This study has some limitations: it was retrospective, may contain possible patient selection bias, USG was only performed once and by a single radiologist, the operator dependency of the USG is an inherent limitation, the authors had a small sample size and a heterogeneous population, and tumor stage was not considered. Low-stage tumors may have increased the accuracy of both methods. Nevertheless, the common endpoint for all patients was surgical resection, which attenuated the heterogeneity aspect of our sample. Both the internal and the external validities of this study still seem to be important, since most of the sample (39 out of 50 cases) consisted of children with the most common pediatric neoplasms such as Wilms' tumor and neuroblastoma. This study brings to light important data regarding the reliability of abdominal CT and compares their findings with those of USG.16 In underdeveloped countries, MRI is less frequently available than CT, which in turn is not as available as USG.
In conclusion, the results of this study demonstrate that USG can be complementary to sph-CT in the preoperative evaluation of children with an abdominal tumor. In several hospitals worldwide that do not possess a pediatric radiology unit, the CT scan protocols include multiphase CT imaging rather than single-phase CT imaging for evaluation of abdominal tumors in children. Our study detected a high accuracy of ultrasonography (USG) in the evaluation of solid abdominal tumors in children. Thus, it seems that this piece of evidence could serve to help the surgeons in surgical planning. In other words, since the combination of single-phase CT and USG provides very high accuracy, the authors defend that multiphase CT scans should be avoided in children.
Study conducted at the Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil.