To measure the prevalence of vitamin D deficiency (through the 25-hydroxyvitamin D metabolite) in pediatric patients using antiepileptic drugs.
Source of dataMeta-analysis of studies identified through search in the PubMed, Embase, LILACS, and Cochrane Library databases, on February 19, 2019.
Summary of dataA total of 748 articles were identified, 29 of which were relevant to the objectives of this study. The prevalence of vitamin D deficiency found was 0.32 (95% CI=0.25–0.41; I2=92%, p<0.01). In the subgroup analyses, the most significant results were observed in the group of patients using cytochrome P450-inducing antiepileptic drugs, with a prevalence of 0.33 (95% CI=0.21–0.47; I2=86%, p<0.01) and, considering the study design, in the subgroup of cohort studies, with a prevalence of 0.52 (95% CI=0.40–0.64; I2=76%, p<0.01).
ConclusionsTaking into account the deleterious effects of vitamin D deficiency on the bone health of individuals using antiepileptic drugs, it is suggested to include in their care 25-hydroxyvitamin D monitoring, cholecalciferol supplementation, and treatment of the deficiency, when present.
Mensurar a prevalência de deficiência de vitamina D (através do metabólito 25-hidroxivitamina D) em pacientes pediátricos em uso de fármacos antiepilépticos.
Fonte dos dadosMetanálise de estudos identificados por meio de pesquisa nas bases de dados Pubmed, Embase, LILACS e Cochrane em 19 de fevereiro de 2019.
Síntese dos dadosForam identificados 748 artigos, dos quais 29 mostraram-se relevantes aos objetivos deste estudo. A prevalência de deficiência de vitamina D encontrada foi de 0,32 (IC 95%=0,25 – 0,41) (I2=92%, p<0,01). Nas análises por subgrupos, os resultados mais expressivos foram observados no grupo de pacientes em uso de fármacos antiepilépticos indutores do citocromo P450, que apresentou prevalência de 0,33 (IC 95%=0,21 – 0,47) (I2=86%, p<0,01). Considerou-se o delineamento dos estudos, no subgrupo de estudos de coorte, com prevalência de 0,52 (IC 95%=0,40 – 0,64) (I2=76%, p<0,01).
ConclusõesLevando-se em consideração os efeitos deletérios da deficiência de vitamina D na saúde óssea dos sujeitos em uso de fármacos antiepilépticos, sugere-se incluir em seu atendimento, o monitoramento de 25-hidroxivitamina D, suplementação com colecalciferol e tratamento de deficiência quando existente.
The prevalence of epilepsy in the pediatric age group in developing countries can reach up to ten cases per 1000 children.1 Treatment with antiepileptic drugs is necessary for a long period in most patients2 and is associated with several adverse effects, such as gingival hyperplasia, gastrointestinal disorders, osteoporosis, osteomalacia, bone marrow toxicity, teratogenicity, hepatotoxicity, and nephrotoxicity, as well as endocrine, neurological, psychiatric, and dermatological disorders.3
Regarding vitamin D deficiency, a population-based study estimates a prevalence of 15% in the general pediatric population, with data referring to the United States.4 Considering the subjects using antiepileptic drugs, there is evidence that supports a prevalence above 70% in the pediatric population.5 Subclinical disease is characterized by biochemical abnormalities (reduced serum levels of calcium and 25-hydrovitamin D and high parathyroid hormone levels), reduction in bone mineral density and changes in bone biopsy findings.6,7 In a study conducted with members of the board of directors of the American Academy of Neurology, it was found that around 40% of pediatric neurologists routinely screen for bone mineral disease, 40% prescribe calcium and vitamin D to patients who already have the disease, and only 9% prophylactically prescribe calcium and vitamin D to subjects using antiepileptic drugs.7
The association between antiepileptic drug use and bone mineral disease was first reported in 1960.8 Since then, several studies have been performed aiming to understand the effect of antiepileptic drugs on bone metabolism, the factors that corroborate bone disease in individuals with epilepsy, and the real need for treatment and prevention in these patients.9–12
Taking into account the classification of antiepileptic drugs as inducers and non-inducers of cytochrome P450 (CYP450) enzyme system, some studies support the idea that the use of inducers is related to higher levels of vitamin D deficiency,12,13 since they can accelerate the hepatic metabolism of vitamin D, with a consequent reduction in its serum level.5
The current meta-analysis aims to measure the prevalence of vitamin D deficiency (measured through 25-hydroxyvitamin D) in pediatric patients using antiepileptic drugs, as well as to verify the prevalence of deficiency in subgroups, considering the study design, antiepileptic drugs used, and patients exclusively with epilepsy (without motor impairment).
MethodsThe literature search, study selection based on title and abstract, and data extraction were carried out independently by two trained reviewers. A senior author was consulted in cases of disagreement. In case of duplicate articles, only one was considered. The report of this meta-analysis followed the recommendations proposed for the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines14 and is registered on the PROSPERO Platform (PROSPERO, University of York, England) under numberCRD42020143322.
Search strategyThe studies were identified through research in the PubMed, Embase, LILACS, and Cochrane Library databases, performed on February 19, 2019. For the search, the term “antiepileptic drug” was used in combination with “vitamin D,” through structured Medical Subject Headings (MeSH) keywords for PubMed, Emtree for Embase, and Health Sciences Descriptors (DeCS) for LILACS. The terms used in the search and the number of articles found per database are depicted in Table S1 of the Supplementary Material.
Eligibility criteriaThe inclusion criteria were as follows: studies published in Portuguese, English, or Spanish, published on any date, and evaluating patients aged 0 to 18 years who were using antiepileptic drugs. Data related to patients who were receiving vitamin D supplementation or medications that act on the metabolism of this vitamin (e.g., glucocorticoids), those with a diagnosis of comorbidities that alter vitamin D metabolism (e.g., kidney, liver, gastrointestinal or endocrine disease), review articles, and studies with incomplete or data not published in full were excluded.
Data collectionAfter assessing the title, abstract, and full text of the studies according to the eligibility criteria, the data of interest were collected using a standard form. The following information was collected: authors, study place, design, year of publication, mean age, 25-hydroxy vitamin D levels characterized as deficiency, prevalence of vitamin D deficiency (measured using 25-hydroxyvitamin D), number of patients exclusively with epilepsy (without motor disability), antiepileptic drugs used, and their minimum time of use. The antiepileptic drugs were categorized according to their effect on the CYP450 system as inducers (carbamazepine, phenobarbital, phenytoin, topiramate, oxcarbazepine, and primidone), non-inducers (valproic acid and clobazam), or not metabolized by this system (levetiracetam, gabapentin, ethosuximide, vigabatrin, zonisamide).15,16 The type of study was recorded according to the interpretation of the data by the researchers. The choice of selecting patients exclusively with epilepsy was because motor impairment is considered an independent factor for 25-hydroxyvitamin D deficiency.8
Statistical analysisThe studies were grouped in a meta-analysis. The dichotomous variables were expressed as proportions (percentage) and the continuous variables as means and standard deviations. The summary measure of the prevalence of vitamin D deficiency was computed as the proportion (and respective 95% confidence interval) of patients with deficiency over the total sample, weighted by the study weight, using the random effects model. The inconsistency test (I2) was used to assess heterogeneity between studies. A p-value <0.05 was considered to be statistically significant. The statistical analysis was performed using the “meta” package of the R program (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, version 3.5.1, Vienna, Austria).
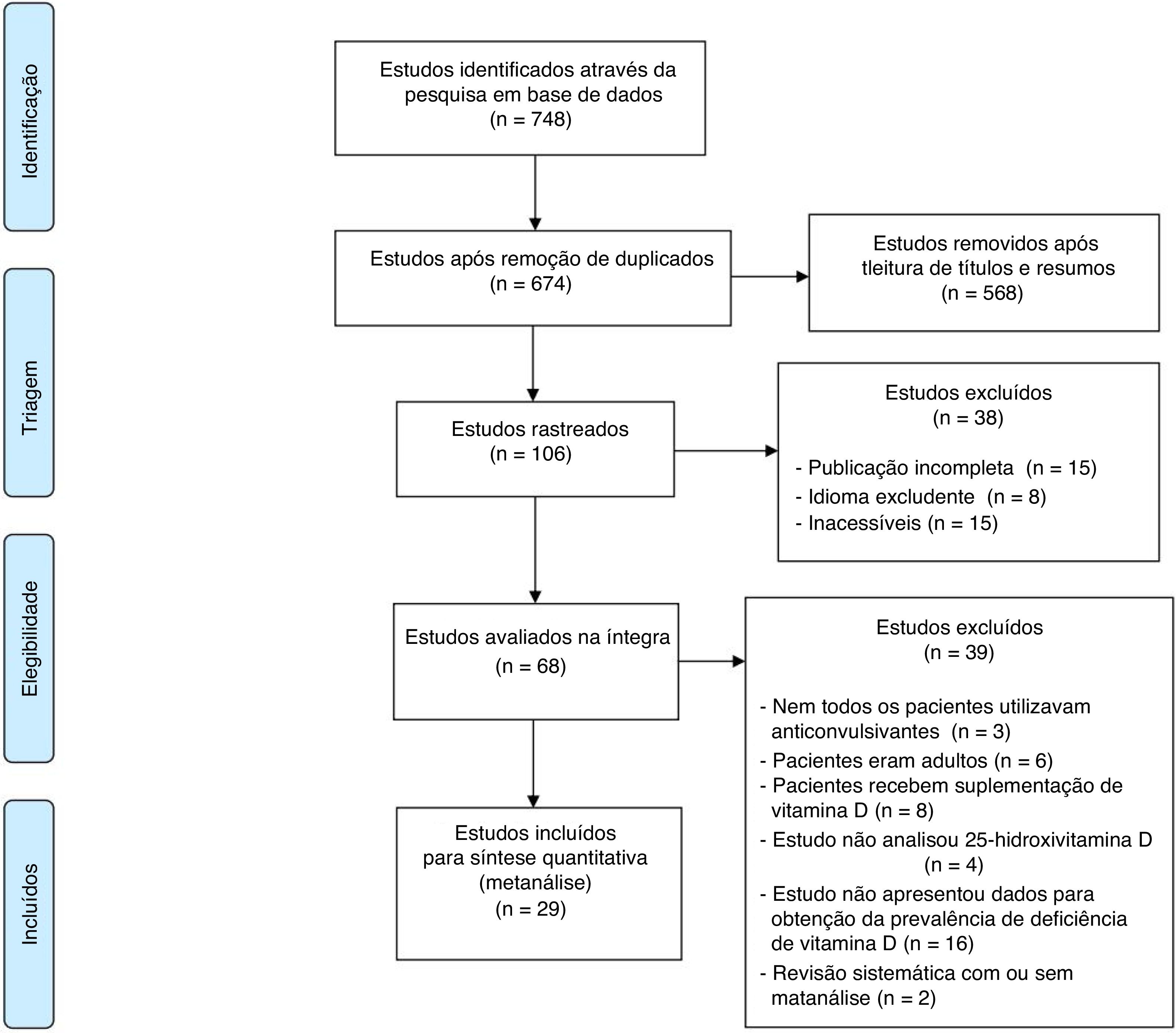
ResultsThe flowchart showing the search results and selection details is depicted in Fig. 1.17 During the search, 748 articles were found, of which 29 were relevant to the objectives of this study (Table 1).18–39 A total of 2368 children were included.
Flowchart with the search results and selection details.
Adapted from Moher et al.17
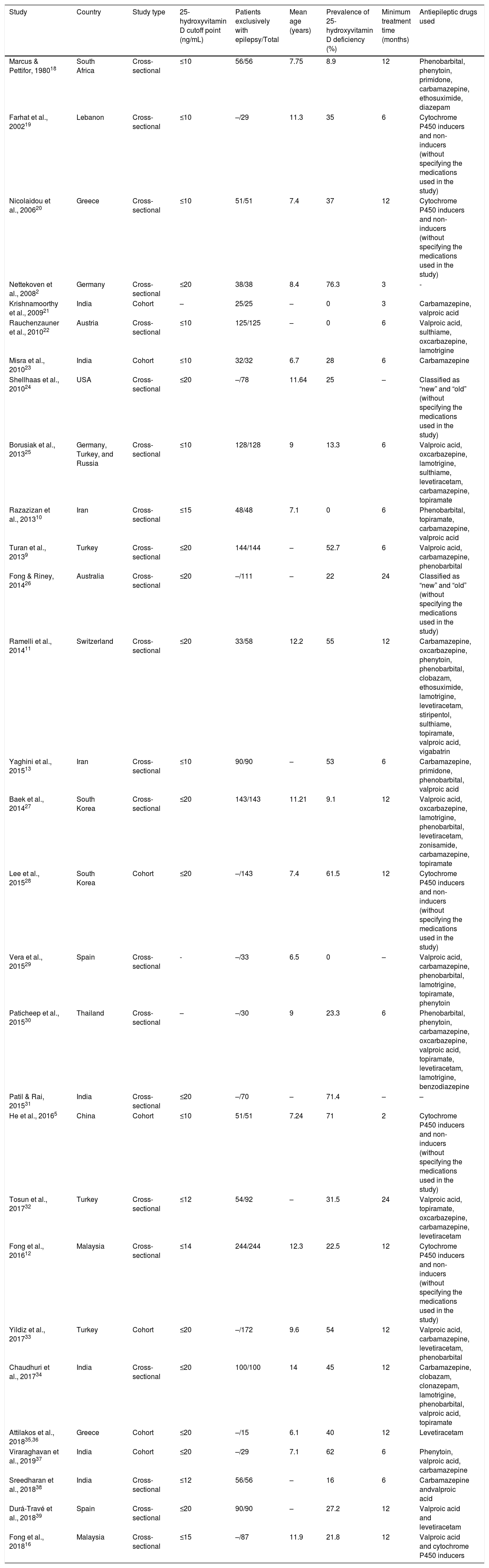
Characteristics of the studies included in the final analysis.
| Study | Country | Study type | 25-hydroxyvitamin D cutoff point (ng/mL) | Patients exclusively with epilepsy/Total | Mean age (years) | Prevalence of 25-hydroxyvitamin D deficiency (%) | Minimum treatment time (months) | Antiepileptic drugs used |
|---|---|---|---|---|---|---|---|---|
| Marcus & Pettifor, 198018 | South Africa | Cross-sectional | ≤10 | 56/56 | 7.75 | 8.9 | 12 | Phenobarbital, phenytoin, primidone, carbamazepine, ethosuximide, diazepam |
| Farhat et al., 200219 | Lebanon | Cross-sectional | ≤10 | –/29 | 11.3 | 35 | 6 | Cytochrome P450 inducers and non-inducers (without specifying the medications used in the study) |
| Nicolaidou et al., 200620 | Greece | Cross-sectional | ≤10 | 51/51 | 7.4 | 37 | 12 | Cytochrome P450 inducers and non-inducers (without specifying the medications used in the study) |
| Nettekoven et al., 20082 | Germany | Cross-sectional | ≤20 | 38/38 | 8.4 | 76.3 | 3 | - |
| Krishnamoorthy et al., 200921 | India | Cohort | – | 25/25 | – | 0 | 3 | Carbamazepine, valproic acid |
| Rauchenzauner et al., 201022 | Austria | Cross-sectional | ≤10 | 125/125 | – | 0 | 6 | Valproic acid, sulthiame, oxcarbazepine, lamotrigine |
| Misra et al., 201023 | India | Cohort | ≤10 | 32/32 | 6.7 | 28 | 6 | Carbamazepine |
| Shellhaas et al., 201024 | USA | Cross-sectional | ≤20 | –/78 | 11.64 | 25 | – | Classified as “new” and “old” (without specifying the medications used in the study) |
| Borusiak et al., 201325 | Germany, Turkey, and Russia | Cross-sectional | ≤10 | 128/128 | 9 | 13.3 | 6 | Valproic acid, oxcarbazepine, lamotrigine, sulthiame, levetiracetam, carbamazepine, topiramate |
| Razazizan et al., 201310 | Iran | Cross-sectional | ≤15 | 48/48 | 7.1 | 0 | 6 | Phenobarbital, topiramate, carbamazepine, valproic acid |
| Turan et al., 20139 | Turkey | Cross-sectional | ≤20 | 144/144 | – | 52.7 | 6 | Valproic acid, carbamazepine, phenobarbital |
| Fong & Riney, 201426 | Australia | Cross-sectional | ≤20 | –/111 | – | 22 | 24 | Classified as “new” and “old” (without specifying the medications used in the study) |
| Ramelli et al., 201411 | Switzerland | Cross-sectional | ≤20 | 33/58 | 12.2 | 55 | 12 | Carbamazepine, oxcarbazepine, phenytoin, phenobarbital, clobazam, ethosuximide, lamotrigine, levetiracetam, stiripentol, sulthiame, topiramate, valproic acid, vigabatrin |
| Yaghini et al., 201513 | Iran | Cross-sectional | ≤10 | 90/90 | – | 53 | 6 | Carbamazepine, primidone, phenobarbital, valproic acid |
| Baek et al., 201427 | South Korea | Cross-sectional | ≤20 | 143/143 | 11.21 | 9.1 | 12 | Valproic acid, oxcarbazepine, lamotrigine, phenobarbital, levetiracetam, zonisamide, carbamazepine, topiramate |
| Lee et al., 201528 | South Korea | Cohort | ≤20 | –/143 | 7.4 | 61.5 | 12 | Cytochrome P450 inducers and non-inducers (without specifying the medications used in the study) |
| Vera et al., 201529 | Spain | Cross-sectional | - | –/33 | 6.5 | 0 | – | Valproic acid, carbamazepine, phenobarbital, lamotrigine, topiramate, phenytoin |
| Paticheep et al., 201530 | Thailand | Cross-sectional | – | –/30 | 9 | 23.3 | 6 | Phenobarbital, phenytoin, carbamazepine, oxcarbazepine, valproic acid, topiramate, levetiracetam, lamotrigine, benzodiazepine |
| Patil & Rai, 201531 | India | Cross-sectional | ≤20 | –/70 | – | 71.4 | – | – |
| He et al., 20165 | China | Cohort | ≤10 | 51/51 | 7.24 | 71 | 2 | Cytochrome P450 inducers and non-inducers (without specifying the medications used in the study) |
| Tosun et al., 201732 | Turkey | Cross-sectional | ≤12 | 54/92 | – | 31.5 | 24 | Valproic acid, topiramate, oxcarbazepine, carbamazepine, levetiracetam |
| Fong et al., 201612 | Malaysia | Cross-sectional | ≤14 | 244/244 | 12.3 | 22.5 | 12 | Cytochrome P450 inducers and non-inducers (without specifying the medications used in the study) |
| Yildiz et al., 201733 | Turkey | Cohort | ≤20 | –/172 | 9.6 | 54 | 12 | Valproic acid, carbamazepine, levetiracetam, phenobarbital |
| Chaudhuri et al., 201734 | India | Cross-sectional | ≤20 | 100/100 | 14 | 45 | 12 | Carbamazepine, clobazam, clonazepam, lamotrigine, phenobarbital, valproic acid, topiramate |
| Attilakos et al., 201835,36 | Greece | Cohort | ≤20 | –/15 | 6.1 | 40 | 12 | Levetiracetam |
| Viraraghavan et al., 201937 | India | Cohort | ≤20 | –/29 | 7.1 | 62 | 6 | Phenytoin, valproic acid, carbamazepine |
| Sreedharan et al., 201838 | India | Cross-sectional | ≤12 | 56/56 | – | 16 | 6 | Carbamazepine andvalproic acid |
| Durá-Travé et al., 201839 | Spain | Cross-sectional | ≤20 | 90/90 | – | 27.2 | 12 | Valproic acid and levetiracetam |
| Fong et al., 201816 | Malaysia | Cross-sectional | ≤15 | –/87 | 11.9 | 21.8 | 12 | Valproic acid and cytochrome P450 inducers |
The studies were carried out in 17 different countries, most of them located in the northern hemisphere. Turkey and India were the countries with the most publications. Regarding the cutoff point used to define 25-hydroxyvitamin D deficiency, they ranged from 10 to 20ng/mL, with the value of 20ng/mL being the most frequently used (13 studies).
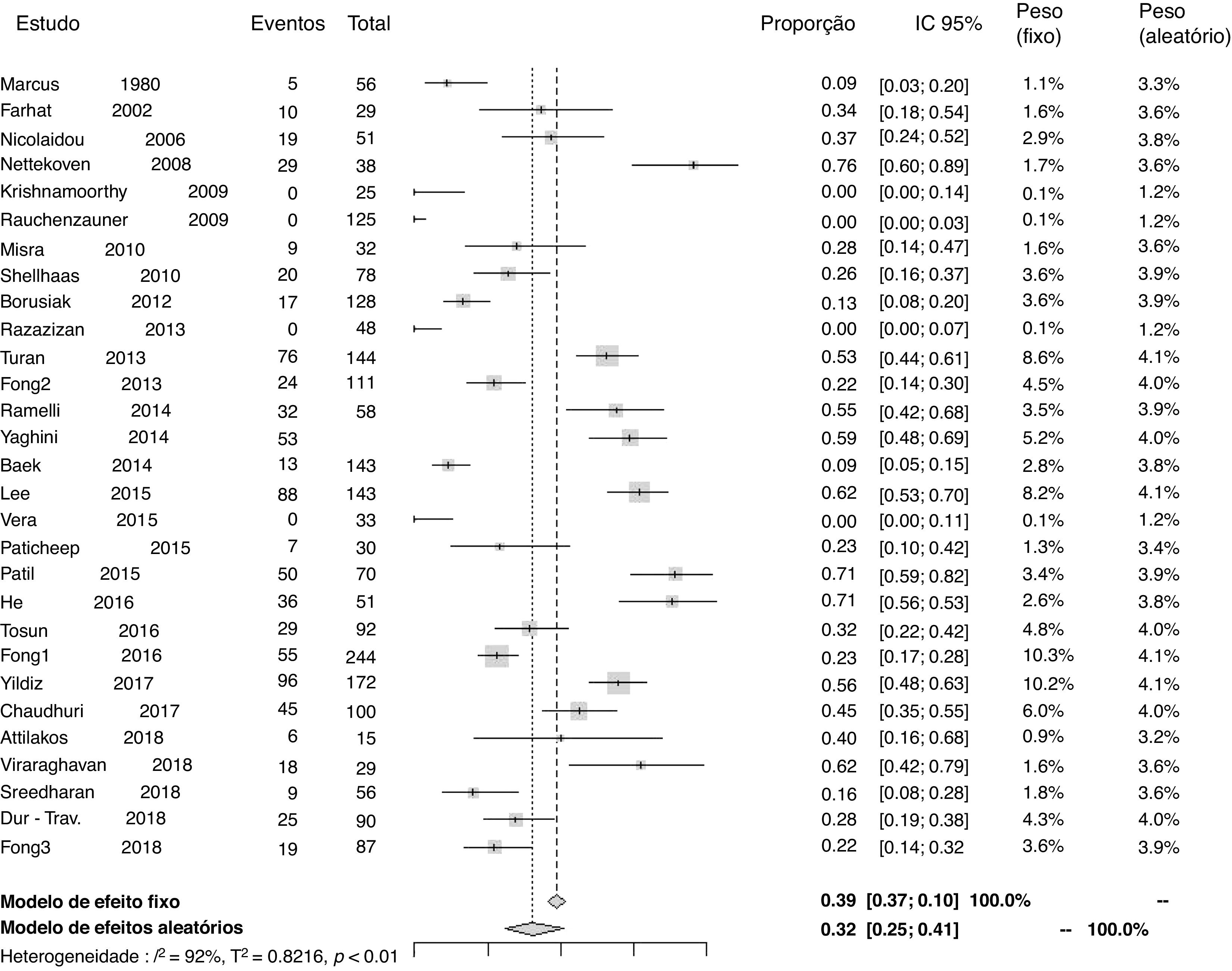
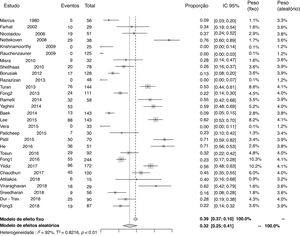
Prevalenceof25-hydroxyvitamin D deficiencyTwenty-nine studies reported data to calculate the prevalence of 25-hydroxyvitamin D deficiency. The proportion of patients using antiepileptic drugs who developed deficiency in relation to the total number of patients was 0.32 (95% CI=0.25–0.41; I2=92%, p<0.01; Fig. 2). A funnel plot analysis showed the presence of publication bias.
Prevalence of 25-hydroxyvitamin D deficiency by subgroupsThe prevalence of 25-hydroxyvitamin D deficiency was analyzed by subgroups, according to the type of study (cross-sectional and cohort), considering patients exclusively with epilepsy (without motor impairment) and according to the type of antiepileptic drug used (CYP450 inducers, non-inducers of CYP450, or not metabolized by this route). The results were as follows: in the sample of cross-sectional studies (22 articles), 0.28 (95%CI=0.21–0.37; I2=92%, p<0.01); in the sample of cohort studies (seven articles), 0.52 (95%CI=0.40–0.64; I2=76%, p<0.01); in the analysis of patients exclusively with epilepsy, 0.29 (95%CI=0.20–0.40; I2=92%, p<0.01); in the group of patients using antiepileptic drugs that were CYP450 inducers, 0.33 (95% CI=0.21–0.47; I2=86%, p<0.01); in the group of patients using antiepileptic drugs that were not CYP450 inducers, 0.24 (95% CI=0.15–0.36; I2=82%, p<0.01); in the group of patients using other antiepileptic drugs, which have no effect related to liver metabolism through CYP450, 0.25 (95%CI=0.11–0.46; I2=64%, p<0.01). The analysis of prevalence was also performed, disregarding the four studies that had zero patients with 25-hydroxyvitamin D deficiency.10,21,22,29 In the analysis, the proportion of patients who developed 25-hydroxyvitamin D deficiency in relation to the total number of patients was 0.37 (95%CI=0.29–0.45; I2=92%, p<0.01). Funnel plots showed publication bias in the subgroup analyses.
DiscussionThis meta-analysis summarized the data from 29 studies that assessed 25-hydroxyvitamin D deficiency in pediatric patients using antiepileptic drugs, comprising a total of 2368 patients. Approximately 760 had vitamin D deficiency at the time of assessment – a prevalence of 32%. This result is consistent with what the medical literature has shown for some decades, that the use of antiepileptic drugs may result in 25-hydroxyvitamin D deficiency and consequent worsening of bone health in patients with epilepsy.8,40
It is known that sun exposure and diet are some of the main factors involved in the metabolism and plasma concentrations of 25-hydroxyvitamin D. Therefore, a higher prevalence of 25-hydroxyvitamin D deficiency is expected in countries located at higher latitudes41 and in places with a diet low in vitamin D2 and D3.42 Taking into account that Turkey was the country that generated the most studies for this review, a reference for the 25-hydroxyvitamin D value in that population was sought. Hocaoglu-emre et al.43 evaluated a sample of 640 children living in Istanbul (Turkey) and found a mean 25-hydroxyvitamin D value of 25.95ng/mL and a deficiency prevalence of 5.6%, considering values <12ng/mL. This information suggests that the prevalence found in this meta-analysis may actually be related to the use of antiepileptic drugs.
Conditions that determine motor impairment, such as cerebral palsy, are also independently associated with bone disease.8 Aiming to exclude a possible confounding factor, a meta-analysis of the subgroup of patients exclusively with epilepsy was performed, i.e., those without motor disabilities. Unlike what some studies in the literature previously showed,28,32 the subgroup analysis showed no difference in the prevalence of 25-hydroxyvitamin D deficiency in relation to that performed for the total sample of studies. The fact that most patients included in the total analysis had only epilepsy contributed to this finding.
Another factor reported as a risk for 25-hydroxyvitamin D deficiency and worsening bone health in these patients is the use of hepatic metabolism-inducing antiepileptic drugs through CYP450.5,12,13 Therefore, an assessment of the prevalence of vitamin D deficiency was also carried out according to the subgroups related to the profile of the antiepileptic drugs used. The subgroup of patients who used antiepileptic drugs that were inducersshowed a higher prevalence of 25-hydroxyvitamin D (33%) compared to the subgroup that used antiepileptic drugs that were non-inducers (24%) and the subgroup that used antiepileptic drugs that were not metabolized through this system (25%).
Antiepileptic drugs can also be divided into potent (phenobarbital, phenytoin, and carbamazepine) or weak inducers (topiramate at doses above 200mg/day and oxcarbazepine)44; however, this subanalysis was not conducted in this study. It is likely that for each antiepileptic drug there is more than one mechanism responsible for its effect on bone metabolism,45 as well as that for each subject there is an individual response to the antiepileptic drug used, which is genetically determined.46
Considering the design of the articles included, all were observational studies. It is known that meta-analyses of observational studies have particular characteristics due to inherent biases and different study designs.14 In an attempt to make the analysis more homogeneous, sub-analyses were performed according to the type of study. In the analysis of the cohort studies, there was a prevalence of 25-hydroxyvitamin D deficiency of 52% and slightly less heterogeneity among the studies in comparison with the analysis of the cross-sectional studies, which maintained values similar to those observed in the evaluation in the total sample of the studies.
Regarding the analysis of the prevalence of 25-hydroxyvitamin D, disregarding the studies that showed no patients with 25-hydroxyvitamin D deficiency, it was observed that there was no difference in the value related to the heterogeneity when compared to the total sample. The possible explanations found for the lack of patients with deficiency in these studies were as follows: Rauchenzauner et al.22 included only monotherapy patients with non-enzyme-inducing antiepileptic drugs or minimally enzyme-inducing antiepileptic drugs; Krishnamoorthy et al.21 and Vera et al.29 did not disclose the cutoff point used for 25-hydroxyvitamin D deficiency and their studies have a small sample size; and finally, Razazizan et al.10 characterized their sample as monotherapy patients, who had normal physical activity and were adequately exposed to sunlight.
Finally, it is emphasized that the main innovation of this research was the outcome used. Previous meta-analyses used the bone mineral density outcome or considered the 25-hydroxyvitamin D variation with the use of the antiepileptic drug, which, despite being a significant data point, does not always result in clinical significance.8,47,48
LimitationsIt is understood that this meta-analysis has some limitations. Moreover, the studies used different methodologies to assess this metabolite, limitations inherent to the included studies. In the analysis of the main outcome, there is no uniformity between studies regarding the definition of the cutoff point for 25-hydroxyvitamin D deficiency. Undoubtedly, these factors are involved in the high heterogeneity observed in practically all analyses, including by subgroups. The articles also lack data related to the type of epilepsy, cause of the epilepsy, family history of osteoporosis, and physical activity. Keeping in mind that changes in vitamin D metabolism related to the use of antiepileptic drugs seem to have a multifactorial etiology,8 all of these data are possible confounding factors in the analysis.
No sub-analyses were performed according to the classification of antiepileptic drugs as potent or weak inducers of cytochrome P450. Therefore, drugs such as phenobarbital, phenytoin, and carbamazepine could be related to even more significant values of vitamin D deficiency than those determined in this meta-analysis.
Although only articles in Portuguese, Spanish, and English were included for the convenience of the researchers, the impact was minimal, as only eight studies were excluded due to this criterion.
ConclusionVitamin D deficiency and bone disease are neglected clinical situations in the context of long-term use of antiepileptic drugs in childhood. It can be observed that, even though there is a consistent pathophysiological basis for changes in the metabolism of 25-hydroxyvitamin D due to the use of medication, the data evidenced in this meta-analysis do not reliably indicate the existence of a related deficiency. However, as it is a complex, multifactorial disease, it is believed that efforts are still needed toward a better understanding of possible related factors in the process, as well as the standardization of vitamin D deficiency assessment parameters in the studies.
Taking into account the deleterious effects of vitamin D deficiency on bone health and, consequently, on the quality of life of these individuals, it is suggested to include the monitoring of 25-hydroxyvitamin D levels, supplementation with cholecalciferol, and treatment of the deficiency in25-hydroxyvitamin D when it exists. This approach is in line with the principle of not only treating epileptic seizures, but patients with epilepsy, who sometimes have motor impairments, are rarely exposed to sunlight, and do not consume a sufficient daily intake of vitamins D2 and D3. It is also suggested to consider patients using antiepileptic drugs that are cytochrome P450inducers as the group most at risk for developing vitamin D deficiency.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Junges C, Machado TD, Nunes Filho PR, Riesgo R, Mello ED. Vitamin D deficiency in pediatric patients using antiepileptic drugs: systematic review with meta-analysis. J Pediatr (Rio J). 2020;96:559–68.
Study conducted at Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.