Infant sleep problems can affect the child's health. Maternal characteristics have been associated with the quality of infant sleep, but few studies have investigated the impact of intrauterine conditions. The aim of the study was to evaluate the association between adverse intrauterine environments (maternal smoking, hypertension, diabetes, and intrauterine growth restriction) and extrauterine factors on infant sleep in the first 6 months of life.
MethodsProspective cohort study, including singleton and at-term infants. Mothers were interviewed after delivery and at 30 days, 3 months, and 6 months of life. Socioeconomic, breastfeeding, and sleep data were self-reported by mothers using semi-structured interviews. Maternal stress (Perceived Stress Scale) and postpartum depression symptoms (Edinburgh Postpartum Depression Scale) were assessed.
ResultsThere was no statistically significant association between intrauterine environments and the sleep of infants of the 359 mother–child dyads investigated. Total infant sleep time decreased from approximately 13–11h from 30 days to 6 months of age (p<0.001) and the longest period of uninterrupted sleep increased from approximately 4–6h during the same period (p<0.001). Breastfed infants slept longer in 24-h periods in the first month, but they woke up more often throughout the night when compared to infants receiving formula. Mothers with depressive symptoms reported increased sleep latency time.
ConclusionsAdverse intrauterine environments did not significantly affect sleep measures in the first 6 months of life. Maternal characteristics and practices, however, were associated with infant sleep, suggesting that environmental factors significantly contribute to sleep quality early in life.
Sleep plays an important role throughout human growth and development. Sleep problems early in life can have negative repercussions on the health of individuals in the short, medium, and long term. Sleep is a period of considerable neurological and physiological activity, involving intense brain activity and increased cortical function.1 In this sense, the fact that infants spend most of their time sleeping in the first months of life highlights the importance of sleep for brain development and somatic growth.1
Complaints related to infant sleep affect a significant number of families. Parents may report short duration of sleep at night, prolonged sleep latency, and frequent nocturnal awakenings.2 When significant, sleep problems may lead to impairments in the child's emotional, physical, cognitive, and social development.3
Several lines of evidence now suggest that early events in the perinatal period, both intra- and extrauterine, affect the development of the individual's health and illness throughout life.4 Absence of breastfeeding, for example, has been shown to increase the risk of overweight and diabetes in adulthood5 and is associated with lower intelligence quotient (IQ) and higher rates of mental health problems in adolescence.6
Adverse intrauterine environments have also been recognized to affect not only neonatal health, but also the future development of metabolic and cardiovascular diseases.7 Conversely, it is known that the intrauterine environment itself is subject to the influence of maternal, fetal, and placental factors,8 such as the socioeconomic status of the pregnant woman, maternal smoking, and the quality of prenatal care.
Infant sleep can both be affected by maternal health and affect the future health status of those faced with early sleep problems. The identification of factors that influence the quality and quantity of the infant's sleep can also help to counsel and prevent sleep problems during the gestational period and after birth, as well as providing elements for the planning of future interventions. In this perspective, the present study aimed to carry out a prospective analysis of the influence of intra- and extrauterine factors on infant sleep during the first 6 months of life.
MethodsThis study is part of the Impact of Perinatal Different Intrauterine Environments on Child Growth and Development in the First Six Months of Life – IVAPSA birth cohort (IVAPSA), whose main objective was to evaluate the effects of different intrauterine environments on child growth, behavior, and development. This is a thematic, prospective, longitudinal birth cohort with the aim to assess the interactions between the maternal phenotype during gestation (maternal smoking, hypertension, diabetes), the maternal/fetal genotype, and their associations with outcomes related to growth, behavior, and neurodevelopment. A convenience sample was chosen due to the difficulty of obtaining mother–child pairs who met the inclusion criteria for the different groups. Study protocols and detailed project information have been previously published9 as well as some baseline results.10
IVAPSA is therefore a prospective controlled multiple cohort of mothers and newborns seen at two hospitals located in the city of Porto Alegre, capital of the state of Rio Grande do Sul, Brazil (Grupo Hospitalar Conceição and Hospital and Hospital de Clínicas de Porto Alegre). Both hospitals serve the public health system (SUS), with similar care characteristics, and are referral centers for high-risk pregnancies.
All mother–infant pairs born at term from single pregnancies were included in the study. Exclusion criteria were HIV-positive mothers, preterm birth (<37 weeks), twin pregnancy, and infants with congenital diseases or need of hospitalization. After inclusion in the study, the mother–infant pairs were classified into five groups: (1) newborns born to smoking mothers who reported having smoked during pregnancy, regardless of the duration of exposure or the number of cigarettes smoked; (2) newborns born to mothers with diabetes who had been diagnosed with diabetes mellitus (type 1, type 2, or gestational); (3) newborns born to hypertensive mothers diagnosed with hypertensive disorders (pre-eclampsia, eclampsia, pre-eclampsia superimposed on chronic hypertension, chronic hypertension, or gestational hypertension); (4) newborns with idiopathic intrauterine growth restriction (IUGR) who had a birth weight below the 5th percentile for fetal growth, according to the curve proposed by Alexander et al.11 and (5) a control group – mothers who did not have any of the defining conditions of the other intrauterine exposure groups. For the groups (2) and (3), conditions were defined as gestational if diagnosed during the current pregnancy and as chronic if diagnosed during that period or earlier. All these clinical conditions were diagnosed by physicians during the prenatal/birth and obtained from hospital records.
It is important to highlight that the inclusion of mother–infant pairs in each of the five distinct groups only occurred when they met the criteria exclusively. That is, the mother–infant pairs included in each group could not have more than one gestational clinical condition. Therefore, these five groups can be considered as “pure.”
Data were collected through six interviews (at birth, 7 days, 15 days, 30 days, 3 months, and 6 months), some of which were performed at home and others at the Clinical Research Center of Hospital de Clínicas de Porto Alegre. The project was approved by the Institutional Review Board of both participating hospitals. The protocol numbers were 11,0097 at Hospital de Clínicas de Porto Alegre and 11,027 at Grupo Hospitalar Conceição.
Study variablesSleepSleep was evaluated by maternal report at three time points (30 days, 3 months, and 6 months of life), considering the period prior to the last interview. Four independent questions were drawn and adapted from the Sleep Behavior Questionnaire12,13: (1) What is the total duration of your child's sleep during the day? (24-h sleep); (2) What is the total duration of your child's sleep at night? (nocturnal sleep); (3) During the night, how many hours does your child sleep without waking up? (uninterrupted sleep); (4) How long does it take for your child to fall asleep at night? (sleep latency). Based on these questions, three quantitative variables of infant sleep characterization were determined: total sleep time (hours of daytime and nighttime sleep), uninterrupted sleep time, and sleep latency.
Maternal depressionThe presence of maternal depressive symptoms in the postpartum period was evaluated at the 1st, 3rd and 6th months postpartum through the Edinburgh Postpartum Depression Scale (EPDS).14 The EPDS has a sensitivity of 72% and a specificity of 88%, and consists of ten self-reporting items, referring to the last seven days. According to the presence and intensity of the symptoms, scores range from 0 to 30, with a value equal or superior to 10 being considered positive for depressive symptomatology.
Maternal stressThe Perceived Stress Scale (PSS 14)15 was used to quantify the degree to which mothers appraised their situation as stressful. The scale is a self-reported questionnaire, composed of 14 items, with scores ranging from zero (no stress) to 56 (extreme stress). The scale was applied only in the first month after delivery.
Statistical analysisDescriptive statistics was used to establish the main characteristics of the examined variables and were expressed as mean and standard deviations. Analysis of variance (ANOVA) was used to compare the means among the five groups of the study. In case of asymmetry, the Kruskal–Wallis test was applied. Spearman's correlation coefficient was employed to assess the association between sleep scores and other quantitative variables. To compare the growth, sleep evolution, and breastfeeding pattern during the first six months of the baby's life, the generalized estimating equations (GEE) model was applied, complemented with Bonferroni's multiple comparisons test. To control for confounding factors, a multivariate linear regression model was used for the variables that presented a p-value <0.20 in the preliminary bivariate analysis. The significance level adopted was 5% (p≤0.05), and the analyses were performed in SPSS v. 18.0.
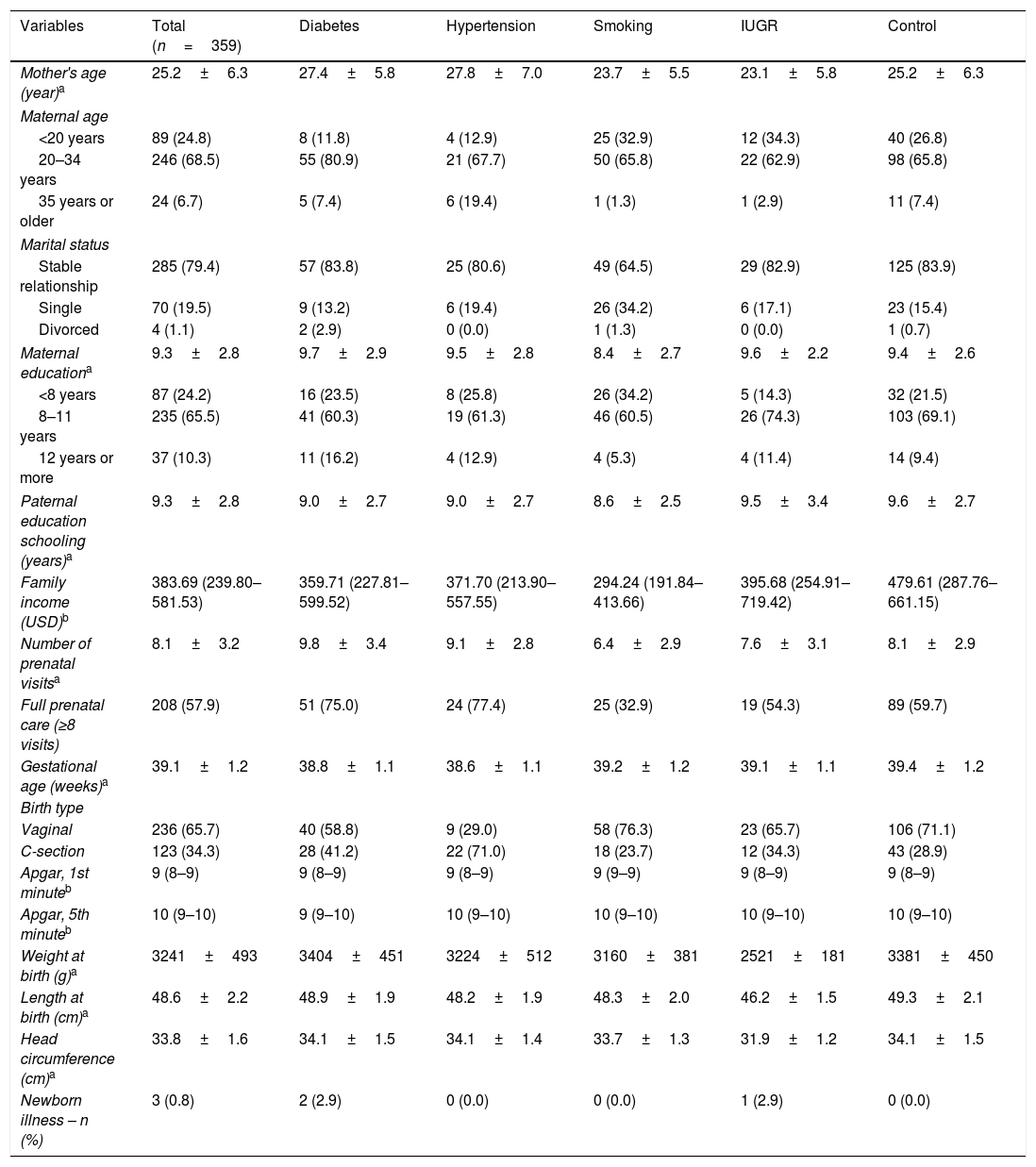
ResultsThe sample consisted of 359 pairs (mother–infant). The mean age of the mothers was 25.2 (±6.3) years, 79.4% of whom were married or in a stable relationship. Mean maternal educational level was 9.3 (±2.8) years of study, similar to that of the parents. Only 10.3% had completed high school or higher education. The median household income was approximately USD 380. Regarding prenatal care, the mean number of prenatal visits was 8.1 (±3.2) (Table 1). The majority of the babies were born vaginally (65.7%), with a mean birth weight of 3241g (±493). Three children (0.8%) presented clinical alterations at the first month of life (hydronephrosis, heart murmur, and cyanosis), which were followed up with clinical investigation. In the first six months, 97.2% of the infants were cared for by their mothers (Table 1).
Sociodemographic and maternal-fetal characteristics of the sample.
| Variables | Total (n=359) | Diabetes | Hypertension | Smoking | IUGR | Control |
|---|---|---|---|---|---|---|
| Mother's age (year)a | 25.2±6.3 | 27.4±5.8 | 27.8±7.0 | 23.7±5.5 | 23.1±5.8 | 25.2±6.3 |
| Maternal age | ||||||
| <20 years | 89 (24.8) | 8 (11.8) | 4 (12.9) | 25 (32.9) | 12 (34.3) | 40 (26.8) |
| 20–34 years | 246 (68.5) | 55 (80.9) | 21 (67.7) | 50 (65.8) | 22 (62.9) | 98 (65.8) |
| 35 years or older | 24 (6.7) | 5 (7.4) | 6 (19.4) | 1 (1.3) | 1 (2.9) | 11 (7.4) |
| Marital status | ||||||
| Stable relationship | 285 (79.4) | 57 (83.8) | 25 (80.6) | 49 (64.5) | 29 (82.9) | 125 (83.9) |
| Single | 70 (19.5) | 9 (13.2) | 6 (19.4) | 26 (34.2) | 6 (17.1) | 23 (15.4) |
| Divorced | 4 (1.1) | 2 (2.9) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (0.7) |
| Maternal educationa | 9.3±2.8 | 9.7±2.9 | 9.5±2.8 | 8.4±2.7 | 9.6±2.2 | 9.4±2.6 |
| <8 years | 87 (24.2) | 16 (23.5) | 8 (25.8) | 26 (34.2) | 5 (14.3) | 32 (21.5) |
| 8–11 years | 235 (65.5) | 41 (60.3) | 19 (61.3) | 46 (60.5) | 26 (74.3) | 103 (69.1) |
| 12 years or more | 37 (10.3) | 11 (16.2) | 4 (12.9) | 4 (5.3) | 4 (11.4) | 14 (9.4) |
| Paternal education schooling (years)a | 9.3±2.8 | 9.0±2.7 | 9.0±2.7 | 8.6±2.5 | 9.5±3.4 | 9.6±2.7 |
| Family income (USD)b | 383.69 (239.80–581.53) | 359.71 (227.81–599.52) | 371.70 (213.90–557.55) | 294.24 (191.84–413.66) | 395.68 (254.91–719.42) | 479.61 (287.76–661.15) |
| Number of prenatal visitsa | 8.1±3.2 | 9.8±3.4 | 9.1±2.8 | 6.4±2.9 | 7.6±3.1 | 8.1±2.9 |
| Full prenatal care (≥8 visits) | 208 (57.9) | 51 (75.0) | 24 (77.4) | 25 (32.9) | 19 (54.3) | 89 (59.7) |
| Gestational age (weeks)a | 39.1±1.2 | 38.8±1.1 | 38.6±1.1 | 39.2±1.2 | 39.1±1.1 | 39.4±1.2 |
| Birth type | ||||||
| Vaginal | 236 (65.7) | 40 (58.8) | 9 (29.0) | 58 (76.3) | 23 (65.7) | 106 (71.1) |
| C-section | 123 (34.3) | 28 (41.2) | 22 (71.0) | 18 (23.7) | 12 (34.3) | 43 (28.9) |
| Apgar, 1st minuteb | 9 (8–9) | 9 (8–9) | 9 (8–9) | 9 (9–9) | 9 (8–9) | 9 (8–9) |
| Apgar, 5th minuteb | 10 (9–10) | 9 (9–10) | 10 (9–10) | 10 (9–10) | 10 (9–10) | 10 (9–10) |
| Weight at birth (g)a | 3241±493 | 3404±451 | 3224±512 | 3160±381 | 2521±181 | 3381±450 |
| Length at birth (cm)a | 48.6±2.2 | 48.9±1.9 | 48.2±1.9 | 48.3±2.0 | 46.2±1.5 | 49.3±2.1 |
| Head circumference (cm)a | 33.8±1.6 | 34.1±1.5 | 34.1±1.4 | 33.7±1.3 | 31.9±1.2 | 34.1±1.5 |
| Newborn illness – n (%) | 3 (0.8) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
Data presented as n (%), except when otherwise indicated.
IUGR, intrauterine growth restriction.
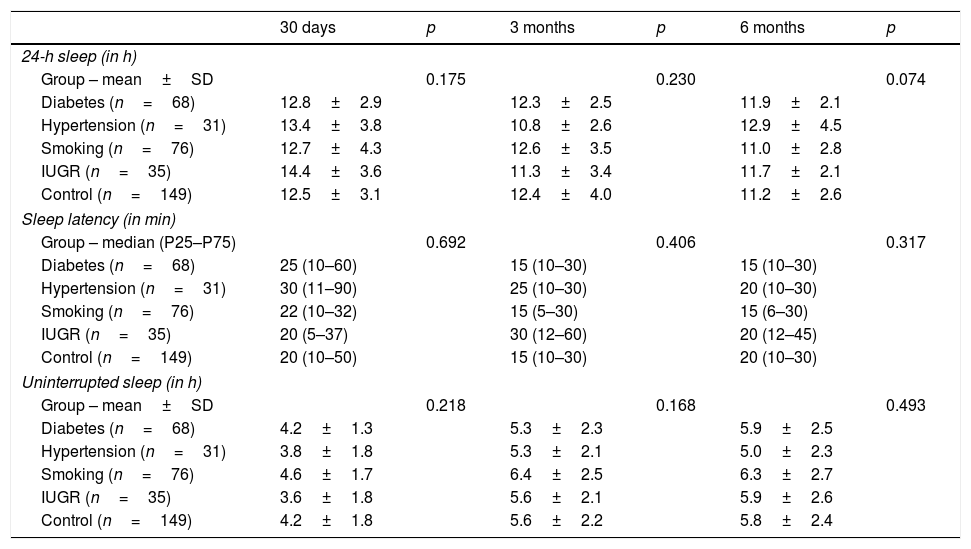
There were no significant differences in infant sleep according to intrauterine environment. Infant sleep, assessed by three different quantitative measures (total sleep time in 24h, sleep latency, and uninterrupted sleep time) in the first, third, and sixth months, did not reveal any significant difference between the five different groups of adverse intrauterine environments, both when analyzed as one whole group of adverse environments or when considered individually (Table 2).
Association between different intrauterine environments during pregnancy and sleep in infants in the first 6 months of life.
| 30 days | p | 3 months | p | 6 months | p | |
|---|---|---|---|---|---|---|
| 24-h sleep (in h) | ||||||
| Group – mean±SD | 0.175 | 0.230 | 0.074 | |||
| Diabetes (n=68) | 12.8±2.9 | 12.3±2.5 | 11.9±2.1 | |||
| Hypertension (n=31) | 13.4±3.8 | 10.8±2.6 | 12.9±4.5 | |||
| Smoking (n=76) | 12.7±4.3 | 12.6±3.5 | 11.0±2.8 | |||
| IUGR (n=35) | 14.4±3.6 | 11.3±3.4 | 11.7±2.1 | |||
| Control (n=149) | 12.5±3.1 | 12.4±4.0 | 11.2±2.6 | |||
| Sleep latency (in min) | ||||||
| Group – median (P25–P75) | 0.692 | 0.406 | 0.317 | |||
| Diabetes (n=68) | 25 (10–60) | 15 (10–30) | 15 (10–30) | |||
| Hypertension (n=31) | 30 (11–90) | 25 (10–30) | 20 (10–30) | |||
| Smoking (n=76) | 22 (10–32) | 15 (5–30) | 15 (6–30) | |||
| IUGR (n=35) | 20 (5–37) | 30 (12–60) | 20 (12–45) | |||
| Control (n=149) | 20 (10–50) | 15 (10–30) | 20 (10–30) | |||
| Uninterrupted sleep (in h) | ||||||
| Group – mean±SD | 0.218 | 0.168 | 0.493 | |||
| Diabetes (n=68) | 4.2±1.3 | 5.3±2.3 | 5.9±2.5 | |||
| Hypertension (n=31) | 3.8±1.8 | 5.3±2.1 | 5.0±2.3 | |||
| Smoking (n=76) | 4.6±1.7 | 6.4±2.5 | 6.3±2.7 | |||
| IUGR (n=35) | 3.6±1.8 | 5.6±2.1 | 5.9±2.6 | |||
| Control (n=149) | 4.2±1.8 | 5.6±2.2 | 5.8±2.4 | |||
IUGR, intrauterine growth restriction.
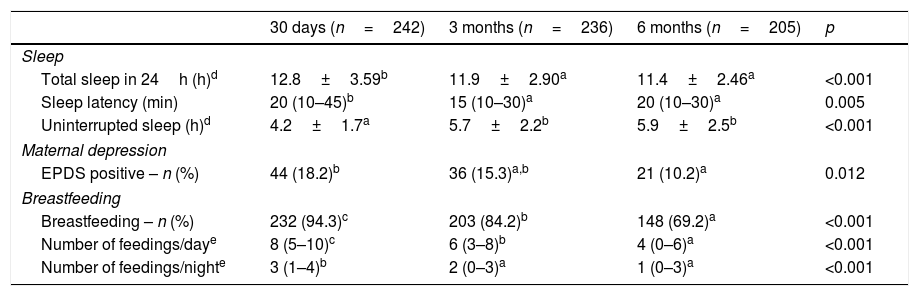
Reported total sleep time and sleep latency had significant reductions from the first to the third month of life (p<0.001) and from the third to the sixth month (p=0.005), as expected. Accordingly, uninterrupted sleep time showed an increase in the number of hours from the first to the third month, which remained in the sixth month post-partum (p<0.001) (Table 3). Postpartum depression symptoms also showed a progressive reduction during the first six months after delivery, as 18.2% of the mothers presented symptoms suggestive of depression in the 1st month, against 15.3% in the 3rd and 10.2% the 6th (p=0.012). Perceived maternal stress in the 1st month was 36.9 (±8.6) points, on a scale ranging from zero (no stress) to 56 (extreme stress) – data not shown in table.
Sleep, maternal depressive symptoms, and breastfeeding pattern in the first, third and sixth months of life.
| 30 days (n=242) | 3 months (n=236) | 6 months (n=205) | p | |
|---|---|---|---|---|
| Sleep | ||||
| Total sleep in 24h (h)d | 12.8±3.59b | 11.9±2.90a | 11.4±2.46a | <0.001 |
| Sleep latency (min) | 20 (10–45)b | 15 (10–30)a | 20 (10–30)a | 0.005 |
| Uninterrupted sleep (h)d | 4.2±1.7a | 5.7±2.2b | 5.9±2.5b | <0.001 |
| Maternal depression | ||||
| EPDS positive – n (%) | 44 (18.2)b | 36 (15.3)a,b | 21 (10.2)a | 0.012 |
| Breastfeeding | ||||
| Breastfeeding – n (%) | 232 (94.3)c | 203 (84.2)b | 148 (69.2)a | <0.001 |
| Number of feedings/daye | 8 (5–10)c | 6 (3–8)b | 4 (0–6)a | <0.001 |
| Number of feedings/nighte | 3 (1–4)b | 2 (0–3)a | 1 (0–3)a | <0.001 |
EPDS, Edinburgh Postnatal Depression Scale.
a,b,c Equal letters do not differ by the Bonferroni test at 5% significance.
Breastfeeding was associated with longer total sleep time in the first month of life (13.0±3.7 vs. 10.8±2.6, p=0.048). Higher family income (r=0.173, p=0.009) and maternal education (r=0.154, p=0.015) were associated with longer sleep latencies. In the third (r=0.133, p=0.041) and sixth month (r=0.152, p=0.030), higher depressive symptomatology was also positively correlated with sleep latency, although the strength of these correlations was weak.
Subsequently, the multivariate linear regression analysis showed that uninterrupted sleep time was associated with the number of breastfeedings per night, from the first (b=−0.14, p=0.005) to the third (b=−0.32, p=0.001) and sixth month (b=−0.49, p<0.001) of life. None of the analyzed factors showed association with the variables total sleep in 24h and sleep latency in the first, third, and sixth months of life.
DiscussionThis study found no associations between intrauterine environments and sleeping time of infants in the first six months of life. Extrauterine factors, such as breastfeeding and maternal depressive symptoms as well as sociodemographic characteristics, appear to affect infant's sleep patterns more directly. Reported sleep time decreased throughout the study period, in accordance with the literature. Full-term infants typically sleep longer than any other age group, sometimes up to 20h/day, with rapid eye movement (REM) and non-rapid eye movement (NREM) sleep cycles more evenly distributed, and shorter in duration compared to older children. Gradually, from 3 to 6 months, the infants sleep will typically become more like that of a child, and by about 6 months, all four sleep stages (N1, N2, N3, and REM) are defined, although infants may still wake for feeding and comfort every four to 6h.
The present study found that breastfed children sleep more hours in the first month of life during the 24-h period compared to those receiving other types of milk (formulas or cow's milk). Breastfed children, however, wake up more often at night compared to children who are not breastfed in the first, third, and sixth months. Maternal mental health also appears to have a significant impact, as infants whose mothers presented depressive symptoms took longer to fall asleep. Although this could represent an information bias, longer latencies were also reported for infants whose parents had higher income or higher education levels. In sum, the results suggest that, in the first semester of life, infant sleep is more affected by environmental factors, including habits and family characteristics, than by biological, prenatal factors.
The overall average sleep of the infant at the three evaluated moments was approximately 12h, which can preliminarily be considered low in this age group. However, the variation of this average sleep was between 10h and 15h in 68% of these infants. On the other hand, a recent American study evaluated sleep in 6, 15, and 24-month-old infants through actigraphy compared with mother-reported sleep perceptions for their children. The results showed that the mean sleep measured by actigraphy was 11.3–11.5h of sleep per day, while the mother-report was 14.1–14.8.16 Similar to the results of this research, a cohort study conducted in southern Brazil found an average of 13.33h of sleep in the infant's 3rd month of life, according to the mothers’ reports.17 Thus, it is possible that mothers overestimate the number of sleep hours of their children, which, if applied in the present study, would still suggest low sleep time in the evaluated infants. However, cultural and age differences should be considered when comparing these studies.
The relationship between sleep and breastfeeding still finds inconclusive results in the literature. In this study, in the first month, newborns who were breastfed slept for more hours during the 24-h period. This association, however, disappeared later, in the third and sixth months. Another study in a similar context found that, at 3 months of age, exclusively breastfed infants spent more hours sleeping in the 24-h period than mixed-fed infants.18 In addition to the number of hours of sleep, the present results showed that breastfed children had a greater number of awakenings in the first, third, and sixth months. The reason for the higher number of arousals may be breastfeeding itself, with infants waking up more often at night to be breastfed. The relation between breastfeeding and the higher number of awakenings had already been suggested by other studies,19–21 as well as a shorter sleep period between zero and 5 months at night compared to formula-fed infants.20 The explanation for this association may be related to the greater digestibility of breast milk when compared to formula or cow's milk, providing a shorter period of satiation and sleep in exclusively breastfed infants. Another explanatory hypothesis suggests that breast-fed infants usually have a closer temporal association between their demands and maternal responses compared to bottle-fed infants, reinforcing night awakenings.
Regarding extrauterine factors, maternal stress showed no association with infants’ sleep in the first month. Previous studies, however, suggest that family stress may play an important role in the development of sleep disorders.22 Field found that increased emotional availability, combined with less physical contact with parents at bedtime, resulted in more hours of sleep.23 Moreover, mothers with depressive symptoms have fewer positive interactions with their children, presenting difficulties to regulate affection and to understand and respond to the needs of their children and, thus, are more inconsistent and ineffective in managing the child.24,25 They are also more prone to not breastfeed, as well as failing to provide adequate care.26
In this study, children of mothers who presented depressive symptoms took more time to fall asleep. Nevertheless, when uninterrupted sleep and 24-h sleep were evaluated, no associations were found with maternal depressive symptoms measured by the EPDS. In the U.S.-based sample of Teti and Crosby,27 a positive association between maternal depressive symptoms and nocturnal awakenings of the child was found. More recently, in addition to depression, maternal anxiety symptoms were also observed to be related to an increase in the number of nocturnal awakenings in childhood.28,29
Although maternal education and family income were associated with longer sleep latency in the crude model, this effect disappeared due to the inclusion of other variables in the adjusted model. Similar findings were found in the cohort study conducted in southern Brazil where in general there were no consistent associations between sleep duration and sociodemographic characteristics.17 This reinforces that there are several other factors, in addition to sociodemographic factors alone, which overlap with the influence on infant sleep, such as exposure to screen media, co-sleeping, and breastfeeding itself.
This study must be interpreted in light of some limitations. First, most measures were subjectively ascertained, based on self-report or time estimates, without objective measurements such as polysomnography, making these measures subject to imprecision and information bias, particularly at the time of delivery. Secondly, only four questions were used from the Sleep Behavior Questionnaire – designed by Cortesi et al. and translated and adapted to Portuguese by Batista et al., which is not specific to the age group investigated (infants).12,13 Nevertheless, the finding of a consistent progressive decrease throughout the six-month period, in the mean number of hours slept in 24-h (from 12.5h to 11.2h from the first to the sixth month), matching what is consistently reported in the literature,19 suggests that these subjective time estimates are in fact valid. Finally, among the limitations, depressive symptoms and maternal stress were measured by screening questionnaires, without clinical confirmation, thereby limiting the interpretation of the findings. Even so, the instruments used for the evaluation of depressive symptoms and maternal stress are validated worldwide and their use has already been established with screening tools for these conditions.
Nonetheless, among the strengths, the study is pioneering in investigating the influence of adverse intrauterine environments on the sleep pattern of infants, observing depressive symptoms and maternal stress as mediators. It should also be noted that this sleep pattern assessment was performed longitudinally and in the first half of the infant's life. During this period, it is possible to identify early risk factors for sleep disorders in childhood and, therefore, to develop interventions for their prevention.
This study investigated the influence of several intrauterine adverse environments (diabetic, hypertensive, smoking, or intrauterine growth-restrictive mothers) on the pattern and sleep quality of newborns and infants, without finding significant associations. In light of the results, it is suggested that sleep, even in the first months of life, is more influenced by the environment and family characteristics than by genetic conditions or intrauterine factors.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at Universidade Federal do Rio Grande do Sul (UFRGS), Hospital de Clínicas de Porto Alegre, Faculdade de Medicina, Programa de Pós-Graduação em Saúde da Criança e do Adolescente, Departamento de Pediatria, Porto Alegre, RS, Brazil.