Perform a systematic review and meta-analysis to assess the effectiveness and complications caused by the use of the high-flow nasal cannula in relation to the post-extubation continuous positive airway pressure system in preterm newborns.
Data SourcesThe searches were performed from January 2013 to December 2018 in the PubMed and Embase databases, as well as a manual search on the internet.
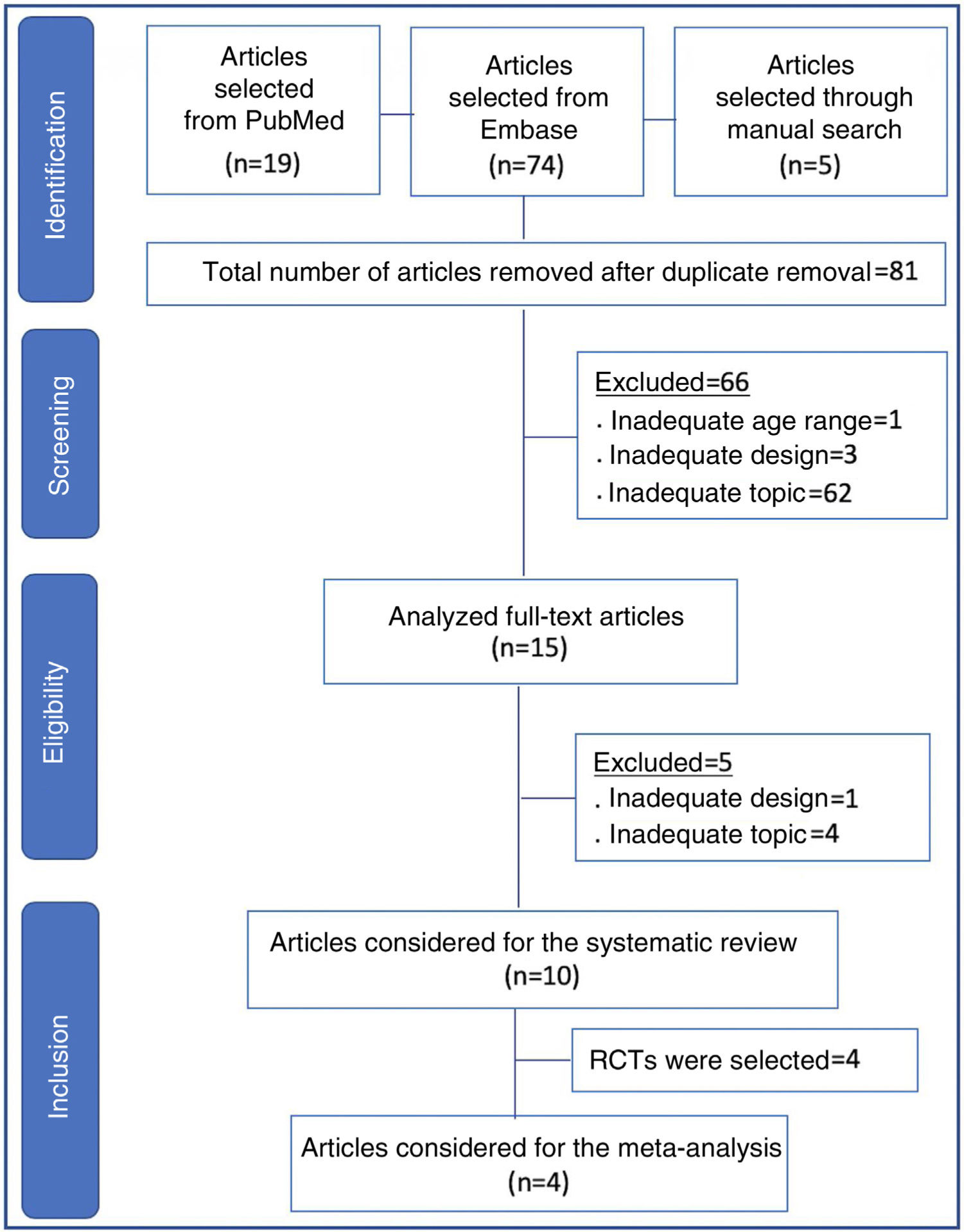
Data SynthesisTwo reviewers independently conducted the search, and a third reviewer resolved questions that arose. Ninety-eight articles from the chosen sources were evaluated, and 66 were discarded because they did not meet the inclusion criteria (inadequate topic, age range, or design, in addition to the duplicates). Fifteen articles were read in full, and five more were discarded due to inadequacy to the topic or design. There were ten articles left for systematic review and four for meta-analysis. The study showed non-inferiority in terms of therapeutic failure of the high-flow nasal cannula in relation to continuous positive airway pressure after extubation of preterm newborns. In the meta-analysis, nasal trauma was significantly lower in patients submitted to the high-flow nasal cannula compared to those using continuous positive airway pressure (p<0.00001).
ConclusionThe high-flow nasal cannula is not inferior to continuous positive airway pressure for post-extubation respiratory support in preterm newborns with a gestational age of 32 weeks or less and greater than 28 weeks, in addition to resulting in less nasal trauma.
Realizar revisão sistemática e metanálise para avaliar efetividade e complicações decorrentes do uso da cânula nasal de alto fluxo em relação ao sistema de pressão positiva contínua de vias aéreas no período pós-extubação em recém-nascidos prematuros.
Fontes dos dadosAs buscas foram feitas de janeiro de 2013 a dezembro de 2018 nas bases de dados PubMed, Embase e busca manual em arquivos da internet.
Resumo dos dadosDois revisores fizeram a busca de forma independente, um terceiro revisor ficou para dirimir dúvidas. Foram avaliados 98 artigos das fontes escolhidas, 66 descartados por não se enquadrar nos critérios de inclusão (tema, faixa etária ou desenho inadequados, além dos duplicados). Foram lidos 15 artigos na íntegra, foram descartados mais 5 por inadequação ao tema ou desenho. Restaram 10 artigos para revisão sistemática e 4 para metanálise. O estudo evidenciou não inferioridade em termos de falha terapêutica da cânula nasal de alto fluxo em relação ao sistema de pressão positiva contínua de vias aéreas na pós-extubação de recém-nascidos prematuros. Na metanálise, foi significativamente menor o trauma nasal nos pacientes em cânula nasal de alto fluxo em relação ao que usaram sistema de pressão positiva contínua de vias aéreas (p<0,00001).
ConclusãoA cânula nasal de alto fluxo não é inferior ao sistema de pressão positiva contínua de vias aéreas para o suporte respiratório pós-extubação de recém-nascidos prematuros com idade gestacional igual a ou menor do que 32 semanas e maior do que 28 semanas, além de provocar menos trauma nasal.
Acute respiratory failure requiring invasive mechanical ventilation (IMV) or non-invasive respiratory support is a common problem in preterm newborns (NBs) after birth.1
Recent studies suggest that increased survival rates of extremely premature infants (<28 weeks gestational age) have been associated with a greater need for respiratory support.1 Until recently, invasive support through an endotracheal tube was the most commonly used type of primary respiratory support. However, the benefits of non-invasive ventilation (NIV) support, especially with the use of continuous positive airway pressure (CPAP), have shown benefits and advantages over IMV.2 In addition to its use as primary respiratory support, it is also well established that CPAP is a respiratory support transition device after tracheal extubation.3,4
The high-flow nasal cannula (HFNC) is a more recent respiratory support device introduced for NBs and has gained popularity among physicians worldwide, although the evidence supporting its use is not fully established.4•6 Several clinical trials throughout the last decade have reported data on the use of HFNC in preterm infants, both as a primary mode of respiratory support at birth and after IMV tracheal extubation, with mixed results.4•9
The authors performed a systematic review and meta-analysis on the use of HFNC as a respiratory support in preterm infants after tracheal extubation, with the primary objective of evaluating the possible non-inferiority of the method in relation to nasal CPAP in terms of therapeutic success. As secondary objectives, this review assessed the risks of some possible method-related complications.
MethodsThe study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10 For the search, structured Medical Subject Headings (MeSH) words were used for PubMed; Emtree was used for the Embase databases. The search strategy was concentrated on the period from January 2013 to December 2018, without language filters, using the keywords: (high flow) AND (CPAP OR nCPAP) AND extubation AND (prematurity OR preterm).
A manual search was also performed in internet repositories, congress abstracts, and Google Scholar to identify possible studies not identified by the two main searched databases.
Eligibility criteriaFor the systematic review, studies in which respiratory support through a high-flow nasal cannula (>1L/min) were used compared to nasal CPAP immediately after extubation in premature infants (<37 weeks) were considered eligible.
The randomized controlled trials were considered eligible for the meta-analysis.
Study selection and data extractionTwo investigators independently carried out the electronic database search and extracted the information. A third investigator was called in to participate in case of disagreement between the first two investigators.
The titles and abstracts of all articles were read as initial screening and, after disregarding the duplicates, those unrelated to the topic, case reports, letters to the editor, experimental studies, questionnaires, expert consensuses, and editorials were excluded. The remaining articles were read in full.
Patient recruitment periods and areas were evaluated to avoid possible duplicate counting of patients included in more than one report by the same authors/working groups.
Categorical data for the outcomes were extracted for each intervention group (HFNC and CPAP), and odds ratios with a 95% confidence interval (95% CI) were calculated. Each event was considered as having a fixed effect using the Mantel-Haenszel method.
To evaluate non-inferiority, absolute risk differences for therapeutic failures and reintubation rates were calculated, with 95% CIs. For the treatment with HFNC to be considered non-inferior, the upper limit of the 95% confidence interval was required to be less than 20%, and the lower limit, negative.11
The analysis was performed for fixed effects, and the weights are based on the inverse of variances. Variances, in turn, are primarily determined by sample sizes. Thus, the study by Manley,12 which has the largest sample (n=303), contributes greater weight in the observed outcomes and adverse events. In this type of analysis, there may be a risk that the results of smaller studies can be essentially ignored, but due to the near absence of heterogeneity, the authors believe the assessment of fixed effects is the most appropriate for this meta-analysis.
For the comparison of gestational ages, means and standard deviations were used to calculate the weighted mean difference. The meta-analysis was carried out with the program Rev Man (Review Manager Web, version 5.3. The Cochrane Collaboration, 2019). Available at revman.cochrane.org.
Bias risk assessmentThe bias risk in the studies was assessed by consensus between the investigators.
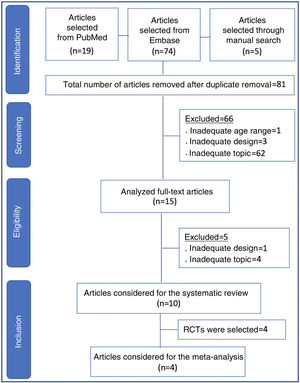
ResultsIn all, 98 articles were found (19 in PubMed, 74 in Embase, and five in references identified through the manual search). After the initial screening and removal of articles in duplicate, 81 articles remained. Sixty-six articles were discarded, including those that were unrelated to the topic, those with inadequate design for review (case reports, letters to the editor, experimental studies, questionnaires, expert consensus, and editorials), as well as an article with full-term newborns.
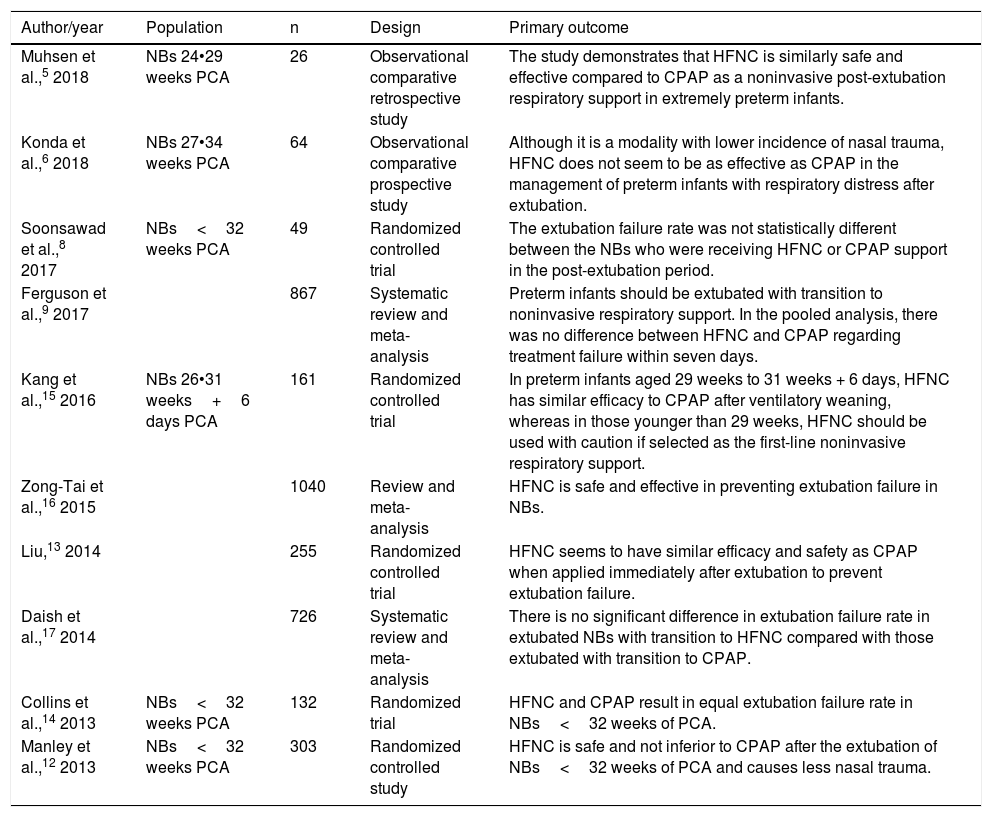
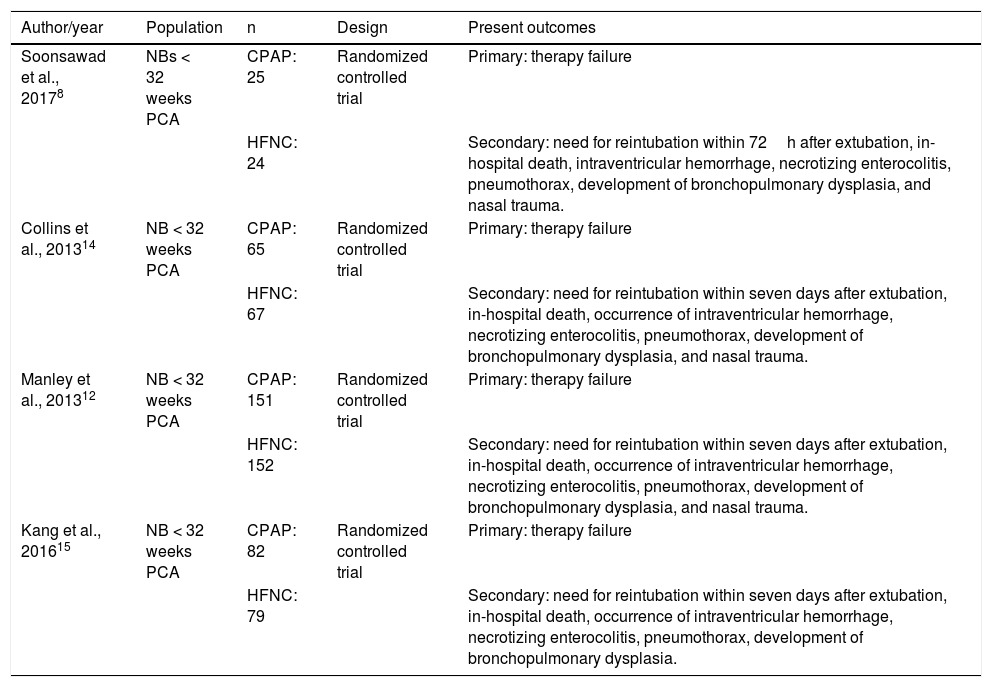
The remaining 15 articles were read in full and ten final articles were selected for the systematic review (Fig. 1). The characteristics of each study • including main author, year of study publication, study design, and primary outcome • are shown in Table 1. Of the studies selected for the systematic review, four were randomized controlled trials and were included for meta-analysis, with a total of 645 patients. The data are summarized in Table 2.
Characteristics of studies included in the systematic review.
| Author/year | Population | n | Design | Primary outcome |
|---|---|---|---|---|
| Muhsen et al.,5 2018 | NBs 24•29 weeks PCA | 26 | Observational comparative retrospective study | The study demonstrates that HFNC is similarly safe and effective compared to CPAP as a noninvasive post-extubation respiratory support in extremely preterm infants. |
| Konda et al.,6 2018 | NBs 27•34 weeks PCA | 64 | Observational comparative prospective study | Although it is a modality with lower incidence of nasal trauma, HFNC does not seem to be as effective as CPAP in the management of preterm infants with respiratory distress after extubation. |
| Soonsawad et al.,8 2017 | NBs<32 weeks PCA | 49 | Randomized controlled trial | The extubation failure rate was not statistically different between the NBs who were receiving HFNC or CPAP support in the post-extubation period. |
| Ferguson et al.,9 2017 | 867 | Systematic review and meta-analysis | Preterm infants should be extubated with transition to noninvasive respiratory support. In the pooled analysis, there was no difference between HFNC and CPAP regarding treatment failure within seven days. | |
| Kang et al.,15 2016 | NBs 26•31 weeks+6 days PCA | 161 | Randomized controlled trial | In preterm infants aged 29 weeks to 31 weeks + 6 days, HFNC has similar efficacy to CPAP after ventilatory weaning, whereas in those younger than 29 weeks, HFNC should be used with caution if selected as the first-line noninvasive respiratory support. |
| Zong-Tai et al.,16 2015 | 1040 | Review and meta-analysis | HFNC is safe and effective in preventing extubation failure in NBs. | |
| Liu,13 2014 | 255 | Randomized controlled trial | HFNC seems to have similar efficacy and safety as CPAP when applied immediately after extubation to prevent extubation failure. | |
| Daish et al.,17 2014 | 726 | Systematic review and meta-analysis | There is no significant difference in extubation failure rate in extubated NBs with transition to HFNC compared with those extubated with transition to CPAP. | |
| Collins et al.,14 2013 | NBs<32 weeks PCA | 132 | Randomized trial | HFNC and CPAP result in equal extubation failure rate in NBs<32 weeks of PCA. |
| Manley et al.,12 2013 | NBs<32 weeks PCA | 303 | Randomized controlled study | HFNC is safe and not inferior to CPAP after the extubation of NBs<32 weeks of PCA and causes less nasal trauma. |
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; NBs, newborns; PCA, post-conceptual age.
Summary of studies included in the meta-analysis.
| Author/year | Population | n | Design | Present outcomes |
|---|---|---|---|---|
| Soonsawad et al., 20178 | NBs < 32 weeks PCA | CPAP: 25 | Randomized controlled trial | Primary: therapy failure |
| HFNC: 24 | Secondary: need for reintubation within 72h after extubation, in-hospital death, intraventricular hemorrhage, necrotizing enterocolitis, pneumothorax, development of bronchopulmonary dysplasia, and nasal trauma. | |||
| Collins et al., 201314 | NB < 32 weeks PCA | CPAP: 65 | Randomized controlled trial | Primary: therapy failure |
| HFNC: 67 | Secondary: need for reintubation within seven days after extubation, in-hospital death, occurrence of intraventricular hemorrhage, necrotizing enterocolitis, pneumothorax, development of bronchopulmonary dysplasia, and nasal trauma. | |||
| Manley et al., 201312 | NB < 32 weeks PCA | CPAP: 151 | Randomized controlled trial | Primary: therapy failure |
| HFNC: 152 | Secondary: need for reintubation within seven days after extubation, in-hospital death, occurrence of intraventricular hemorrhage, necrotizing enterocolitis, pneumothorax, development of bronchopulmonary dysplasia, and nasal trauma. | |||
| Kang et al., 201615 | NB < 32 weeks PCA | CPAP: 82 | Randomized controlled trial | Primary: therapy failure |
| HFNC: 79 | Secondary: need for reintubation within seven days after extubation, in-hospital death, occurrence of intraventricular hemorrhage, necrotizing enterocolitis, pneumothorax, development of bronchopulmonary dysplasia. |
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; NBs, newborns; PCA, post-conceptual age.
All studies excluded infants with suspected respiratory obstruction, or severe airway or cardiopulmonary malformations. It was decided not to use data from the study by Liu13 in the metanalysis, because it includes non-preterm newborns.
Observed bias risksDue to differences between devices, the interventions could not be blinded in either study. There were no attempts to blind the analyses for secondary prognoses, with the only exception being intraventricular hemorrhages in the study by Collins et al.,14 where ultrasound imaging assessments were performed without identification by radiologists.
The decision on reintubation was left to the discretion of the attending physicians in all studies. Changes in flow rates were also left to the discretion of the attending physicians in the studies by Collins et al.14 and Manley et al.,12 which may have generated non-described differences between the groups. There is no description of the flow application method in the other studies.
As for the allocation, the studies by Collins et al.,14 Soonsawad et al.,8 and Manley et al.12 describe adequate randomization by computer-generated random sequences and allocation by opening opaque,sealed envelopes at the time of extubation. There is no description of the randomization and allocation process in the study by Kang et al.15
There is no record, in any of the studies, on how to obtain secondary prognosis of the studies, except for severe adverse events (pneumothorax) in the study by Manley et al.,12 which were reported to the investigator, and nasal lesions in the study by Collins et al.,14 assessed by their own score. The assessment of secondary outcomes through medical records, i.e., retrospectively upon discharge or death, has the potential to result in biases.
Assessed outcomesThe primary outcome evaluated was therapeutic method failure in all studies. The criteria for therapeutic failure were similar: apnea (respiratory pause >20s), requiring return to CPAP (in case of HFNC), BIPAP (BI-level Positive Airway Pressure) or reintubation in case of CPAP; respiratory acidosis, with pH<7.20•7.25 and PCO2>60∧66mmHg; sustained increase in FiO2>15•20% after extubation.
Secondary outcomes present in the studies were the need for reintubation, hospital death, intraventricular hemorrhage, necrotizing enterocolitis, pneumothorax, development of bronchopulmonary dysplasia, and nasal trauma.
Bronchopulmonary dysplasia was defined in all studies as the need for respiratory support or supplemental oxygen at 36 weeks post-gestational age. Necrotizing enterocolitis has a clear definition in Collins et al.,14 with the cases being considered due to surgical or radiographic evidence of intestinal pneumatosis, or hepatobiliary or peritoneal gas; cases were reported using the Bell criteria (≥2) in other articles.
All reported bleeding events were grade 3 (intraventricular) and grade 4 (periventricular). The criteria for nasal lesions are clear only in Collins et al.,14 using their own score. Unfortunately, this study does not report the numbers of lesions in each group, only the average scores, and thus cannot be compared to other studies. In Soonsawad et al.,8 nasal trauma is defined as any sign of hyperemia or rupture of the skin in contact with the device. Failure to apply a score can lead to important heterogeneity.
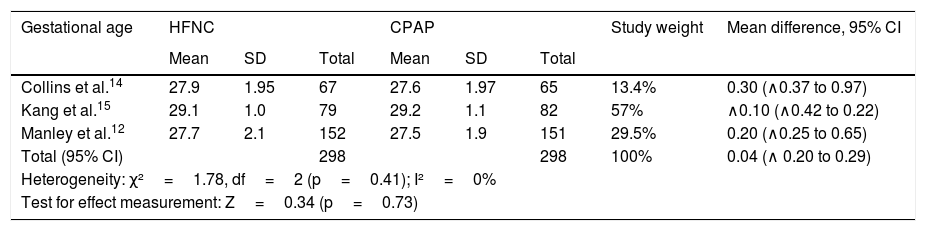
Gestational ageAs shown in Table 3, there were no significant mean differences in post-menstrual gestational ages in three studies, with no significant heterogeneity. The study by Soonsawad et al.8 shows the median and interquartile range values for age (27.5 weeks, IQR: 26•30 in the HFNC group; 28 weeks, IQR: 25•29.5 in the CPAP group, p=0.72) and inclusion in this comparison was not possible.
Comparison between gestational ages of premature infants at randomization.
| Gestational age | HFNC | CPAP | Study weight | Mean difference, 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | |||
| Collins et al.14 | 27.9 | 1.95 | 67 | 27.6 | 1.97 | 65 | 13.4% | 0.30 (∧0.37 to 0.97) |
| Kang et al.15 | 29.1 | 1.0 | 79 | 29.2 | 1.1 | 82 | 57% | ∧0.10 (∧0.42 to 0.22) |
| Manley et al.12 | 27.7 | 2.1 | 152 | 27.5 | 1.9 | 151 | 29.5% | 0.20 (∧0.25 to 0.65) |
| Total (95% CI) | 298 | 298 | 100% | 0.04 (∧ 0.20 to 0.29) | ||||
| Heterogeneity: χ²=1.78, df=2 (p=0.41); I²=0% | ||||||||
| Test for effect measurement: Z=0.34 (p=0.73) | ||||||||
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; SD, standard deviation; CI, confidence interval.
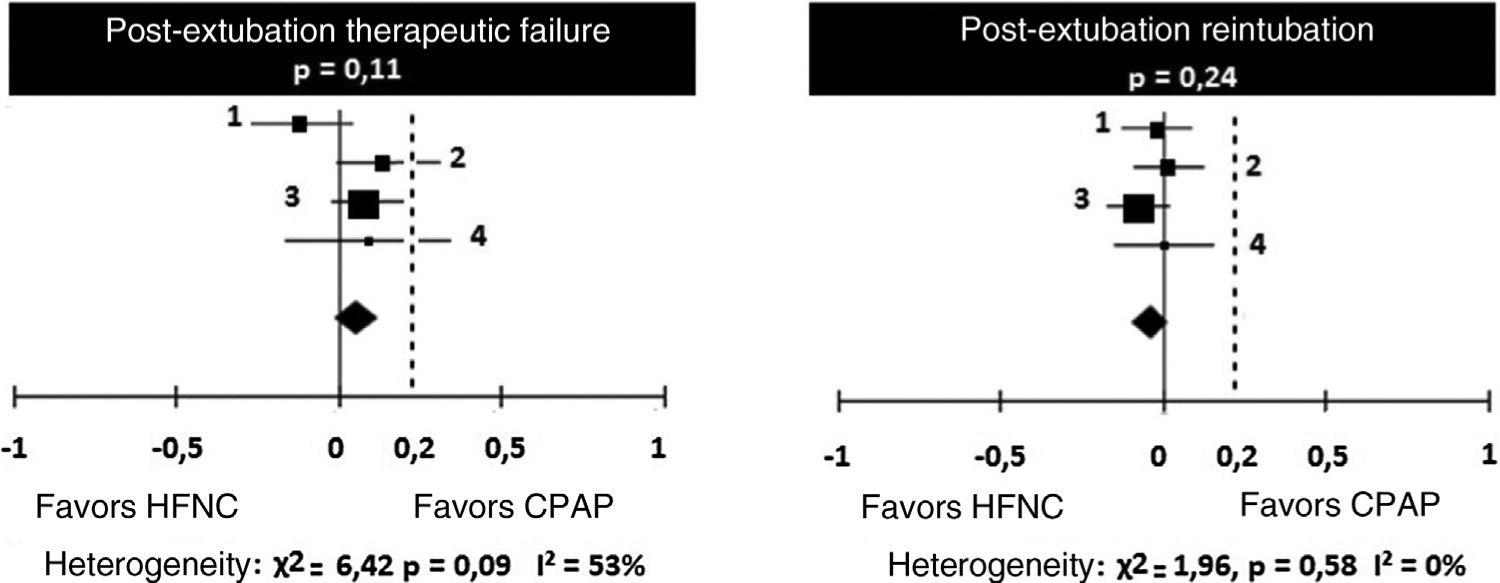
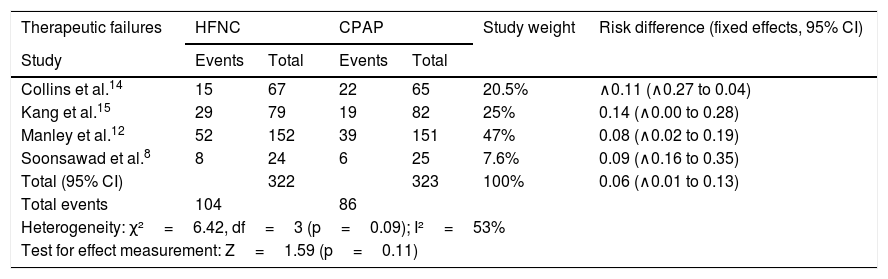
Regarding treatment failures, the analysis of the four studies showed substantial, though not significant, heterogeneity (Table 4). When analyzing the reintubation rates, however, heterogeneity disappears. There is no significant difference between the number of failures in patients receiving HFNC or CPAP, with a 9% risk difference (95% CI: ∧1% to 13%) favoring CPAP (p=0.11). However, in two studies (Kang et al.15 and Soonsawad et al.8) the upper limit of the 95% confidence interval exceeds the 20% established as a parameter for non-inferiority (28% and 35%, respectively). For the number of re-intubations, the risk difference is ∧3% favoring HFNC (95% CI, ∧9% to 2%, p=0.24, Fig. 2).
Risk differences for therapeutic failures and number of re-intubations after extubation by the Mantel-Haenszel method. Negative values favor HFNC and positive values show the superiority of CPAP.
| Therapeutic failures | HFNC | CPAP | Study weight | Risk difference (fixed effects, 95% CI) | ||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Collins et al.14 | 15 | 67 | 22 | 65 | 20.5% | ∧0.11 (∧0.27 to 0.04) |
| Kang et al.15 | 29 | 79 | 19 | 82 | 25% | 0.14 (∧0.00 to 0.28) |
| Manley et al.12 | 52 | 152 | 39 | 151 | 47% | 0.08 (∧0.02 to 0.19) |
| Soonsawad et al.8 | 8 | 24 | 6 | 25 | 7.6% | 0.09 (∧0.16 to 0.35) |
| Total (95% CI) | 322 | 323 | 100% | 0.06 (∧0.01 to 0.13) | ||
| Total events | 104 | 86 | ||||
| Heterogeneity: χ²=6.42, df=3 (p=0.09); I²=53% | ||||||
| Test for effect measurement: Z=1.59 (p=0.11) | ||||||
| Number of re-intubations | ||||||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Collins et al.14 | 7 | 67 | 8 | 65 | 20.5% | ∧0.02 (∧0.13 to 0.09) |
| Kang et al.15 | 11 | 79 | 10 | 82 | 25% | 0.02 (∧0.09 to 0.12) |
| Manley et al.12 | 27 | 152 | 38 | 151 | 47% | ∧0.07 (∧0.17 to 0.02) |
| Soonsawad et al.8 | 2 | 24 | 2 | 25 | 7.6% | 0.00 (∧0.15 to 0.16) |
| Total (95% CI) | 322 | 323 | 100% | ∧0.03 (∧0.09 to 0.02) | ||
| Total events | 47 | 58 | ||||
| Heterogeneity: χ²=1.96, df=3 (p=0.58); I²=0% | ||||||
| Test for effect measurement: Z=1.18 (p=0.24) | ||||||
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; CI, confidence interval.
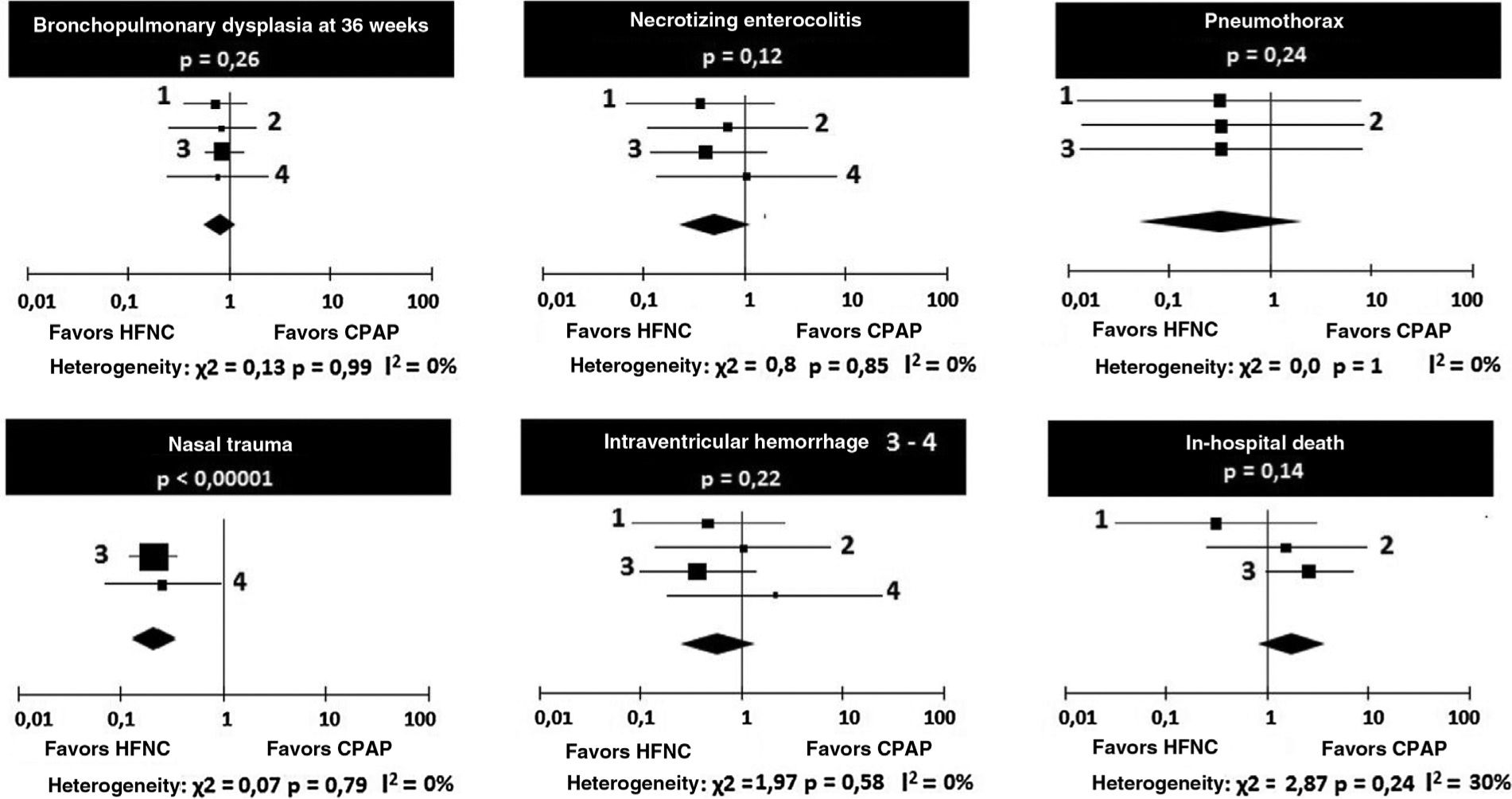
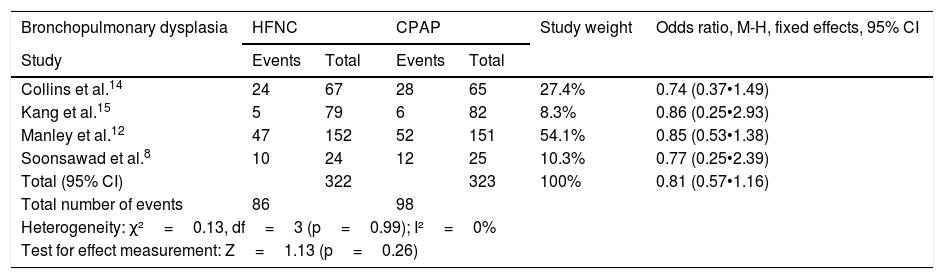
Regarding the occurrence of bronchopulmonary dysplasia and necrotizing enterocolitis, no heterogeneity was observed between the four studies, and the chances of occurrence of the events were similar in the groups receiving HFNC or CPAP (Table 5).
Odds ratios for the occurrence of bronchopulmonary dysplasia and necrotizing enterocolitis.
| Bronchopulmonary dysplasia | HFNC | CPAP | Study weight | Odds ratio, M-H, fixed effects, 95% CI | ||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Collins et al.14 | 24 | 67 | 28 | 65 | 27.4% | 0.74 (0.37•1.49) |
| Kang et al.15 | 5 | 79 | 6 | 82 | 8.3% | 0.86 (0.25•2.93) |
| Manley et al.12 | 47 | 152 | 52 | 151 | 54.1% | 0.85 (0.53•1.38) |
| Soonsawad et al.8 | 10 | 24 | 12 | 25 | 10.3% | 0.77 (0.25•2.39) |
| Total (95% CI) | 322 | 323 | 100% | 0.81 (0.57•1.16) | ||
| Total number of events | 86 | 98 | ||||
| Heterogeneity: χ²=0.13, df=3 (p=0.99); I²=0% | ||||||
| Test for effect measurement: Z=1.13 (p=0.26) | ||||||
| Necrotizing enterocolitis | ||||||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| Collins et al.14 | 2 | 67 | 5 | 65 | 29.9% | 0.37 (0.07•1.98) |
| Kang et al.15 | 2 | 79 | 3 | 82 | 17.4% | 0.68 (0.11•4.21) |
| Manley et al.12 | 3 | 152 | 7 | 151 | 41.8% | 0.41 (0.11•1.63) |
| Soonsawad et al.8 | 2 | 24 | 2 | 25 | 10.9% | 1.05 (0.14•8.08) |
| Total (95% CI) | 322 | 323 | 100% | 0.52 (0.23•1.18) | ||
| Total number of events | 9 | 17 | ||||
| Heterogeneity: χ²=0.80, df=3 (p=0.85); I²=0% | ||||||
| Test for effect measurement: Z=1.57 (p=0.12) | ||||||
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; CI, confidence interval. Values of OR<1 favor HFNC, and >1, favor CPAP. M-H, Mantel-Haenszel.
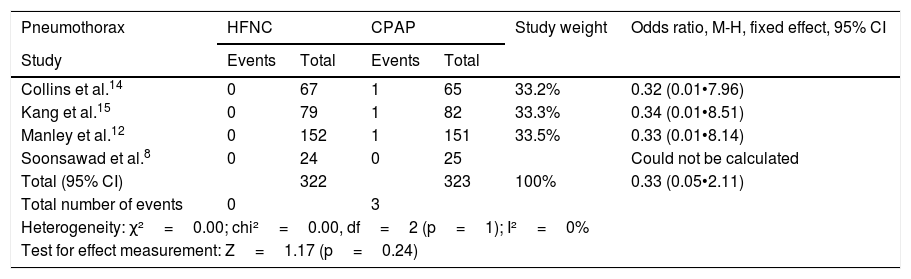
The occurrence of pneumothorax after extubation was small in all the studies, with no heterogeneity or difference between CPAP and HFNC (Table 6). Regarding the occurrence of nasal trauma in absolute numbers, it was substantially higher in patients receiving CPAP (51.7%) than in those receiving HFNC (18.7%), p<0.0001. It is emphasized that it was possible to make the comparison in only two studies.
Odds ratios for pneumothorax and nasal trauma.
| Pneumothorax | HFNC | CPAP | Study weight | Odds ratio, M-H, fixed effect, 95% CI | ||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Collins et al.14 | 0 | 67 | 1 | 65 | 33.2% | 0.32 (0.01•7.96) |
| Kang et al.15 | 0 | 79 | 1 | 82 | 33.3% | 0.34 (0.01•8.51) |
| Manley et al.12 | 0 | 152 | 1 | 151 | 33.5% | 0.33 (0.01•8.14) |
| Soonsawad et al.8 | 0 | 24 | 0 | 25 | Could not be calculated | |
| Total (95% CI) | 322 | 323 | 100% | 0.33 (0.05•2.11) | ||
| Total number of events | 0 | 3 | ||||
| Heterogeneity: χ²=0.00; chi²=0.00, df=2 (p=1); I²=0% | ||||||
| Test for effect measurement: Z=1.17 (p=0.24) | ||||||
| Nasal trauma | ||||||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Manley et al.12 | 29 | 152 | 80 | 151 | 87.9% | 0.21 (0.12•0.35) |
| Soonsawad et al.8 | 4 | 24 | 11 | 25 | 12.1% | 0.25 (0.07•0.97) |
| Total (95% CI) | 176 | 176 | 100% | 0.21 (0.13•0.35) | ||
| Total number of events | 33 | 91 | ||||
| Heterogeneity: χ²=0.07;df=1 (p=0.79); I²=0% | ||||||
| Test for effect measurement: Z=6.27 (p<0.00001) | ||||||
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; CI, confidence interval; M-H, Mantel-Haenszel.
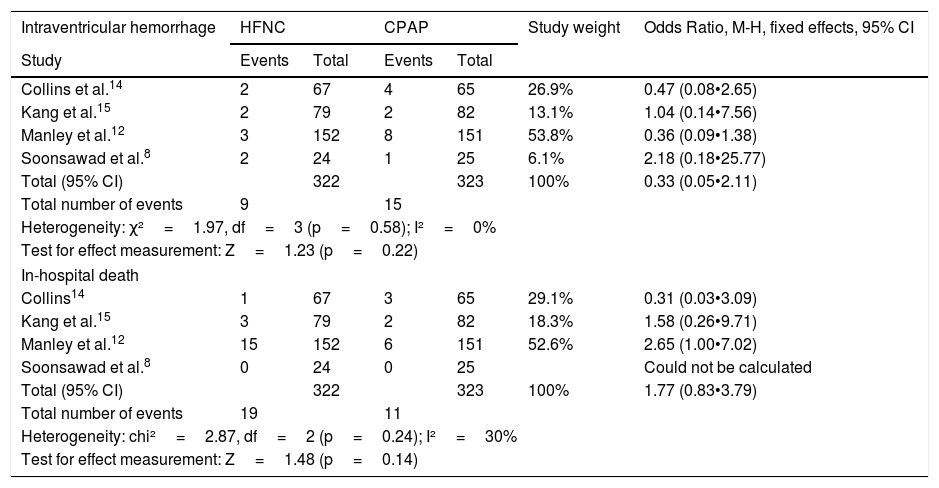
There were no significant heterogeneity or differences between the chances of intraventricular hemorrhage and in-hospital deaths in patients receiving HFNC or CPAP (Table 7). The odds ratios are depicted graphically in Fig. 3.
Odds ratios for intraventricular hemorrhage and hospital death.
| Intraventricular hemorrhage | HFNC | CPAP | Study weight | Odds Ratio, M-H, fixed effects, 95% CI | ||
|---|---|---|---|---|---|---|
| Study | Events | Total | Events | Total | ||
| Collins et al.14 | 2 | 67 | 4 | 65 | 26.9% | 0.47 (0.08•2.65) |
| Kang et al.15 | 2 | 79 | 2 | 82 | 13.1% | 1.04 (0.14•7.56) |
| Manley et al.12 | 3 | 152 | 8 | 151 | 53.8% | 0.36 (0.09•1.38) |
| Soonsawad et al.8 | 2 | 24 | 1 | 25 | 6.1% | 2.18 (0.18•25.77) |
| Total (95% CI) | 322 | 323 | 100% | 0.33 (0.05•2.11) | ||
| Total number of events | 9 | 15 | ||||
| Heterogeneity: χ²=1.97, df=3 (p=0.58); I²=0% | ||||||
| Test for effect measurement: Z=1.23 (p=0.22) | ||||||
| In-hospital death | ||||||
| Collins14 | 1 | 67 | 3 | 65 | 29.1% | 0.31 (0.03•3.09) |
| Kang et al.15 | 3 | 79 | 2 | 82 | 18.3% | 1.58 (0.26•9.71) |
| Manley et al.12 | 15 | 152 | 6 | 151 | 52.6% | 2.65 (1.00•7.02) |
| Soonsawad et al.8 | 0 | 24 | 0 | 25 | Could not be calculated | |
| Total (95% CI) | 322 | 323 | 100% | 1.77 (0.83•3.79) | ||
| Total number of events | 19 | 11 | ||||
| Heterogeneity: chi²=2.87, df=2 (p=0.24); I²=30% | ||||||
| Test for effect measurement: Z=1.48 (p=0.14) | ||||||
HFNC, high-flow nasal cannula; CPAP, continuous positive airway pressure; CI, confidence interval; M-H, Mantel-Haenszel.
There were no deaths and occurrence of pneumothorax in the study by Soonsawad et al.8 Only Manley et al.12 and Soonsawad et al.8 reported nasal trauma in a comparable manner.
DiscussionThe use of HFNC is becoming widespread in neonatal intensive care units. Among the possibilities of use is respiratory support after tracheal extubation in preterm newborns. Among the studies selected for this review, the meta-analyses stand out. This review identified four randomized controlled trials with a very similar design, and it was possible to analyze outcomes of interest between the two interventions performed.
Although there are still some disagreements about the effectiveness of HFNC over CPAP, as shown by the prospective study by Konda et al.,6 who reported that “although it is a modality with a lower incidence of nasal trauma, HFNC does not seem to be as effective as CPAP in the management of preterm infants with respiratory distress,” the present review shows that HFNC performance is non-inferior to that of CPAP in preterm newborn patients submitted to extubation.
However, it was not possible to analyze subgroups, for instance, of children under 27 weeks of gestation, which was the average observed in the studies. Therefore, this meta-analysis, and the studies analyzed here, do not answer the question of which is the lowest safe gestational age for the method to be used.
Manley et al.12 suggested caution when using the method in extremely preterm infants, with age younger than 26 weeks, because the study did not have the power to assess efficacy in this subgroup. In the study by Kang et al.,15 the subgroup analysis was performed in newborns with gestational ages between 26 and 28 weeks + 6 days and compared with newborns with gestational ages between 29 and 31 weeks + 6 days. The more preterm newborn group showed significantly higher percentage of therapeutic failures (45% vs. 30%, p=0.03).
Ferguson et al.9 analyzed 867 patients enrolled in the studies and concluded that “preterm infants should be extubated with transition to noninvasive respiratory support, and the pooled analysis showed no difference between HFNC and CPAP regarding treatment failure within seven days.” Zong-Tai et al.16 analyzed 1040 patients included in the studies and concluded that “HFNC is safe and effective in preventing extubation failure in newborns.” Daish et al.17 analyzed 726 patients included in the studies and concluded that “there is no significant difference in extubation failure rate in neonates extubated to HFNC when compared to those extubated to CPAP.”
Another noteworthy aspect regarding the possibility of using HFNC is the lower risk of nasal lesions in patients undergoing this respiratory support compared to the use of CPAP.18 In the studies by Collins et al.,14 Manley et al.,12 and Soonsawad et al.,8 the use of HFNC was associated with a significant reduction in nasal trauma risk. Lesion to the nasal mucosa may be an unexplained cause of sepsis in preterm infants.19 It is noteworthy that not applying a score may lead to significant heterogeneity.
In all studies, in cases of therapeutic failure under HFNC, newborns were allowed to receive another type of noninvasive respiratory support such as CPAP. In the study by Manley et al.,12 CPAP with non-synchronized frequency prevented reintubation in almost half of the newborns who failed while receiving HFNC. This return to CPAP may have influenced some of the secondary outcomes in the group using HFNC. In the study by Konda et al.,6 despite a higher number of cases with therapeutic failure in the group using HFNC, the number of re-intubations was equal between the two groups. There was not a sufficient number of cases using CPAP vs. nasal intermittent positive pressure ventilation (NIPPV) to make a comparison.
There is insufficient evidence on whether or not the use of HFNC increases the oxygen delivery time to preterm infants and, consequently, whether it is responsible for an increase in the number of cases of bronchopulmonary dysplasia and longer hospital length of stay.20 As inherent in this population, there was a large number of cases of bronchopulmonary dysplasia (28.5%), but the meta-analysis did not detect heterogeneity and differences in the odds ratios. Therefore, it can be affirmed that there are no differences in the risk for this condition between HFNC and CPAP, and the same can be understood about the risk of reintubation and in-hospital death. For events such as necrotizing enterocolitis, intraventricular hemorrhage, and pneumothorax, the occurrence of few cases may compromise the power of analysis but, apparently, there is no risk difference. According to the meta-analysis data, one can consider HFNC as a safe respiratory support modality unrelated to an increased number of cases of bronchopulmonary dysplasia.
Within the four studies included in the meta-analysis for the primary outcome, there was a total of 645 patients, and the risk differences showed that HFNC was not inferior to CPAP in post-extubation respiratory support in preterm infants younger than 32 weeks. The upper limit of 13% of the difference observed in the joint analysis seems reasonable to affirm the effectiveness of the method in relation to CPAP.
There are still few good-quality studies on the subject, opening the possibility for multicenter randomized controlled trials that can better determine the efficacy of HFNC over other forms of noninvasive respiratory support.
This review is limited by the small number of randomized controlled trials available for analysis. The studies were methodologically adequate, although the non-concealment of allocation could not be verified in one of the studies used in the meta-analysis (Kang et al.15). The possible biases observed, such as the absence of reintubation criteria, which was left to the attending physicians tm) discretion, may have led to unidentified differences between the groups; however, it reflects the daily clinical practice in neonatal intensive care units.
The strengths include the almost uniform assessment of primary and secondary outcomes and the use of similar methods for delivery of the gas mixture into the high-flow cannulas with more modern equipment. The studies by Manley et al.,12 Soonsawad et al.,8 and Kang et al.,15 used Fisher &Paykel¨r) equipment (Fisher & Paykel Healthcare, CA, USA), whereas the study by Collins14 used Vapotherm¨r) equipament (Vapotherm, NH, USA), with no evidence that one commercial model is superior to the other.
ConclusionHFNC is non-inferior to CPAP as respiratory support after extubation of preterm newborns with gestational age of 32 weeks or less, and has similar reintubation rates, although its use should be cautious in extremely preterm newborns with gestational age <26 weeks, as it is not yet possible to determine its efficacy in this age group. The lower risk of nasal trauma should be taken into account when choosing between therapies. The use of HFNC appears to be safe and efficient. Multicenter studies with different gestational ranges are required.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Colleti Junior J, Azevedo R, Araujo O, Carvalho WB. High-flow nasal cannula as a post-extubation respiratory support strategy in preterm infants: a systematic review and meta-analysis. J Pediatr (Rio J). 2020;96:422–31.
Study conducted at Hospital Santa Catarina, São Paulo, SP, Brazil.