To evaluate the psychometric properties of the Brazilian version of health-related quality-of-life questionnaires of children with food allergy and their parents.
MethodsThe translation and cultural adaptation processes were previously performed, according to the method proposed by the World Health Organization. After this stage, the questionnaires were applied to 201 parents of children under 6 years of age with food allergy. The assessment of the psychometric properties included: evaluation of the internal consistency by Cronbach's alpha coefficient; of the reproducibility by the intraclass correlation coefficient between test and retest; and of the construct, using Spearman's correlation coefficient, comparing the obtained scores with those of generic questionnaires that evaluate health-related quality of life.
ResultsThe means of the obtained scores were 2.44 and 3.35, for the children and their parents, respectively. Cronbach's alpha coefficients were 0.85 and 0.91, respectively, which showed good internal consistency of the tools. The intraclass correlation coefficients between test and retest were 0.87 and 0.84 for children and their parents, respectively, showing good reproducibility for both questionnaires. The correlation between the specific and the generic questionnaires was significant (−0.27 for the children, −0.64 for their parents, p<0.05).
ConclusionsThe specific questionnaires to evaluate the health-related quality of life of children with food allergy and of their parents were satisfactorily validated to be used in Brazil.
Avaliar as propriedades psicométricas da versão brasileira dos questionários de qualidade de vida relacionada à saúde de crianças com alergia alimentar e de seus pais.
MétodoOs processos de tradução e adaptação cultural foram feitos previamente, de acordo com o método proposto pela Organização Mundial da Saúde. Após essa etapa, os questionários foram aplicados a 201 pais de crianças menores de 6 anos com alergia alimentar. A avaliação das propriedades psicométricas incluiu: avaliação da consistência interna, pelo coeficiente alfa de Cronbach; da reprodutibilidade, pelo coeficiente de correlação intraclasse entre teste e reteste; e do constructo, empregou-se o coeficiente de correlação de Spearman, comparando os escores obtidos com os de questionários genéricos que avaliam a qualidade de vida relacionada à saúde.
ResultadosAs médias dos escores obtidos foram 2,44 e 3,35, para as crianças e seus pais, respectivamente. Os coeficientes alfa de Cronbach foram 0,85 e 0,91, respectivamente, o que demonstrou boa consistência interna dos instrumentos. Os coeficientes de correlação intraclasse entre os testes e os retestes foram 0,87 e 0,84, para crianças e seus pais, respectivamente, demonstraram boa reprodutibilidade para ambos os questionários. A correlação entre os questionários específicos e genéricos foi significante (−0,27 para as crianças; −0,64 para os pais; p<0,05).
ConclusõesOs questionários específicos para avaliar a qualidade de vida relacionada à saúde de crianças com alergia alimentar e de seus pais foram satisfatoriamente validados para uso no Brasil.
Health-related quality of life (HRQoL) is defined as an individual perception about the effects of a disease and the consequences of its treatment, considering physical, social, and psychological aspects.1
HRQoL can be evaluated through generic or disease-specific tools. The former are useful because they allow comparisons between different diseases, whereas the specific tools, although not allowing such comparisons, are more sensitive and, thus, more likely to detect minor alterations.2
Since 2003, some researchers have developed questionnaires to evaluate the HRQoL of individuals with food allergy (FA) in different age groups. One of the most frequently used tools in recent years has been the Food Allergy Quality of Life Questionnaire-Parent Form (FAQLQ-PF), which has already been translated and adapted to different languages and has shown adequate validation in different cultures.3 This questionnaire is answered by the children's parents (proxy). Another widely used tool is the Food Allergy Quality of Life-Parental Burden (FAQL-PB), which reports the quality of life of the parents of children with FA.4
The identification of the factors that affect the HRQoL of individuals with FA and their family members can help to choose the best ways to approach the diagnosis and direct the treatment of these patients.5
The present study aims to evaluate the psychometric properties of the Brazilian version of the FAQLQ-PF and FAQL-PB questionnaires.3,4
MethodsThe evaluation of the psychometric properties of the Brazilian version of the FAQLQ-PF and FAQL-PB questionnaires was performed according to the method commonly used for HRQoL tools.6 The translation and cultural adaptation processes of the tools were previously performed, according to the method proposed by the World Health Organization, and have been already published.7,8
ParticipantsRecruitingThe present study was carried out in four different locations: three referral health services and a distribution center that offers free supply of special formulas for children with cow's milk allergy, maintained by the Government of the State of São Paulo.
Parents of children up to 6 years of age, with a previously established clinical diagnosis of FA (IgE-mediated, unmediated, and mixed forms) and were being treated and/or followed at outpatient clinics where the interviews were carried out were invited to participate in the study.
The exclusion criteria were: doubtful medical history about FA, such as those based only on subjective and/or non-reproducible symptoms; reports that the child had overcome the FA; and presence of another underlying disease, such as diabetes, asthma, among other chronic diseases that could interfere with the HRQoL of children and their parents.
The clinical and demographic characteristics of the children were obtained through a standardized questionnaire developed for this study.
Sample size calculationThe FAQLQ-PF has 30 questions categorized into three domains: emotional impact, food anxiety, and social limitations. Parents of children aged 0–3 years should answer only the first 14 questions; parents of those aged 4–6 years old should answer up to question 26; and parents of those aged 7–12 years should answer all the questions.9
To evaluate the psychometric properties of the Brazilian version of the FAQLQ-PF, it was estimated that it would be necessary to interview at least 102 parents of children aged 0–3 years and 98 parents of children aged 4–6 years. The sample size was calculated using the statistical software PASS (Power Analysis and Sample Size System, version 2008, UT, USA) – NCS. Detection was considered, with 90% power, the difference between the α-Cronbach coefficient having as null hypothesis a coefficient value of 0.60 (poor consistency) against the alternative hypothesis with a value of 0.75 (acceptable consistency).10
The FAQL-PB questionnaire was developed for parents of children and adolescents aged 0–17 years. The tool contains 17 questions, categorized into two domains: life limitations (six items) and emotional stress (11 items). To evaluate of the psychometric properties of the Brazilian version of the FAQL-PB, the sample size was not calculated, admitting that it would be sufficient to interview at least 100 parents of children with FA.
Sample sizeA total of 201 parents of children with FA participated in this study, of which 183 were parents of children under 4 years of age and 18 of children aged 4–6 years. All answered the Brazilian version of the FAQLQ-PF and 148 answered the Brazilian version of the FAQL-PB.
Ethical considerationsThis study was approved by the Ethics Committee of the university where it was carried out (CAAE: 19515413.8.1001.5505). Participants were included in the study only after they signed the free and informed consent form.
MeasurementsThe scores of the questionnaires were obtained through the mean values of the answers, by domains as well as totals. Only the first 14 items were considered for the calculation of the total score of the FAQLQ-PF.
The questionnaires FAQLQ-PF and FAQL-PB have a rating scale of 0–6 points. For these questionnaires, the lower the obtained score, the better the HRQoL of the individual.
For the FAQLQ-PF, the scores reported on the scale from 0 to 6 were converted to the most typically used scale of 1–7 points (thus, zero-value responses became 1, 1-value responses became 2, and so on), to be more easily compared with previous publications by other authors who used this tool.
The Pediatric Quality of Life Inventory™ (PedsQL™) 4.0 and the PedsQL – Family Impact Module questionnaires have an evaluation scale of 0–4 points (0=never; 4=always). To determine the HRQoL scores according to these questionnaires, the results obtained from the 0-to-4 point scale were transformed into a scale from 0 to 100, where 0=100, 1=75, 2=50, 3=25, and 4=0. After this transformation, the interpretation is that the lower the score obtained, the worse the HRQoL of the interviewed individual. The scale transformation was performed according to the scaling and scoring of the PedsQL™, available at http://www.pedsql.org/PedsQL-Scoring.pdf.
Statistical analysisDescriptive statistics and graphs (histograms and box-plots) were used to analyze data distribution. As distributions were asymmetrical, data were presented as medians and interquartile ranges (25th percentile–75th percentile).
Evaluation of psychometric propertiesThe internal consistency of the two questionnaires was assessed using the α-Cronbach coefficient, considering values ≥0.75 as acceptable.11
The test–retest was performed to evaluate the reproducibility of the questionnaires, i.e., the questionnaires were applied at a first moment and reapplied after 7–30 days. This second application was performed through an electronic questionnaire sent by e-mail, or by a printed questionnaire, returned by mail, according to the participant's choice. To facilitate their return by mail, envelopes were delivered with stamps and the return address.
The intraclass correlation coefficient (ICC) was used for statistical analysis, and only those questionnaires whose parents said they had not observed any change in their quality of life and their children's since the first application of the questionnaires were used. To interpret the magnitude of the correlations, the following classification was adopted: values ≤0.40, poor correlation; 0.41–0.60, moderate correlation; 0.61–0.80, good correlation; and 0.81–1.00, excellent correlation.11
The participants answered the generic HRQoL questionnaires PedsQL™ 4.0 and PedsQL – Family Impact Module for construct validation.12–14 Spearman's correlation coefficient calculation was used to analyze the correlation between the scores of the generic and specific questionnaires. Correlations were made by both domain and total score. For the interpretation of the magnitude of the correlations, the classification proposed by Hulley et al. was used: correlation coefficients <0.3 (weak magnitude correlation), ≥0.3 to <0.6 (moderate magnitude), and ≥0.6 (strong magnitude).15 Weak to moderate correlations were expected.
The data obtained with the filling out the Brazilian versions of the FAQLQ-PF and FAQL-PB questionnaires were entered into a database and the statistical analyses were then performed. The evaluation of the psychometric properties was performed using STATISTICA software (StatSoft® South America, version 12.7, SP, Brazil). In all tests, 5% was used as the rejection level of the null hypothesis.
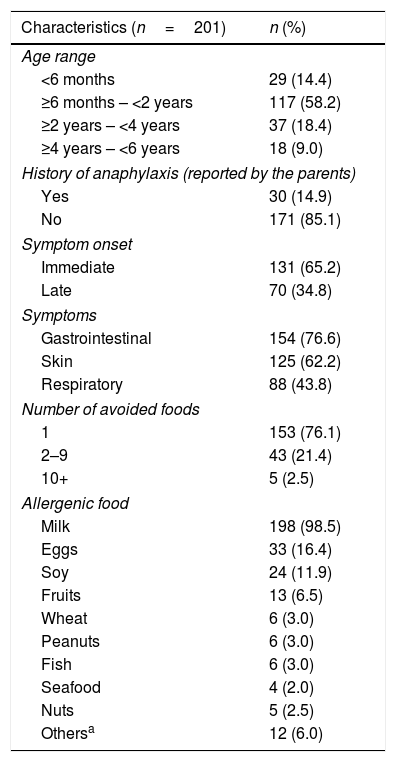
ResultsCharacteristics of the study populationOf the 201 subjects included in the study, 75.6% (152) were mothers. The mean age of the interviewees was 33.5 years. Regarding the children, 52.2% (n=105) were boys. Age ranged from 2 months to 5.9 years (median 17 months). The median age for the establishment of the FA diagnosis was 4 months. Thirty interviewees (14.9%) reported that their children had already had anaphylaxis; in 15 cases (7.5%) the children had systemic reactions, and the use of self-injectable epinephrine was prescribed for five children (2.5%). Most children (98.5%, n=198) had cow's milk allergy and 76.1% (n=153) were allergic to only one type of food. The clinical and demographic characteristics are shown in Table 1.
Clinical and demographic characteristics of the children included in the study.
| Characteristics (n=201) | n (%) |
|---|---|
| Age range | |
| <6 months | 29 (14.4) |
| ≥6 months – <2 years | 117 (58.2) |
| ≥2 years – <4 years | 37 (18.4) |
| ≥4 years – <6 years | 18 (9.0) |
| History of anaphylaxis (reported by the parents) | |
| Yes | 30 (14.9) |
| No | 171 (85.1) |
| Symptom onset | |
| Immediate | 131 (65.2) |
| Late | 70 (34.8) |
| Symptoms | |
| Gastrointestinal | 154 (76.6) |
| Skin | 125 (62.2) |
| Respiratory | 88 (43.8) |
| Number of avoided foods | |
| 1 | 153 (76.1) |
| 2–9 | 43 (21.4) |
| 10+ | 5 (2.5) |
| Allergenic food | |
| Milk | 198 (98.5) |
| Eggs | 33 (16.4) |
| Soy | 24 (11.9) |
| Fruits | 13 (6.5) |
| Wheat | 6 (3.0) |
| Peanuts | 6 (3.0) |
| Fish | 6 (3.0) |
| Seafood | 4 (2.0) |
| Nuts | 5 (2.5) |
| Othersa | 12 (6.0) |
The time to answer each of the FA-specific questionnaires ranged from 2 to 4min.
Evaluation of tool reliability and validityTo analyze the internal consistency of the Brazilian version of the FAQLQ-PF, only data from children younger than 4 years (n=183) were considered. Using the α-Cronbach coefficient, the authors observed α=0.85, which demonstrated good internal consistency among the 14 items that comprise the questionnaire for this age group.
For the analysis of the internal consistency of the Brazilian version of the FAQL-PB, the data of the 148 interviewees who completed this questionnaire were considered. The α-Cronbach coefficient was α=0.91, which showed excellent internal consistency among the 17 items of the tool.
To evaluate the reproducibility, 84 individuals participated in the retest of the questionnaires, with 28 (1/3) returning the printed retest by mail and 56 (2/3) by answering the retest in electronic format. The intraclass correlation coefficient (ICC) between the FAQLQ-PF test and retest was 0.874 (95% confidence interval [95% CI]: 0.793–0.923) and between the FAQL-BP test and retest, it was 0.836 (95% CI: 0.734–0.899).
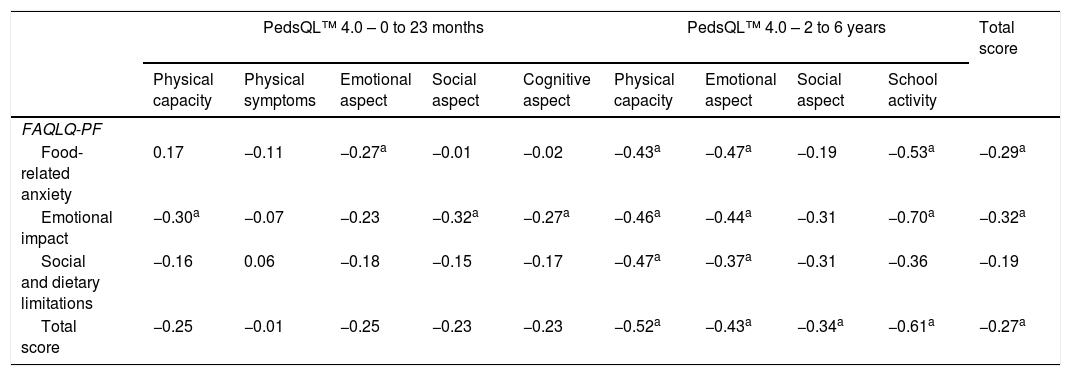
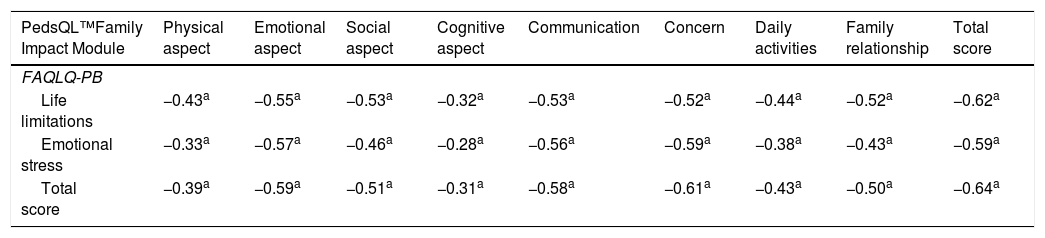
At the evaluation of the construct, as expected, a low correlation was found between the total scores of FAQLQ-PF and PedsQL™ 4.0, r=−0.27 (p<0.05; low correlation) (Table 2). The correlation between the FAQL-PB and PedsQL – Family Impact Module scores showed r=−0.64 (p<0.05; strong correlation) (Table 3). The correlation between the specific and generic questionnaires was also performed for each of the domains that comprised the tools (Tables 2 and 3). The inverse association between the scores can be explained by the fact that, for the specific questionnaires, lower values are associated with better HRQoL, while for the generic ones, the scale is the opposite, i.e., lower values represent worse HRQoL.
Spearman's correlation coefficient between the FAQLQ-PF and PedsQL™ 4.0 scores, according to domains, by pediatric range.
| PedsQL™ 4.0 – 0 to 23 months | PedsQL™ 4.0 – 2 to 6 years | Total score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical capacity | Physical symptoms | Emotional aspect | Social aspect | Cognitive aspect | Physical capacity | Emotional aspect | Social aspect | School activity | ||
| FAQLQ-PF | ||||||||||
| Food-related anxiety | 0.17 | −0.11 | −0.27a | −0.01 | −0.02 | −0.43a | −0.47a | −0.19 | −0.53a | −0.29a |
| Emotional impact | −0.30a | −0.07 | −0.23 | −0.32a | −0.27a | −0.46a | −0.44a | −0.31 | −0.70a | −0.32a |
| Social and dietary limitations | −0.16 | 0.06 | −0.18 | −0.15 | −0.17 | −0.47a | −0.37a | −0.31 | −0.36 | −0.19 |
| Total score | −0.25 | −0.01 | −0.25 | −0.23 | −0.23 | −0.52a | −0.43a | −0.34a | −0.61a | −0.27a |
Spearman's correlation coefficient between the FAQL-PB and PedsQL™ Family Impact Module, according to the different domains.
| PedsQL™Family Impact Module | Physical aspect | Emotional aspect | Social aspect | Cognitive aspect | Communication | Concern | Daily activities | Family relationship | Total score |
|---|---|---|---|---|---|---|---|---|---|
| FAQLQ-PB | |||||||||
| Life limitations | −0.43a | −0.55a | −0.53a | −0.32a | −0.53a | −0.52a | −0.44a | −0.52a | −0.62a |
| Emotional stress | −0.33a | −0.57a | −0.46a | −0.28a | −0.56a | −0.59a | −0.38a | −0.43a | −0.59a |
| Total score | −0.39a | −0.59a | −0.51a | −0.31a | −0.58a | −0.61a | −0.43a | −0.50a | −0.64a |
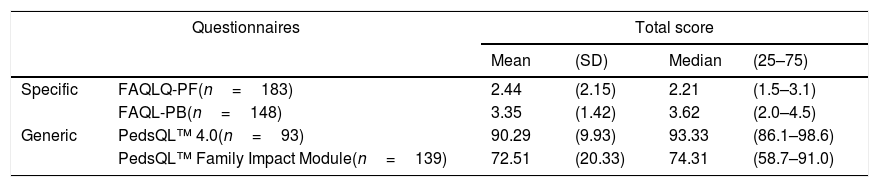
The median score of the HRQoL questionnaires of children with FA was 1.21 (1.5–3.1) (on the scale of 1–7) and the median score of the questionnaires that assessed the parents’ quality of life was 3.6 (2.0–4.5) (on the scale of 0–6) (Table 4).
Quality of life score, according to the applied questionnaire.
| Questionnaires | Total score | ||||
|---|---|---|---|---|---|
| Mean | (SD) | Median | (25–75) | ||
| Specific | FAQLQ-PF(n=183) | 2.44 | (2.15) | 2.21 | (1.5–3.1) |
| FAQL-PB(n=148) | 3.35 | (1.42) | 3.62 | (2.0–4.5) | |
| Generic | PedsQL™ 4.0(n=93) | 90.29 | (9.93) | 93.33 | (86.1–98.6) |
| PedsQL™ Family Impact Module(n=139) | 72.51 | (20.33) | 74.31 | (58.7–91.0) | |
FAQLQ-PF, scale from 1 to 7. FAQL-PB, scale from 0 to 6.
The medians of the generic questionnaire scores were 93.3 (86.1–98.6) and 74.3 (59.7–91.0) for those that assessed the HRQoL of children and parents, respectively (Table 4).
DiscussionThe FAQLQ-PF, one of the most commonly used questionnaires in HRQoL studies of individuals with FA, has been translated into nine languages and validated for use in more than ten countries in Europe and the USA, and also in Japan. Other FAQLQ-PF validation studies are ongoing in Australia, Russia, and Norway.16,17
Couto et al. translated and culturally adapted the FAQLQ-PF for use in Portugal.16 Although this questionnaire is in Portuguese (Portuguese culture), it is not culturally adapted to be used with the Brazilian population, since there are differences regarding the spelling of some words and certain expressions.7 Therefore, it would not be appropriate to use in Brazil the same version used in Portugal, but the results of the studies carried out by applying each version in their respective countries can be perfectly compared, as well as the results obtained in the other places where this questionnaire was applied.
The Brazilian versions of FAQLQ-PF and FAQL-PB showed good internal consistency and reliability. Similar results were observed by other researchers when carrying out the validation of these questionnaires in different cultures.
For example, in the original FAQLQ-PF publication involving 30 Irish children and ten children from the USA under the age of 4, the α-Cronbach coefficient was 0.89 and 0.92, respectively, for each country.3 As for the study carried out in Japan, the α-Cronbach coefficient found for the same age group was 0.87 for the total score, ranging from 0.77 to 0.97 between the different domains.9 The literature on the validation of the FAQL-PB questionnaire shows that the α-Cronbach coefficient was 0.95 or higher, as seen in the study carried out by Cohen et al. in the United States,4 involving 352 parents of children and adolescents with FA whose age ranged from 2 months to 17 years (median 5.8 years); the study by Knibb and Stalker,17 seeking the validation of the same questionnaire for the population of the United Kingdom, based on responses of 699 parents of allergic children (mean age 8.9 years), observed an α-Cronbach of 0.95.18
The present study showed good reproducibility for both tested questionnaires and there were no differences between the retests obtained by mail or electronic means. Similar results were observed in the original validation studies of these questionnaires, as well as in cultural adaptation studies.
In the study by DunnGalvin et al., the ICC was 0.78 for Irish children younger than 4 years (n=30).3 In a Japanese study on the use of the FAQLQ-PF, the ICC was 0.80 for the same age group.9 Comparing these results, the reproducibility of the questionnaire applied in the present study was excellent.
Cohen et al. observed an ICC of 0.93 between the FAQL-PB test and retest. In the present study, the result was lower, but still enough to consider that there was a good reproducibility of the questionnaire (ICC>0.7).4
The correlations between the generic and specific questionnaires were statistically significant (p<0.05), but poor (below 0.3) to moderate (between 0.3 and 0.6), probably because generic questionnaires do not have the same sensitivity to detect problems related to FA. This highlights the importance of applying specific questionnaires for each disease.
Similar results were observed by Morou et al., whose objective was the translation, cultural adaptation, and validation of the FAQLQ-child form (children aged 8–12 years) for use in Greece.19 Correlating the scores with those of the PedsQL™ 4.0 questionnaire for construct evaluation, the correlation between the total scores of the questionnaires was poor and that for the “emotional impact” subscale was moderate.
As a study limitation, it should be mentioned that the inclusion criterion was based on a previously established clinical diagnosis of FA, not necessarily confirmed by an oral provocation test (OPT). In any case, all the participating families followed the elimination diet and believed that the children were really allergic and, therefore, could have their HRQoL affected by the occurrence of FA. Birdi et al. found that even when the allergy is diagnosed by the parents themselves, they experience a loss in their quality of life, when compared to the parents of children without FA, so it is important to carry out studies also including these individuals, encouraging them to seek medical and psychological help, if necessary.20
Another limitation to be highlighted in the present study is the fact that correlations were made between the scores of generic and specific questionnaires to evaluate the validity of the tools. However, because there were no specific tools validated for use in the food-allergic population in Brazil, there was no alternative but to use the generic questionnaires. This limitation justifies the poor-to-moderate correlations found.
The targeted focus of disease-specific tools makes them especially clinically relevant. A tool developed to address a particular disease must respond to clinically important changes in health and wellbeing that result from interventions aimed at this disease. Disease-specific tools do not contain items that are not relevant or that may introduce biases in the interpretation of results. In comparison to generic tools, they are able to better discriminate and may be more sensitive in detecting and quantifying small changes that are important for physicians or patients and for the clinical outcomes. As the specific tools have a clear relevance for patients with the respective disease, their acceptance is usually very high.
Some studies aimed to verify the impact of OPT on the HRQoL of children with suspected FA and of their parents. It was observed that after the OPT was performed, there was an improvement in the HRQoL scores of the children and their parents, regardless of the test result.21–23 Therefore, it is worthwhile to consider the usability of the tools validated here in future cohort studies, involving objective diagnostic tests and evaluation of tolerance acquisition, which can better clarify the clinical manifestations to be expected, thus reducing the anxiety of patients and their family members. Moreover, a well-defined diagnosis allows the elimination diet to be well targeted and treatment-related educational interventions be carried out to help improve the HRQoL of patients and their family members.
With the increasing international collaboration in medical research, the availability of culturally applicable tools for the evaluation of HRQoL in research and clinical trials is important, particularly when considering the increase of large multi-center, multinational studies, and studies carried out in immigrant populations. Cross-cultural comparison provides important information, since cultural groups may vary in disease expression and in the use of different health systems.17
ConclusionsAccording to the evaluation of the psychometric properties of the Brazilian versions of the FAQLQ-PF3 and the FAQL-PB,4 the tools showed good validity, reliability, and reproducibility, being considered adequate to be used with parents of Brazilian children with food allergy, aged 0–4 years and 0–6 years, respectively, for the FAQLQ-PF and FAQL-PB.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the parents who participated in this study and the professionals who allowed it to be performed in each of the places where the questionnaires were applied. They also would like to thank the authors of the original version of the tools used in this study, for authorizing the translation and use of the questionnaires they have developed.
Please cite this article as: Mendonça RB, Solé D, DunnGalvin A, Len CA, Sarni RO. Evaluation of the measurement properties of the Brazilian version of two quality-of-life questionnaires in food allergy – for children and their parents. J Pediatr (Rio J). 2020;96:600–6.