To evaluate the complementary feeding practices, food intake, and nutritional status of infants on a cow's milk protein elimination diet.
MethodsA cross-sectional and observational study was conducted to compare infants aged 4–18 months who were on a cow's milk protein elimination diet with a control group of healthy infants without any dietary restrictions. General information on the child's health, demographic data, and food consumption were collected.
ResultsThe study included 96 infants in the elimination diet group and 99 in the control group. In the elimination diet group, the median age (in months) of introduction of solid foods (5.0 × 4.0; p < 0.001) and water (5.5 × 4.0; p < 0.05) was later, consumption of soft drinks and industrialized cookies was less frequent (p < 0.05), and a lower index of complementary feeding inadequacies (2.75 × 3.50; p < 0.001) was observed. The elimination diet group presented lower individual values of Z scores for weight/age, weight/height, and body mass index/age, although they were fed with higher amounts of energy (117.4 × 81.3 kcal/kg of weight; p < 0.001) and macro-and micronutrients, except for vitamin A. In the elimination diet group, breast milk and its substitutes contributed to more than 67% of energy intake. Although calcium consumption was a deficit in 31.5% of the infants, none received supplementation.
ConclusionInfants on an elimination diet presented more adequate complementary feeding practices and higher nutritional intake, despite lower body weight values.
An elimination diet for cow's milk and dairy products is recommended for infants with a suspected or confirmed diagnosis of cow's milk protein allergy (CMPA).1,2 However, the elimination diet is followed by a greater number of infants in the initial phase, before diagnostic confirmation, and also by patients who for various reasons do not undergo a diagnostic confirmation test. An elimination diet should always be adequate to ensure full growth and development.
Until 6 months of age, exclusive breastfeeding should be maintained, and the breastfeeding mother should eliminate cow milk from her diet. Infants who are not breastfed should be fed special formulas without cow's milk protein.1–3 Complementary feeding should be started after 6 months of age. Complementary feeding is defined as all foods offered in addition to breast milk during the period that child will not be exclusively breastfed. The same definition may be used for infants receiving artificial feeding.3,4 According to national and international guidelines, complementary feeding for infants with CMPA should follow the recommendations for healthy children. Hence, no restriction or delay in the introduction of foods, including potentially allergenic foods, should be done1,3,4 as often occurs in healthcare practice.5,6 Complementary feeding is important to define lifelong eating habits and behaviors.4,7
Despite the relevance of the quality of diet for infants on an elimination diet, there are only a few studies on this subject. There are pieces of evidence of dietary nutrient deficiencies, growth deficits, and low food intake.8-12 To the best of the authors's knowledge, no publication on complementary feeding practices of infants on an elimination diet for cow's milk and dairy products is available.
Thus, the objective of this study was to evaluate the complementary feeding practices, dietary intake, and nutritional status classification of infants on a cow's milk protein elimination diet compared to a control group.
MethodsA cross-sectional and observational study approved by the Research Ethics Committee of the Federal University of São Paulo (UNIFESP) was conducted. Infants aged 4–18 months who were on a diet free of cow's milk and dairy products for a minimum period of 1 month owning to suspected or confirmed diagnosis of CMPA were considered for this study. These infants were included regardless of the type of clinical presentation or criteria used to establish the diagnosis. Confirmation of the correct completion of the cow's milk elimination diet was carried out during the individual interview. It was based on a specific question of whether the infant was receiving or not cow's milk. The elimination of cow's milk was also evaluated in the food survey. Infants on the elimination diet for other reasons besides cow's milk allergy, such as vegetarianism, veganism, or galactosemia, were not admitted to the study. A control group consisting of healthy infants without any dietary restrictions was included for comparison purposes.
The parents/caregivers of children in both the groups were aged ≥18 years and they provided written informed consent. In case the volunteers had more than one eligible child, only the youngest child was included. Infants previously diagnosed with other severe diseases or those requiring major dietary changes, healthy infants living with children with CMPA, and infants who weren't correctly complying with the exemption diet were excluded.
The cow's milk protein elimination diet group was recruited in the metropolitan region of São Paulo, at the Food Allergy Clinic of the Pediatric Gastroenterology Department of UNIFESP and at Vila Mariana High-Cost Pharmacy of the Health Department of the State of São Paulo, Brazil, similar to that reported in a previous study.13 The latter is a pharmacy from the Health Public System where special formulas are supplied free of charge to infants on an elimination diet living in São Paulo, which complies with the terms established in legislation (Resolution SS-336, of November 27, 2007). The control group was recruited at Vila dos Remedios Basic Public Health Unit and at a church located in the Butantã neighborhood.
The sample size estimative was based on a previous Brazilian study that revealed that children on a cow's milk protein elimination diet had insufficient intake of energy and other nutrients such as calcium. There were differences of more than 40% in the proportions of children with inadequate intake in the study and control groups.8 Thus, in the present study, the sample size was calculated to identify a smaller difference between the groups (15%) with expectations of intake below the recommendation in 20% of the infants on an elimination diet and 5% of those on a diet without exclusions.
The data were collected between January 2018 and April 2019, through individual interviews using a standardized protocol that contained the parents/caregivers socioeconomic and educational information, the principal clinical presentation that motivated the suspicious or diagnosis of CMPA; and information on feeding, supplementation, and professionals who provided guidance on complementary feeding. The socio-economic classification was performed using the “ABEP - Brazilian Association of Research Companies” questionnaire (2015).14 It was expressed in decreasing order of strata, being class A the highest socioeconomic level, followed by classes B1, B2, C1, C2, and DE (the lowest socioeconomic level).
To evaluate the complementary feeding practices, information regarding the age at which food groups were introduced, forms of preparation, and supply of ultra-processed foods were collected. The index of complementary feeding inadequacies developed in Brazil was calculated.15 This index is specified in Supplementary Material 1. A formula based on a multiple linear regression model was used to estimate the volume of breast milk ingested.16,17 Food intake was evaluated based on the usual daily food intake method.8 Data on food intake and complementary feeding practices were compared to the recommendations of the Brazilian Society of Pediatrics,4 Food Guide for Children under 2 years of age, 7 and Institute of Medicine.18 The DietWin PC19 version 2.5.4.26856 software was used to calculate macro-and micronutrient intake.
The World Health Organization's Anthro software version 3.2.2 was used to evaluate and classify nutritional status based on weight and height data.20
The infant weight and height measurements were performed on a Welmy® pediatric electronic scale, with a division scale of 5 g, a minimum load of 100 g, and capacity of 15 kg, after consultation with a pediatrician and under the supervision of the main researcher. Height was measured using a horizontal stadiometer, with a precision of 1 mm.21 When weight and height could not be checked, values measured and registered by the infants’ pediatricians were used, since the measurements had been performed in the previous 30 days.
The Z scores for weight/age, weight/height, body mass index (BMI)/age, and height/age were calculated according to the World Health Organization standards.20 The Z scores of infants who had their weight and height values extracted from the pediatrician's records were calculated considering the date of weight and height measurement.
The statistical analysis was performed using the Epi-Info 7.2.3.1 (Center of Disease Control and Prevention, Atlanta, GA, USA) and SigmaPlot 12.5 (Systat Software, San Jose, CA, USA) software. A significance level of 5% was considered in all analyses.
ResultsA total of 230 parents/caregivers were interviewed for this study. Twenty infants with severe diseases or conditions that could lead to nutritional deficiencies or dietary restrictions; six with transgressions in the elimination diet; two with siblings already included in the group; and seven with incomplete data collection were excluded. Thus, the study included 96 infants in the elimination diet group and 99 in the control group.
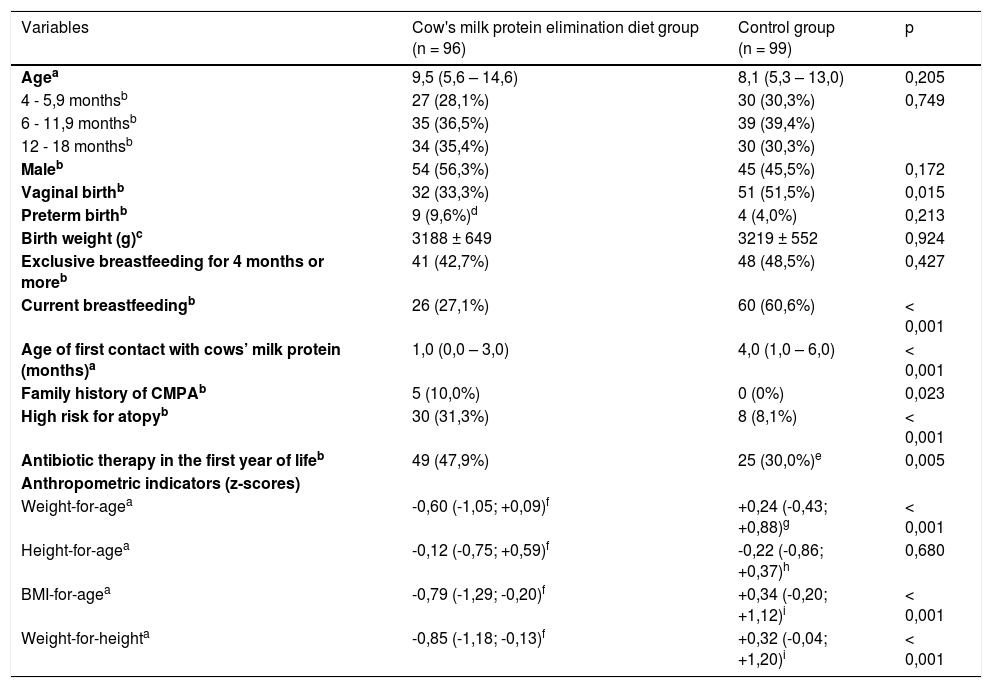
Table 1 shows the demographic characteristics, breastfeeding habits, family history of atopy, and nutritional status of both groups. The groups were similar in terms of age, sex, frequency of premature birth, weight at birth, and frequency of exclusive breastfeeding for a period of 4 months or more. Infants on an elimination diet had a lower frequency of vaginal delivery and breastfeeding, and a lower age at the first contact with the cow's milk protein. Furthermore, these infants had a higher frequency of family history of atopy and CMPA and previous use of antibiotics. Regarding the socio-economic classification, the elimination diet group presented a higher frequency of families belonging to classes A and B (70.8% vs. 37.5%; p < 0.001) and mothers with bachelor's degrees (66.7% vs. 25.0%; p < 0.001).
Sociodemographic characteristics and anthropometric data of infants fed a cow's milk protein elimination diet and an unrestricted diet (control group).
| Variables | Cow's milk protein elimination diet group (n = 96) | Control group (n = 99) | p |
|---|---|---|---|
| Agea | 9,5 (5,6 – 14,6) | 8,1 (5,3 – 13,0) | 0,205 |
| 4 - 5,9 monthsb | 27 (28,1%) | 30 (30,3%) | 0,749 |
| 6 - 11,9 monthsb | 35 (36,5%) | 39 (39,4%) | |
| 12 - 18 monthsb | 34 (35,4%) | 30 (30,3%) | |
| Maleb | 54 (56,3%) | 45 (45,5%) | 0,172 |
| Vaginal birthb | 32 (33,3%) | 51 (51,5%) | 0,015 |
| Preterm birthb | 9 (9,6%)d | 4 (4,0%) | 0,213 |
| Birth weight (g)c | 3188 ± 649 | 3219 ± 552 | 0,924 |
| Exclusive breastfeeding for 4 months or moreb | 41 (42,7%) | 48 (48,5%) | 0,427 |
| Current breastfeedingb | 26 (27,1%) | 60 (60,6%) | < 0,001 |
| Age of first contact with cows’ milk protein (months)a | 1,0 (0,0 – 3,0) | 4,0 (1,0 – 6,0) | < 0,001 |
| Family history of CMPAb | 5 (10,0%) | 0 (0%) | 0,023 |
| High risk for atopyb | 30 (31,3%) | 8 (8,1%) | < 0,001 |
| Antibiotic therapy in the first year of lifeb | 49 (47,9%) | 25 (30,0%)e | 0,005 |
| Anthropometric indicators (z-scores) | |||
| Weight-for-agea | -0,60 (-1,05; +0,09)f | +0,24 (-0,43; +0,88)g | < 0,001 |
| Height-for-agea | -0,12 (-0,75; +0,59)f | -0,22 (-0,86; +0,37)h | 0,680 |
| BMI-for-agea | -0,79 (-1,29; -0,20)f | +0,34 (-0,20; +1,12)i | < 0,001 |
| Weight-for-heighta | -0,85 (-1,18; -0,13)f | +0,32 (-0,04; +1,20)i | < 0,001 |
Family history of CMPA, presence of one or more first-degree relatives with a past or current history of cows’ milk protein allergy; High risk for atopy, presence of two or more first-degree relatives with atopy; BMI, body mass index.
Available data: n = 94d, n = 82e, n = 43f, n = 84g, n = 83h, n = 82i.
The elimination diet group presented lower individual values of Z scores for weight/ age, weight/height, and body mass index/age as presented in Table 1. No statistical difference between the groups regarding height/age z score was observed.
In the elimination diet group, 27.1% (26/96) of the infants were fed breast milk with other foods (21 with special formulas and complementary foods, four with complementary foods only, and one with special formula only). Other 70.8% (68/96) were fed special formulas, of which 61 also received complementary foods. Finally, 2.1% (2/96) of the infants were fed with soy drinks as the only substitute for breast milk, although they already had a medical prescription for soy-based infant formulas and were awaiting supply by the government. Of the 90 infants who were fed special formulas, regardless of whether they received breast milk, 47 (52.2%) received free amino acid formula, 36 (40.0%) received formula based on extensively hydrolyzed milk protein, six (6.7%) were on soy-based infant formula alone, and one (1.1%) was fed a formula based on extensively hydrolyzed rice protein. Only one infant had a soy-based infant formula introduced before 6 months of age.
In the control group, 60.6% (60/99) of the infants were breastfed (eight exclusively, 26 with other dairy sources and complementary foods, and 26 with complementary foods only). Other 23.2% (23/99) received infant formula with complementary foods. Finally, 14.1% (14/99) were fed whole cow's milk, and 2.0% (2/99) received dairy products.
In the elimination diet group, other foods than cow's milk were excluded from the diet of 19 infants. The most commonly reported were eggs (n = 8), wheat (n = 7), and bananas (n = 7).
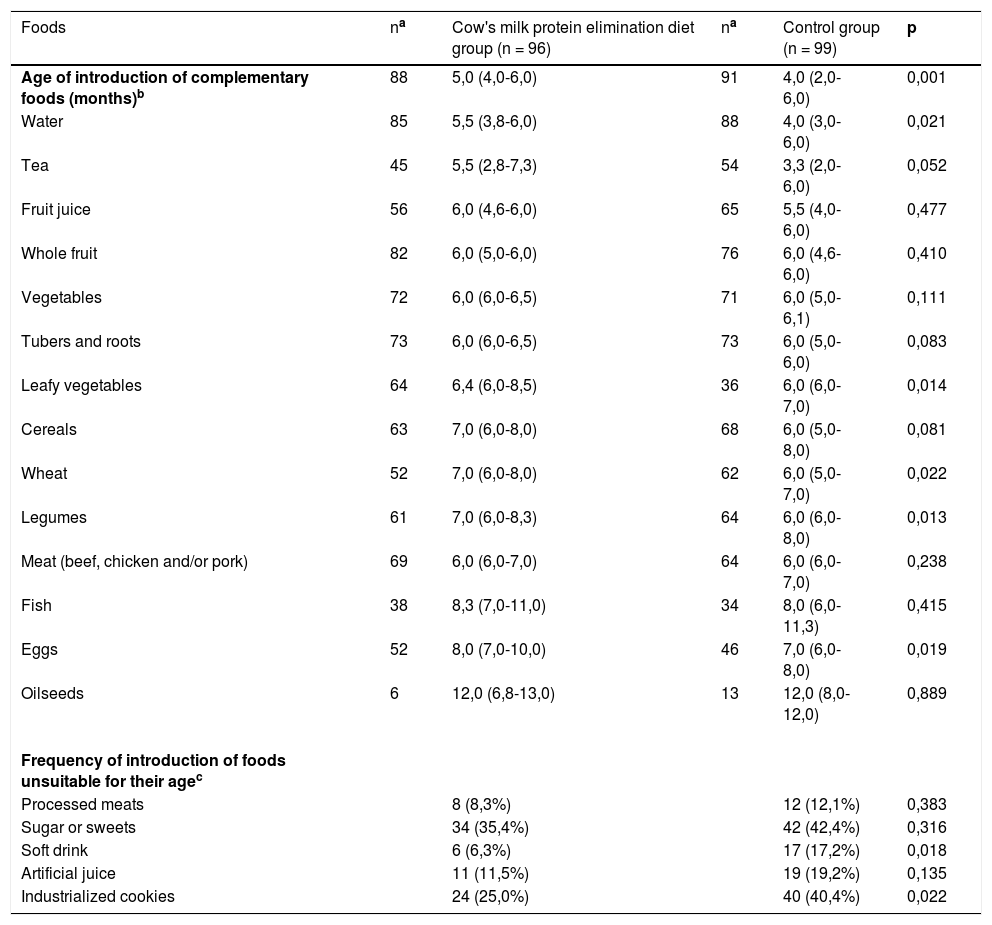
Table 2 presents the results of complementary feeding introduction. Water, leafy vegetables, legumes, wheat, and eggs were introduced later in the elimination diet group. The introduction of tea, tubers, roots, and cereals was also later in this group, although the difference was not statistically significant. Delay was observed in the introduction of potentially allergenic foods, such as egg and wheat. The proportion of children who had already consumed soft drinks and industrialized cookies was higher in the control group.
Age of introduction of complementary foods and frequency of introduction of foods unsuitable for the age of infants fed a cow's milk protein elimination diet and an unrestricted diet (control group).
| Foods | na | Cow's milk protein elimination diet group (n = 96) | na | Control group (n = 99) | p |
|---|---|---|---|---|---|
| Age of introduction of complementary foods (months)b | 88 | 5,0 (4,0-6,0) | 91 | 4,0 (2,0-6,0) | 0,001 |
| Water | 85 | 5,5 (3,8-6,0) | 88 | 4,0 (3,0-6,0) | 0,021 |
| Tea | 45 | 5,5 (2,8-7,3) | 54 | 3,3 (2,0-6,0) | 0,052 |
| Fruit juice | 56 | 6,0 (4,6-6,0) | 65 | 5,5 (4,0-6,0) | 0,477 |
| Whole fruit | 82 | 6,0 (5,0-6,0) | 76 | 6,0 (4,6-6,0) | 0,410 |
| Vegetables | 72 | 6,0 (6,0-6,5) | 71 | 6,0 (5,0-6,1) | 0,111 |
| Tubers and roots | 73 | 6,0 (6,0-6,5) | 73 | 6,0 (5,0-6,0) | 0,083 |
| Leafy vegetables | 64 | 6,4 (6,0-8,5) | 36 | 6,0 (6,0-7,0) | 0,014 |
| Cereals | 63 | 7,0 (6,0-8,0) | 68 | 6,0 (5,0-8,0) | 0,081 |
| Wheat | 52 | 7,0 (6,0-8,0) | 62 | 6,0 (5,0-7,0) | 0,022 |
| Legumes | 61 | 7,0 (6,0-8,3) | 64 | 6,0 (6,0-8,0) | 0,013 |
| Meat (beef, chicken and/or pork) | 69 | 6,0 (6,0-7,0) | 64 | 6,0 (6,0-7,0) | 0,238 |
| Fish | 38 | 8,3 (7,0-11,0) | 34 | 8,0 (6,0-11,3) | 0,415 |
| Eggs | 52 | 8,0 (7,0-10,0) | 46 | 7,0 (6,0-8,0) | 0,019 |
| Oilseeds | 6 | 12,0 (6,8-13,0) | 13 | 12,0 (8,0-12,0) | 0,889 |
| Frequency of introduction of foods unsuitable for their agec | |||||
| Processed meats | 8 (8,3%) | 12 (12,1%) | 0,383 | ||
| Sugar or sweets | 34 (35,4%) | 42 (42,4%) | 0,316 | ||
| Soft drink | 6 (6,3%) | 17 (17,2%) | 0,018 | ||
| Artificial juice | 11 (11,5%) | 19 (19,2%) | 0,135 | ||
| Industrialized cookies | 24 (25,0%) | 40 (40,4%) | 0,022 |
The index of complementary feeding inadequacies15 (Supplementary Material 1) was lower in the elimination diet group. Therefore, liquified or sieved soups, which are inadequate inconsistency, had already been consumed by 47.9% and 52.1% of the elimination diet and control groups, respectively.
As shown in Supplementary Material 2A, the elimination diet group had a higher energy and macronutrient intake than the control group. Breast milk and its substitutes provided a higher percentage of energy and macronutrient intake in the elimination diet group than in the control group (Supplementary Material 2B). The median energy and protein intakes per kilogram of body weight were higher in the elimination diet group than in the control group. A higher daily volume of formula was consumed by the infants in the elimination diet group than by those in the control group.
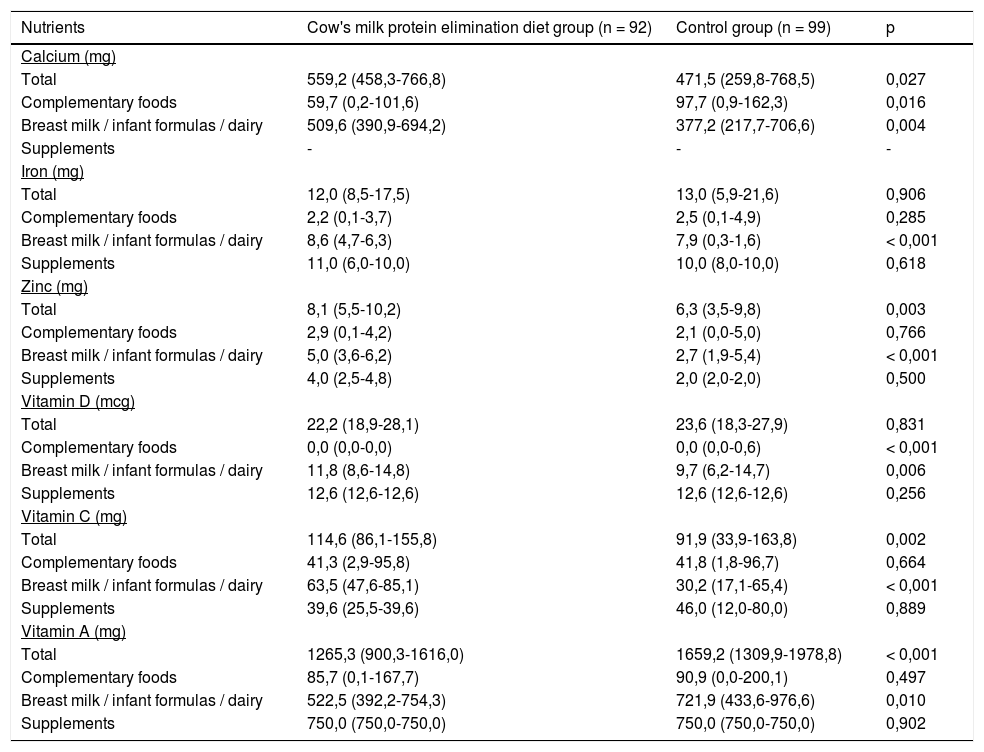
Table 3 shows the consumption of micronutrients provided by breast milk and its substitutes, complementary foods, and supplements. The elimination diet group intake was highest for calcium, zinc, and vitamin C. However, the consumption of vitamin A was lower in the elimination diet group and no difference regarding iron and vitamin D intake was observed between the groups.
Daily intake of calcium, iron, zinc, vitamin A, C, and D of infants fed a cow's milk protein elimination diet and an unrestricted diet (control group).
Values are expressed as the median and 25th and 75th percentiles in parentheses, Mann-Whitney test.
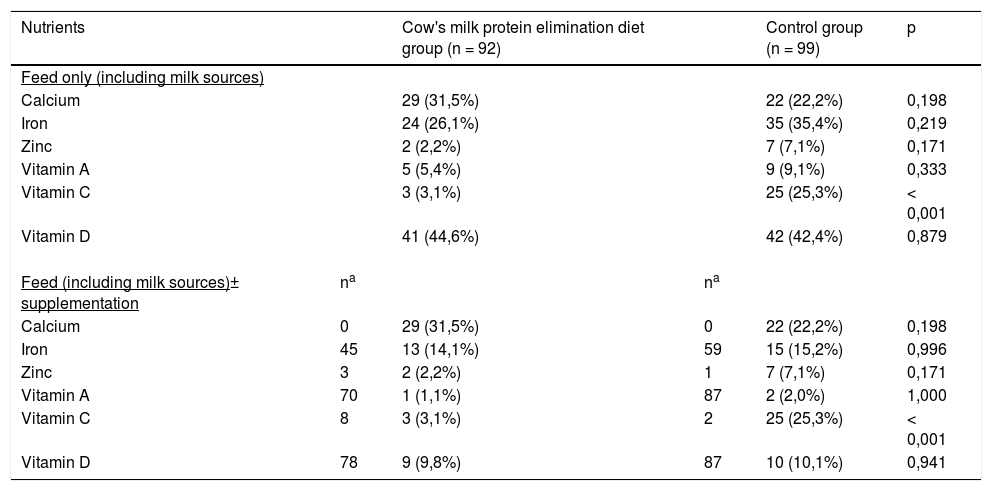
Table 4 shows the number and percentage of infants who had a food intake lower than the Recommended Dietary Allowances18 for calcium, iron, zinc, and vitamin A, C, and D, including oral supplementations. The control group presented a higher percentage of infants with dietary intake below the Recommended Dietary Allowances for vitamin C.
Infants with food intake below the Recommended Daily Intakes (RDA/AI)17 for micronutrients on a cow's milk protein elimination diet and control groups.
| Nutrients | Cow's milk protein elimination diet group (n = 92) | Control group (n = 99) | p | ||
|---|---|---|---|---|---|
| Feed only (including milk sources) | |||||
| Calcium | 29 (31,5%) | 22 (22,2%) | 0,198 | ||
| Iron | 24 (26,1%) | 35 (35,4%) | 0,219 | ||
| Zinc | 2 (2,2%) | 7 (7,1%) | 0,171 | ||
| Vitamin A | 5 (5,4%) | 9 (9,1%) | 0,333 | ||
| Vitamin C | 3 (3,1%) | 25 (25,3%) | < 0,001 | ||
| Vitamin D | 41 (44,6%) | 42 (42,4%) | 0,879 | ||
| Feed (including milk sources)± supplementation | na | na | |||
| Calcium | 0 | 29 (31,5%) | 0 | 22 (22,2%) | 0,198 |
| Iron | 45 | 13 (14,1%) | 59 | 15 (15,2%) | 0,996 |
| Zinc | 3 | 2 (2,2%) | 1 | 7 (7,1%) | 0,171 |
| Vitamin A | 70 | 1 (1,1%) | 87 | 2 (2,0%) | 1,000 |
| Vitamin C | 8 | 3 (3,1%) | 2 | 25 (25,3%) | < 0,001 |
| Vitamin D | 78 | 9 (9,8%) | 87 | 10 (10,1%) | 0,941 |
Values are expressed as the absolute number and percentages in parentheses, Pearson chi-squared test, or Fisher Exact test.
RDA/AI (Recommended Dietary Allowances and Adequate Intakes)17: values corresponding to the recommendations for 0-6 months/ 7-12 months/ 1-3 years: Calcium 200/260/700 mg/day; Iron 0,27/11/7 mg/ day; Zinc 2/3/3 mg/ day; Vitamin A 400/500/300 mcg/ day; Vitamin C 40/50/15 mg/ day; Vitamin D 10/10/15 mcg/day.
Specific guidance on complementary feeding by a nutritionist or physician was more frequent in the elimination diet group (80.2%; 77/96) than in the control group (53.8%; 49/9; p < 0.001).
DiscussionIn the present study, complementary feeding practices of infants on an elimination diet were more closer to the recommendations, although inadequacies were observed in both groups. Infants on an elimination diet had lower weight values according to score Z of weight-for-age, weight-for-height and BMI-for-age but the height was similar to the control group. Breast milk and infant formulas were contributors to more than 50% of the daily energy intake, as well as most nutrients.
In the present study, complementary feeding practices of infants on an elimination diet were more appropriate, although inadequacies were observed in both groups. Infants on an elimination diet had lower weight values, although they were more frequently guided by pediatricians and nutritionists. Breast milk and infant formulas were contributors to more than 50% of the daily energy intake, as well as most nutrients.
As for complementary feeding, liquids, such as water and tea were introduced earlier in the control group, probably owning to the belief that the child feels thirsty and that tea has soothing properties. However, this assumption is unfounded.4,7 The elimination diet group presented a later introduction of leafy vegetables, legumes, eggs, and wheat.
Regarding ultra-processed foods, cookies and soft drinks were introduced less frequently in the elimination diet group, possibly because of the more frequent nutritional guidance of health care professionals and the presence of allergenic fractions of cow's milk in some of these products. The control group also consumed thickening agents (starches) more frequently. Ultra-processed foods are characterized by high sugar, fructose, sodium, and saturated and trans-fat content, besides the presence of color additives and preservatives. They are highly contraindicated, especially for children under 18 months of age.3,4,7
This study also identified a higher complementary feeding inadequacies index in the control group.15 When analyzing individually each item that makes up this index, the elimination diet group showed a greater inadequacy only in the item “late introduction of solid foods (at 7 months of age or more)”. This difference was also observed in a study held in the United Kingdom.22 This practice may be associated with the fear of introducing new foods into the diet of infants with CMPA. There are no studies to support the later introduction of foods, since this conduct may even increase the risk of developing food allergies.1,4,11,23,24 It is important to emphasize that even those children who had adequate practices up to the date of the assessment may still have inadequate practices before reaching the cut-off age. Moreover, it is alarming to observe that half of the infants were fed liquefied or sieved soups, a practice proscribed decades ago.
As for nutritional intake, the results differ from those reported in the literature. Past studies showed lower energy and nutrients intake in infants fed a cow's milk elimination diet.8,12 Possibly, the wide access to special CMPA formulas, associated with the lower occurrence of complementary feeding inadequacies, contributed to the higher dietary intake. Moreover, the nutritional contribution of special CMPA formulas was greater than that of the milk sources in the control group, possibly owning to the higher volume of formula ingested, reinforcing their importance in the absence of breastfeeding.4,7
Therefore, the mandatory provision of special CMPA formulas by the government is extremely important for promoting nutritional safety, especially for those on an elimination diet, as they are quite vulnerable to nutritional deficiencies. The soy-based infant formulas, which are less expensive and could be consumed by infants over 6 months of age without any gastrointestinal symptoms, were consumed only by 6.7% of the infants. Moreover, the amino acid formula was the most consumed formula in the elimination diet group (52.2%), although it is not recommended as the first-line treatment for infants with CMPA.2,3,25 This result is consistent with that of studies conducted in the United Kingdom (45.5%).22 and the Netherlands (51%),26 even though amino acid formulas are more expensive and generally indicated for infants with more complex or severe symptoms, failure to thrive, or when a reaction to extensively hydrolyzed formulas1 is presented.
Although both the groups had met most of their nutritional demands, it is important to note that most infants in the elimination diet group were children of well-educated mothers, who were guided by physicians and nutritionists. The mothers of the infants in the elimination diet group were probably better prepared to perform the complementary feeding correctly than the mothers of the infants in the control group.
None of the groups reported the intake of calcium supplementation, a nutrient often documented as deficient in infants fed an elimination diet. Calcium intake was below the recommended daily value in approximately one-quarter of the cases, indicating that its intake may be underestimated in routine infant healthcare assistance.
Despite the higher nutritional intake, the lower weight values observed in infants on an elimination diet were similar to that reported in previous studies.8,27-29 It is still unclear whether the lower weight values are related to poor adherence, multiple allergies, or to inflammation status, which can lead to decreased nutrients bioavailability, inappetence, increased energy requirement, or nutrients loss.30
The height of infants on an elimination diet showed a slight deviation to the left (median Z score = -0.12). However, the lower height values observed in previous studies were not evidenced8,11. The better socioeconomic status and access to adequate nutrition, provided by the special CMPA formulas supported by the government, may have contributed to this result. The control group also showed a deviation to the left with respect to height (median score Z = -0.22), reinforcing the absence of differences between the groups.
The limitations of this study were the socioeconomic and demographic differences observed between the groups, and anthropometric measurements were not performed on all the infants by the main researcher, although only anthropometric data collected by fully qualified healthcare professionals were considered. The lower frequency of breastfeeding in the elimination diet group may have been influenced by the recruitment of volunteers at the pharmacy where patients get access to the special formulas. The results of inadequate food intake should be interpreted considering the sample size and the possibility that future studies with greater power would identify differences not evidenced in the present study. On the other hand, the present study's results give a suggestion on how to calculate the sample size in future projects.
The strengths of this study were as follows: this was a pioneering study with a considerable sample size. The age range limited to 18 months may have contributed to minimizing memory bias about complementary feeding practices. Food consumption was performed during the four seasons of the year and data collection and coding were performed by a single researcher, decreasing the risk of bias. Another strength of this study was the fact that children from both groups were not being followed up in a single service, which brings the results closer to real life.
This study reinforces the importance of actions to encourage the maintenance of breastfeeding, the provision of infant formulas by the government, and increased educational actions on complementary feeding to prevent nutritional deficiencies in early childhood.
In conclusion, infants on a cow's milk elimination diet presented more adequate complementary feeding practices and higher nutritional intake, despite lower body weight values.
FundingCoordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).