Despite the global impact of the Respiratory Syncytial Virus (RSV) infection in children, only one monoclonal antibody (Palivizumab) has been approved for clinical use. However, advances in the knowledge of RSV immunology may enable the development of safe and effective new vaccines and monoclonal antibodies in a few years. The purpose of this review is to summarize available data on approved and developing passive and active immunizations against RSV in childhood and pregnancy.
Data sourceA non-systematic review of RSV immunoprophylaxis in childhood and pregnancy was carried out in PubMed, path.org and clinical trial registries, without language restrictions, up to September 2022.
Data synthesisThree monoclonal antibodies and 17 active immunization candidates are under development in phase 1 to 3 clinical studies. Regarding the first group, Nirsevimab is a monoclonal antibody with a prolonged half-life whose approval for clinical use is expected in the next months. Among the vaccines under development, six techniques are being used: protein subunit, viral particles, live attenuated virus, recombinant viral vector, chimeric, and mRNA. The first two approaches are being tested primarily in pregnancy, while the others are being developed for the pediatric population.
ConclusionsThe approval of extended half-life monoclonal antibodies is the next expected advance in RSV prevention, although the costs may be a barrier to the implementation. Regarding active immunizations, maternal and infant vaccination are complementary strategies and there are many promising candidates in clinical studies using different platforms.
The Respiratory Syncytial Virus (RSV) is the main agent causing hospitalizations for lower respiratory tract infections (LRTIs) in children, especially those related to bronchiolitis and pneumonia. Contrary to the epidemiological changes observed in vaccine-preventable diseases in recent decades, the impact of LRTIs secondary to RSV has remained relatively unchanged. In children under 5 years of age, annual estimates of the global impact of RSV-related LRTIs is 33 million, with 3.6 million hospitalizations and 26,300 in-hospital deaths. The highest incidence of hospitalization occurs in children under 6 months of age and it is estimated that approximately 99% of deaths occur in low- and middle-income countries.1–4 Some groups are particularly vulnerable, such as preterm infants, those with congenital heart disease, bronchopulmonary dysplasia, genetic syndromes, and neuromuscular diseases.5 The impact of RSV-related LRTIs causing hospitalizations and deaths has also been increasingly recognized in the elderly.6 The most commonly used epidemiological surveillance criteria are based on the detection of the influenza virus, which restricts the actual identification of the impact and seasonality related to the RSV.7
Marked reductions in the incidence of LRTIs due to RSV have been reported in several countries during periods of more controlled social distancing, with activity restrictions aimed at attenuating the COVID-19 pandemic.8–11 Despite robust epidemiological evidence of the effectiveness of non-pharmacological measures in controlling the spread of RSV, maintaining these efforts is not feasible and the incidence of RSV-related LRTIs has increased significantly soon after the resumption of usual activities in most countries.12,13
An effective immunoprophylaxis regimen is expected to be the primary public health measure with the potential to have a significant protective impact on RSV infections in infants. Despite the need for new interventions and major investments to develop safe and effective immunizations against RSV in recent decades, only passive immunization using the monoclonal antibody Palivizumab is currently approved. However, there is an expectation that at least one monoclonal antibody with a long half-life (Nirsevimab) will soon be approved for clinical use.14
The immune response to RSV is one of the most important barriers to the development of active immunizations. The natural immune response against RSV is partial and incomplete. Although there are more concerns with individuals in high-risk groups for severity, there are many available epidemiological data reinforcing that reinfections occur throughout life, including by the same strain.15,16
RSV structureThe RSV is a single-stranded negative-sense RNA virus that belongs to the Paramyxoviridae family, Pneumovirinae subfamily. Its two antigenic subtypes, A and B, circulate simultaneously annually and exhibit many genomic divergences. Among the eight RSV structural proteins, three are surface glycoproteins [small hydrophobic (SH), attachment (G) and fusion (F)], the last two of which are very important for the immune response against RSV and its pathogenesis17 F-glycoprotein is the most promising target in the development of vaccines and monoclonal antibodies due to its crucial role in viral entry into human cells, showing several antigenic sites with the potential to induce neutralizing antibodies (very conserved in A and B subtypes). F-glycoprotein has two conformations, one pre-fusion, and one post-fusion.15 After the RSV binds to the target cell, the F-glycoprotein irreversibly changes from the first to the second conformation, which involves important structural changes that lead to the loss of many important antigenic sites, such as Ø and V. For these reasons, the pre-fusion F glycoprotein (pre-F) is the most promising target for the induction of neutralizing antibodies. Knowledge of the greater potential of pre-F glycoprotein compared to post-fusion (post-F) F glycoprotein for inducing the immune response has revolutionized the prospect of obtaining the RSV vaccine. Although neutralizing antibodies against glycoproteins G and F (pre-F and post-F) have been associated with protection in clinical studies, a precise correlate of protection has not yet been defined.18
History of the RSV vaccine developmentIn the 1960s, an initially well-tolerated, formalin-inactivated RSV vaccine was associated with increased RSV severity in vaccinated infants without previous RSV infection, which led to a markedly increased risk of hospitalizations (it was related to two deaths).19 Many hypotheses were considered for such unfavorable results; the central idea is that there was complement activation and Th2 polarization of the immune response.20 These findings have posed some difficulties regarding the RSV vaccine development related to safety issues, especially in populations without prior RSV immunity. In addition to these disappointing results, the abovementioned factors, such as epidemiological data based on influenza surveillance, the evolution of the immune response knowledge, plus the lack of an ideal animal model (since mice are not susceptible to RSV), can be listed as additional barriers to the development of a vaccine against this agent in recent decades.14,15
A major advance regarding active immunizations was the PREPARE study, a phase 3 clinical trial of a pre-F RSV protein nanoparticle vaccine aimed at pregnant women. A total of 4,636 pregnant women with a gestational age of 28 to 36 weeks were randomized in a 2:1 ratio to receive a single intramuscular dose of vaccine or placebo. The study did not meet the estimated primary endpoint for efficacy, which was at least a 50% reduction in physician-identified RSV-associated lower respiratory tract infections within the first 90 days of life. However, although the overall outcomes were not met (vaccine efficacy was 39.4%; 97.52% confidence interval [CI], -1.0 to 63.7%), the efficacy in South African infants was statistically significant (vaccine efficacy 57.0%; 95%CI 32.7%-72.5%). While the reasons for the differences in efficacy between participants from South Africa and those from other countries have been the subject of extensive debate, this was the first study to demonstrate the effectiveness of a maternal RSV vaccine in any subgroup of participants.21
Several active immunization strategies are currently in clinical studies, which include maternal immunization, viral vector, subunit vaccines, chimeric and live attenuated vaccines.14,15
The main objective of this review is to describe the current status and prospects of active and passive RSV immunizations.
MethodsA non-systematic review was performed to describe current and future strategies for pediatric RSV immunization, targeting vaccines and monoclonal antibodies approved for clinical use or those candidates in development through clinical phase studies. Data search was performed on PubMed, clinical trial registries, and PATH VSR Vaccine and mAb snapshot (last updated September 9, 2022), including immunizations in children under 18 years of age or during pregnancy, with no language restrictions (up to September 10, 2022).22 Studies on vaccines or interventions with immunoprophylaxis in the elderly are beyond the scope of this review and will not be detailed throughout this manuscript.
Overall strategiesThe evolution in knowledge about the immune response against RSV, as well as the increasing identification of the RSV disease burden, have led to an important increase in the number of promising candidates for active and passive immunization. The ideal strategy to protect mainly infants and preschool children includes a combination of approaches, such as active and passive immunization. For infants less than six months of age, maternal immunization of pregnant women proposes protein-based vaccines using both vaccine subunits containing stabilized pre-F, or virus-like particles containing protein F. For older children, active immunization with live vaccines with the attenuated virus, chimeric vaccines, or vaccines based on recombinant vectors is the main strategies. The safety and efficacy of mRNA vaccines against SARS-CoV-2 also reinforced the potential of this platform as a viable strategy for RSV vaccination during childhood.23 The ideal vaccine should prevent not only disease severity but also reduce transmission.
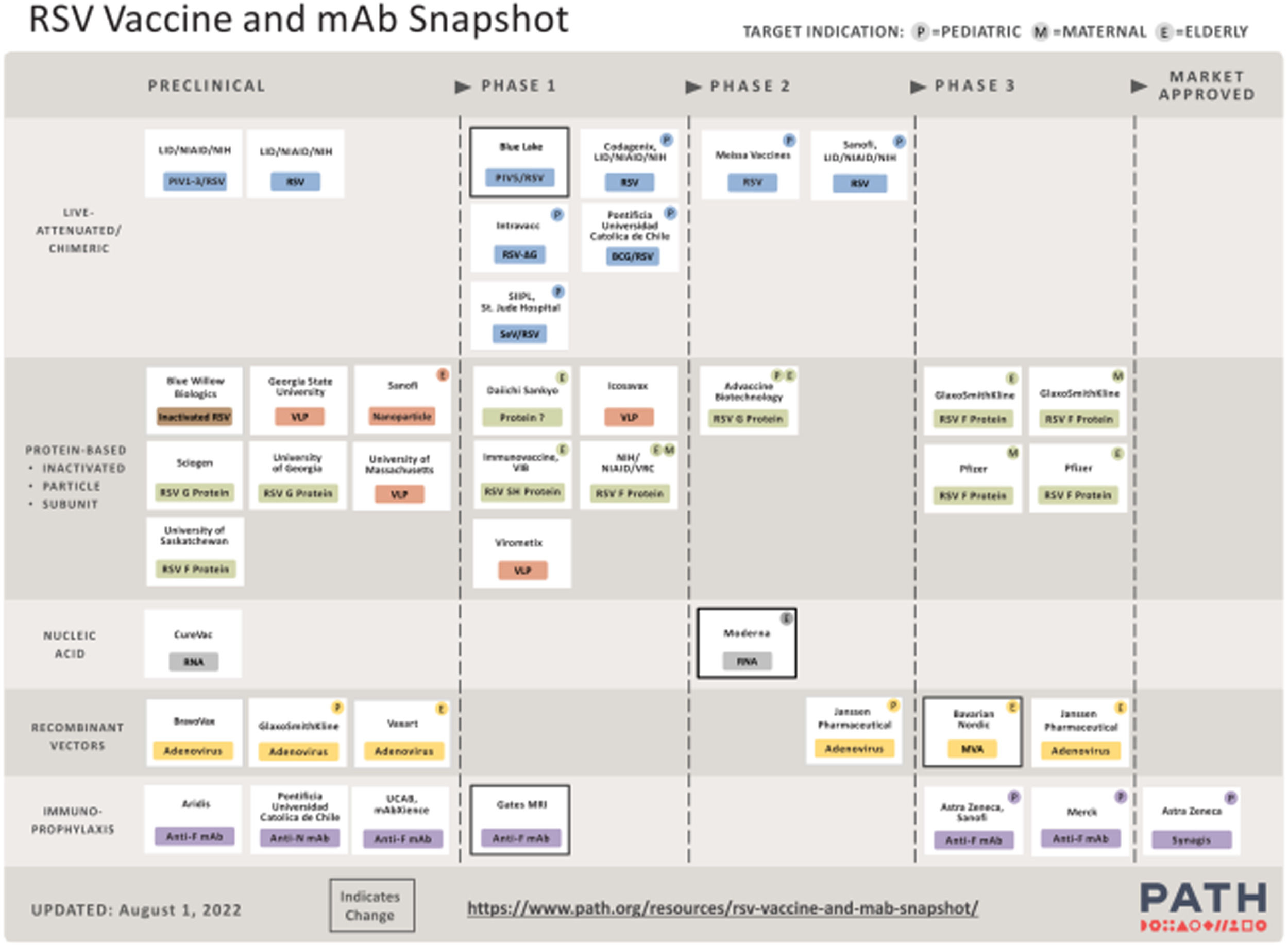
Although the development of active immunization for pregnant women and infants is one of the main goals to control RSV-related LRTI burden in infants, the use of monoclonal antibodies will continue to play a role as a complementary strategy. Transplacental antibodies are transferred mainly in the third trimester of pregnancy and preterm infants, one of the highest-risk groups, cannot be protected by maternal immunization. Moreover, the seasonality of RSV may not coincide with the period of greatest protection of maternal vaccination, making the use of monoclonal antibodies an interesting option to cover the period of greatest risk of the year in situations where other forms of immunization are not feasible. Currently, there are 17 vaccines and three monoclonal antibodies in ongoing or completed clinical trials, as shown in Table 1. Figure 1 summarizes the overview of the main vaccine groups in all preclinical and clinical studies for the different target populations.14–16,22
Summary of RSV immunizations in children and pregnant women undergoing evaluation in clinical trials.
Legend: IV: intravenous, ID: intradermal, IM: intramuscular, IN: intranasal.
Main RSV vaccines and monoclonal antibodies in development.22 *Adapted from https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Reproduced by permission of Path.
Extensive epidemiological evidence highlights the potential of maternal vaccination to protect infants from diseases such as pertussis, influenza, and COVID-19.24 For the prevention of RSV, the objective is to promote reinforcement of previous immunity to RSV through the vaccination of pregnant women.
Particle-based vaccinesThis group of vaccines is at an early stage of development but has the potential to induce a potent immune response, as it can display multiple antigens using F protein-based nanoparticles. As mentioned above, the PREPARE study was the first to report the efficacy of a maternal RSV vaccine in the subset of South African infants, despite the primary efficacy endpoint in the overall groups of participants not being met.21 In addition, a phase 1 study is being conducted in healthy adult women with V-306, a candidate based on the RSV F protein site II antigen, with no results yet available.14
Protein subunitDue to the previous experience of inactivated vaccine induction of exacerbated respiratory disease in children with no previous RSV immunity, potential vaccines using this platform were designed for pregnant women and the elderly. Several attempts to develop a subunit vaccine using post-F antigen have previously failed. Currently, most candidates are based on a stabilized pre-F antigen. The phase 1 study evaluating the DS-Cav1 candidate to the pre-F subunit was published in 2021. Ninety-five healthy adults were enrolled for safety and immunogenicity assessment of three different doses (50, 150 and 500 µg) and comparison of a single dose versus two doses (12-week interval), with or without aluminum hydroxide adjuvant. DS-Cav1 was shown to be safe and well tolerated. Additionally, the vaccine induced a robust and sustained boost in neutralizing antibodies above the baseline for at least 44 weeks, regardless of the dose, number of injections, and use of adjuvants. These results reinforce the potential of this vaccine to provide protection against RSV throughout an entire viral season.25
RSVpreF3, a maternal vaccine using pre-F protein, is already in phase 3 studies (Grace trial). However, although it has been shown in previous phases to attain high titers of neutralizing antibodies, the inclusion of subjects in this study was stopped in February 2022, due to a safety sign.14
A third candidate is an RSV-stabilized pre-fusion F subunit vaccine (RSVpreF). In the phase 2b study, RSVpreF was compared by administering it alone and by co-administering it with Tdap, with or without adjuvant, in healthy non-pregnant adult women. The vaccine showed to be safe and well tolerated. Immune responses against tetanus and diphtheria were non-inferior when co-administered with Tdap, although the non-inferiority criteria were not met for pertussis. The phase 3 study of RSVpreF in pregnant women started in 2021 and recruitment is still ongoing.26
Active pediatric immunizationLive attenuated vaccineThis has been one of the most promising vaccine groups for infants and older children as they were developed to induce a robust immune response through attenuated local infection by stimulating the humoral and cell immune systems. In addition, live attenuated vaccines were not associated with increased RSV disease severity, although they were related to upper airway disease; they are administered intranasally, which also seems to be an advantage from the point of view of acceptance by the family and the children. There are nine candidates currently in phase 1 and 2 clinical trials. In one analysis of seven phases, 1 trial in 239 infants aged 6 to 24 months, evaluating five vaccines in development that induced neutralizing antibody responses in ≥ 80% among those vaccinated, the effectiveness in reducing RSV episodes of acute respiratory illness requiring medical treatment was 67% (95% CI, 24 to 85%), with a reduction in medical care for RSV-related LRTIs of 88% (95% CI, -9 to 99; P = 0.04). A four-fold increase in neutralizing antibody levels was strongly associated with protection against both of the aforementioned clinical outcomes.27
Recombinant viral vector vaccineThis group of vaccines has been widely used against SARS-CoV-2 and consists of viruses with replication-deficient RSV genes. Its rationale is to induce humoral and cell immunity. For children, the main candidate is pre-F.Ad26.RSV, which uses an adenoviral vector for pre-fusion F protein expression. The phase 2 results of the study in previously RSV seropositive infants in the second year of life showed that pre-F.Ad26.RSV was safe and immunogenic. A second similar phase 2 study in previously seronegative children for RSV, also aged between 12 and 24 months, is also being carried out, but the results are not yet available.14
Chimeric vaccineThe main characteristic of this group of vaccines is the expression of the RSV protein in viruses or bacteria with more favorable safety profiles and antigen presentation, compared to vector-based vaccines. One such potential candidate is based on recombinant BCG N protein via intradermal administration (rBCG-N-hRSV); this vaccine was shown to be safe and immunogenic in a phase 1 study in healthy adults.28 The second candidate is SeVRSV, a vaccine based on the Sendai virus (SeV), which is efficient for replicating the gene that carries the RSV fusion protein (F). This vaccine was shown to be safe in phase 1 studies in which the vaccine was administered intranasally to healthy adults.29
mRNA vaccinesThe success of the mRNA vaccine against SARS-CoV-2 makes the use of this platform a very interesting option for the development of vaccines against RSV.23 Interestingly, previous studies identifying pre-F RSV as the preferred vaccine antigen have supported the development of the mRNA-based SARS-CoV-2 vaccine.30 Currently, a phase 1 study of the mRNA-1345 vaccine, which encodes stabilized pre-F RSV, is underway in healthy women of childbearing age, the elderly, and RSV-seropositive children (aged 12 to 59 months); these results are expected by 2023. As an additional benefit, the use of mRNA vaccines against RSV has the potential to be combined with mRNA vaccines against SARS-CoV-2 and influenza, currently being tested in adults.14
Passive immunizationPalivizumab (Synagis®, AstraZeneca AB, Sweden) is currently the only monoclonal antibody clinically approved and used in recent decades. The efficacy and safety of palivizumab were demonstrated in three randomized, placebo-controlled clinical trials. A meta-analysis of 2,831 high-risk neonates (preterm gestation ≤ 35 weeks, bronchopulmonary dysplasia, and congenital heart disease) showed that the use of palivizumab was not related to an increased risk of adverse events and was associated with a reduction in RSV-related hospitalizations from 101 to 50/1,000 (relative risk [RR] 0.49, 95% CI 0.37-0.64) and reductions in pediatric intensive care unit admissions from 34 to 17/1000 (RR 0.5, 95%CI 0.3 to 0.81).31 Palivizumab has been used in many countries and, although indications may vary, most include bronchopulmonary dysplasia, congenital heart disease, and preterm infants born with a gestational age ≤ 28 weeks and 6 days. In post-licensing surveillance studies, the efficacy of palivizumab was similar, although the impact varied in different settings.32 The inclusion of preterm infants born between 29 and 34 weeks of gestational age has been a matter of debate. In 2014, the American Academy of Pediatrics amended the policy, restricting its use in the latter group.33 Although it is not a uniform finding, some studies suggest an increase in RSV-related hospitalization in infants born within the gestational age range in which palivizumab use was restricted.34–36 This debate on a more restrictive use reinforces that, despite the effectiveness and efficacy demonstrated by palivizumab, the need for monthly administration and costs are still important barriers to its universal use, especially in developing countries and in infants without risk factors.16
Two other monoclonal antibody candidates did not pass previous stages of clinical studies. Motavizumab has not been shown to be superior to palivizumab and has been associated with a higher incidence of skin rash in phase 3 studies.37 The development of suptavumab was also discontinued because it did not meet the endpoints of efficacy clinical trials in phase 3 studies, mainly due to a lack of activity against RSV subtype B strains.38
Nirsevimab has phase 3 studies that indicate it is the most promising of these new, long half-life monoclonal antibodies. It focuses on the Ø site of the F protein, with a single dose proposal for protection throughout the season, being a promising candidate for RSV immunoprophylaxis. In 2020, a phase 2b, randomized, placebo-controlled clinical trial evaluated nirsevimab in a single 50mg intramuscular dose in 1,453 healthy preterm infants born at a gestational age between 29 and 34 weeks and 6 days and reported a reduction in the risk of RSV hospitalization of 78.4% (95% CI, 51.9 to 90.3), maintained for 150 days after dose administration.39 In 2022, a phase 3, randomized, placebo-controlled clinical trial evaluating the efficacy of nirsevimab in 1,490 late preterm (at least 35 weeks of gestational age) or full-term neonates reported a 74.1% reduction (CI 95%, 49.6 to 87.1) in medical care related to lower respiratory tract infections caused by RSV, the main outcome of the study.40 Regarding safety, none of the abovementioned studies identified an increase in adverse events in subjects receiving nirsevimab when compared to placebo. Based on these promising results, nirsevimab is expected to gain regulatory approval for clinical use in 2023, possibly being the first effective intervention for RSV since the advent of palivizumab.14,38,40
Clesrovimab (MK-1654) is another monoclonal antibody candidate, with similarities to nirsevimab and targeting site IV of the F protein. Recruitment for this phase 2a study has been completed and phase 2b/3 has started and is expected to be completed in 2024.
All these new options under development can help to reduce costs and increase accessibility, as this is one of the main points related to the use of monoclonal antibodies. RSM-01, which is presently in phase 1, is expected to cost significantly less than the current monoclonal antibodies. Finally, the development of a biosimilar palivizumab, as well as its nasal administration, both in clinical trials with no results yet, can help to reduce costs and increase accessibility, especially in lower-income countries, exactly in places where RSV-related morbidity and mortality rates are higher.14
ConclusionsDespite the great impact of vaccines on the epidemiology of infectious diseases for children in recent decades, the impact of RSV-related LRTIs has remained unchanged. Several challenges, particularly those related to the immune response to this agent, have greatly hindered progress toward attaining consistent immunoprevention of RSV infection. In recent years, several advances have been made regarding the understanding of the immunogenicity of potential vaccines against RSV due to the immunogenic potential of the pre-F conformation of the F glycoprotein, making the development of an effective and long-lasting active immunization more feasible in the coming years. It is expected that several of these strategies, currently under development, will be complementary, with vaccination in infants combined with immunization of pregnant women, aiming to protect infants in their first months of life. For preterm infants, the use of monoclonal antibodies is the main preventive strategy, as transplacental antibodies are transferred to the fetus during the third trimester of pregnancy. Currently, only passive immunization with palivizumab is available in clinical practice, with the duration of protection and high costs being important barriers to achieving a high impact on RSV epidemiology.
The prospect of effective active RSV immunization in the coming years is promising, although the immediate reality for most countries is still the use of monoclonal antibodies in high-risk groups. In an optimistic scenario of new, more effective, long-lasting and accessible interventions being available only in a period that should vary between 3 and 5 years, knowledge of the local epidemiology and seasonality is very important to guide the rational use of resources.