To assess the accuracy of umbilical cord bilirubin values to predict jaundice in the first 48h of life and neonatal infection.
MethodNewborn infants treated at a regional well-baby nursery born at ≥36 weeks of gestation were included in this retrospective cohort study. All infants born in a 3-year period from mothers with O blood type and/or Rh-negative were included and had the umbilical cord bilirubin levels measured. Hyperbilirubinemia in the first 48h was defined as bilirubin levels above the phototherapy threshold. Neonatal infection was defined as any antibiotic treatment before discharge.
ResultsA total of 1360 newborn infants were included. Two hundred and three (14.9%) newborn infants developed hyperbilirubinemia in the first 48h of life. Hyperbilirubinemic infants had smaller birth weight, higher levels of umbilical cord bilirubin, a higher rate of infection and were more often direct antiglobulin test positive. Umbilical cord bilirubin had a sensitivity of 76.85% and a specificity of 69.58% in detecting hyperbilirubinemia in the first 48h, with the cut-off value at 34μmol/L. The area under the receiver operating characteristic curve was 0.80 (95% CI: 0.78–0.82). Umbilical cord bilirubin had a sensitivity of 27.03% and specificity of 91.31% in detecting perinatal infection. The area under the receiver operating characteristic (ROC) curve was 0.59 (95% CI: 0.57–0.63).
ConclusionsA positive correlation was found between umbilical cord bilirubin and hyperbilirubinemia in the first 48h of life. Umbilical cord bilirubin is a poor marker for predicting neonatal infection.
Hyperbilirubinemia is a common and, in most cases, benign problem in the neonatal period that is often physiologic, and interventions are not usually necessary.1 Over 50% of all newborn infants become visibly jaundiced.2 Infants become clinically jaundiced when the bilirubin level reaches about 80μmol/L.3 On the other hand, neonatal jaundice is an important clinical feature as it may be a sign of an underlying disorder (i.e. hemolytic anemia, infection, an inborn error of metabolism or liver disease).4 In severe cases, high unconjugated hyperbilirubinemia can be deposited in the brain, particularly in the basal ganglia, causing kernicterus.2
Early discharge of the healthy-term newborns after delivery has become a common practice because of both medical and social reasons as well as economic constraints.5 Universal follow-up within 1–2 days of early discharge (often an unattainable goal in low-income countries), umbilical cord bilirubin (uCB) concentration at birth, routine pre-discharge serum bilirubin, and transcutaneous bilirubin measurements, as well as the universal clinical assessment of risk factors for developing jaundice, are various strategies to predict significant hyperbilirubinemia.6 Despite these suggested measures, hyperbilirubinemia is still the most common cause of readmission during the early neonatal period.7
Providing practitioners with new insights for predisposing factors, exacerbating or etiologic factors for jaundice could help them with better management.8 One of these factors is an infection, a recognized cause of hyperbilirubinemia in the newborn.9 Some reports suggest that unexplained indirect hyperbilirubinemia may be the only manifestation of sepsis in otherwise healthy-appearing newborns.10 It is well known that clinical manifestations of neonatal infection present as a broad spectrum, ranging from nonspecific signs and symptoms to severe illness presenting with poor feeding, fever, vomiting, renal failure, and respiratory distress syndrome.11,12 Hyperbilirubinemia may be the only manifestation of infection, especially urinary tract infection (UTI) within the neonatal period.13
In this retrospective study, we evaluated whether the cord bilirubin values could be useful in predicting significant hyperbilirubinemia requiring treatment in the first 48h of life and if cord bilirubin values predict neonatal infection.
MethodsThis retrospective cohort study was conducted at a regional well-baby nursery. Data were obtained for all infants with a gestational age of ≥36 weeks admitted to the well-baby nursery and born between January 2018 and December 2019. During this period, there were 3485 live births at our institution. In our institution, there is an established policy that all infants born to blood type O and Rh-negative mothers have their umbilical cord bilirubin measured. Out of 3485 live-born infants, 1360 infants were born to O and/or Rh-negative mothers and had their uCB measurements, blood type, and direct antiglobulin tests (DAT) evaluated.
Clinical data of those infants were collected, including sex, birth weight, gestational age, Apgar scores at 1 and 5min after birth, history of early neonatal infection, blood type, Rh factor, and DAT. Umbilical cord blood bilirubin (uCB), infants’ blood group, and DAT were obtained from all 1360 infants at birth. The maternal blood group was obtained from the maternal medical history. After birth, infants were evaluated daily for hyperbilirubinemia via transcutaneous bilirubin measurement using the Dräger Jaundice Meter JM-105. If higher values were noted, the blood bilirubin measurement was performed. Hyperbilirubinemia in the first 48h was defined as bilirubin levels above phototherapy recommendations in the NICE guidelines.14 Early neonatal infection was defined as any antibiotic treatment before discharge. The study was approved by the hospital’s research ethics committee under protocol Nr. R2-6974/2020.
Statistical analysisMaternal and neonatal clinical data were collected and analyzed using the program R project (www.r-project.org). Categorical data are descriptively presented in absolute and relative frequencies. Differences between categorical variables were examined using the chi-squared test, and for numerical variables the Mann–Whitney U test was used. To derive the complication and outcome prediction model, multivariate logistic regression was applied. The sensitivity, specificity, negative likelihood ratio (NLR), and positive likelihood ratio (PLR) were calculated. Receiver operating characteristic (ROC) curve analysis was performed with the Statistical Package for the Social Sciences (SPSS), version 16.0 (SPSS Inc. – Chicago, IL, USA). P values of <0.05 were considered statistically significant.
ResultsIn the observed two-year period, a total of 3485 newborn infants were admitted to the well-baby nursery at University Hospital Osijek, Croatia. Umbilical cord bilirubin levels were measured in all infants from mothers with blood group O and all Rh-negative mothers.
Our study group included 1360 newborn infants, gestational age ≥36 weeks. Of the total number of infants admitted, 203 (14.9%) infants developed hyperbilirubinemia in the first 48h of life, of which 116 (57%) were female, and 87 (43%) were male. Patient characteristics and differences between hyperbilirubinemic and non-hyperbilirubinemic newborn infants are shown in Table 1.
Patient characteristics and differences between hyperbilirubinemic and non-hyperbilirubinemic newborn infants.
| Parameter | No hyperbilirubinemia (n=1157) | Hyperbilirubinemia (n=203) | p Value |
|---|---|---|---|
| Gestational age (completed weeks) (mean [IQR]) | 39.0 (38–40) | 39.0 (38.0–40.0) | 0.052a |
| Birth weight (g) (mean [SD]) | 3,413.99 (469.99) | 3,318.62 (503.31) | 0.01b |
| Cord bilirubin level (μmol/L) (mean [IQR]) | 31.0 (26–36) | 40.0 (35.0–46.0) | <0.001a |
| Apgar score 1min (mean [IQR]) | 10 (10–10) | 10 (10–10) | 0.8a |
| Apgar score 5min (mean [IQR]) | 10 (10–10) | 10 (10–10) | 0.8a |
| Positive direct antiglobulin tests (DAT) (n [%]) | 12 (1.03) | 26 (12.80) | <0.001a |
| Infection (n [%]) | 21 (1.81) | 16 (7.88) | <0.001a |
SD, standard deviation.
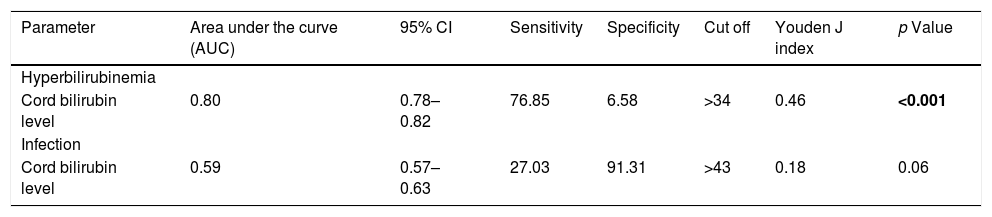
The analysis of the area under the ROC curve demonstrates that amongst all infants, uCB is a moderately good predictor of the development of hyperbilirubinemia at 48h of life [AUC=0.80 (95% CI 0.78–0.82)], but it poorly predicts early perinatal infection [AUC=0.59 (95% CI 0.57–0.63)] (Table 2, Fig. S1).
ROC curve analysis for predicting significant hyperbilirubinemia at 48h of life and development of infection.
| Parameter | Area under the curve (AUC) | 95% CI | Sensitivity | Specificity | Cut off | Youden J index | p Value |
|---|---|---|---|---|---|---|---|
| Hyperbilirubinemia | |||||||
| Cord bilirubin level | 0.80 | 0.78–0.82 | 76.85 | 6.58 | >34 | 0.46 | <0.001 |
| Infection | |||||||
| Cord bilirubin level | 0.59 | 0.57–0.63 | 27.03 | 91.31 | >43 | 0.18 | 0.06 |
CI, confidence interval.
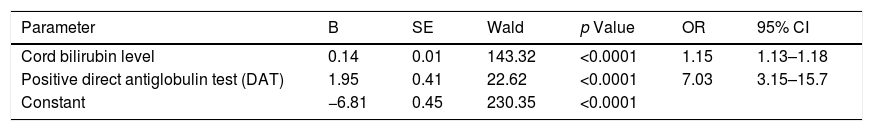
To perform a complication and outcome prediction model, multivariate logistic regression was applied. Two independent predictors made a unique statistically significant contribution to the hyperbilirubinemia prediction model, and those were the umbilical cord bilirubin and positive DAT (Hosmer–Lemeshow test, p=0.65). The model as a whole is statistically significant (χ2=6.02, p<0.001) and generally explains between 16.96% (according to Cox & Snell) and 29.79% (according to Negelkerke) of the variance in the presence of hyperbilirubinemia, and accurately classifies 87.43% of cases (Table 3).
Multivariate regression analysis of the risk parameters impacting the development of hyperbilirubinemia.
| Parameter | B | SE | Wald | p Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Cord bilirubin level | 0.14 | 0.01 | 143.32 | <0.0001 | 1.15 | 1.13–1.18 |
| Positive direct antiglobulin test (DAT) | 1.95 | 0.41 | 22.62 | <0.0001 | 7.03 | 3.15–15.7 |
| Constant | −6.81 | 0.45 | 230.35 | <0.0001 |
B, regression constant; SE, standard error; OR, odds ratio; CI, confidence interval.
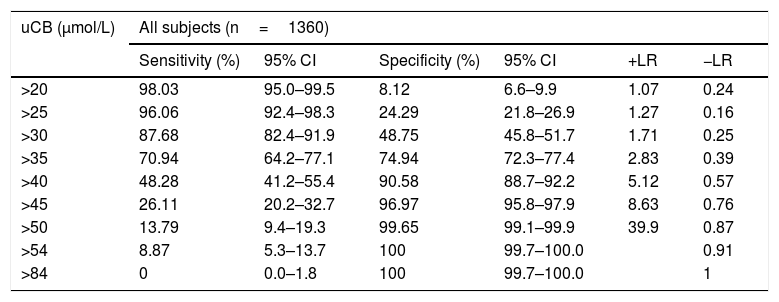
The uCB cut-off levels and sensitivity and specificity for predicting all-cause jaundice and positive likelihood ratio and negative likelihood ratio at the different cut-offs are presented in Table 4.
uCB and all-cause jaundice.
| uCB (μmol/L) | All subjects (n=1360) | |||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | +LR | −LR | |
| >20 | 98.03 | 95.0–99.5 | 8.12 | 6.6–9.9 | 1.07 | 0.24 |
| >25 | 96.06 | 92.4–98.3 | 24.29 | 21.8–26.9 | 1.27 | 0.16 |
| >30 | 87.68 | 82.4–91.9 | 48.75 | 45.8–51.7 | 1.71 | 0.25 |
| >35 | 70.94 | 64.2–77.1 | 74.94 | 72.3–77.4 | 2.83 | 0.39 |
| >40 | 48.28 | 41.2–55.4 | 90.58 | 88.7–92.2 | 5.12 | 0.57 |
| >45 | 26.11 | 20.2–32.7 | 96.97 | 95.8–97.9 | 8.63 | 0.76 |
| >50 | 13.79 | 9.4–19.3 | 99.65 | 99.1–99.9 | 39.9 | 0.87 |
| >54 | 8.87 | 5.3–13.7 | 100 | 99.7–100.0 | 0.91 | |
| >84 | 0 | 0.0–1.8 | 100 | 99.7–100.0 | 1 | |
uCB, umbilical cord bilirubin; CI confidence interval; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
Jaundice is a clinical condition that is often present and constitutes one of the major issues during the neonatal period.15 In this period of early discharge of the mother–infant dyad,16,17 it is important to prevent possible reasons for hospital readmission. Since jaundice is the most common cause of hospital readmission, it is important to identify infants at risk for readmission due to hyperbilirubinemia, especially since some of these infants are at risk of potentially developing catastrophic neurological damage (kernicterus).18
Measurement of umbilical cord bilirubin values is a cheap, readily available, non-invasive procedure19 that has the potential to predict hyperbilirubinemia in otherwise healthy infants and could aid in the decision of early discharge of newborn infants.
In our institution, it is standard practice to sample umbilical cord blood for measuring bilirubin levels of all infants born to group O and/or Rh-negative mothers. After the cord blood bilirubin measurement, bilirubin levels are monitored transcutaneously every day until discharge.
Our study included 1360 infants, gestational age ≥36 weeks; of those, 203 (14.93%) newborn infants had hyperbilirubinemia during the first 48h of life. Those rates of hyperbilirubinemia are similar to those from a study by Chary et al.,20 who identified newborns at risk of developing significant hyperbilirubinemia by using cord blood serum bilirubin levels; out of 282 healthy-term newborn infants, 51 (18.09%) developed significant hyperbilirubinemia.
In our population of healthy newborn infants, compared to infants who did not develop hyperbilirubinemia, hyperbilirubinemic infants had smaller birth weight [3318.62g (±503.31g) vs 3413.99g (±469.99g; p<0.01)], higher levels of umbilical cord bilirubin values, and higher rates of infection, and were more often DAT positive (Table 1).
In our study, ROC curve analysis demonstrates that amongst all infants, umbilical cord bilirubin is an acceptable predictor for the development of hyperbilirubinemia in the first 48h of life, with a cut-off value of uCB of 34μmol/L, sensitivity of 76.85%, and 69.58% specificity [AUC=0.80 (95% CI 0.78–0.85)]. In a study by Guan et al.,21 the cut-off value of umbilical cord bilirubin in the diagnosis of hyperbilirubinemia was 32.1μmol/L, and its sensitivity and specificity were 71.4% and 65.6%, respectively. Similar cut-off values of umbilical cord bilirubin for prediction of hyperbilirubinemia were reported by Zeitoun et al.22 In a study by Ipek et al.23 that had an identical incidence of phototherapy to our study, findings were that to recognise the newborns at high risk for developing hyperbilirubinemia, using an umbilical cord bilirubin cut-off level of 44.4μmol/L had a positive predictive value of 41.18%, negative predictive value of 97.9%, and sensitivity of 50%.
One of the confounding factors that influence cut-off values in some studies is the inclusion of infants with positive DAT in the analysis of subsequent development of hyperbilirubinemia. This issue was addressed in a study by Jones et al.,19 who found that ROC curve analysis demonstrates that amongst all infants, uCB strongly predicts the development of DAT jaundice [area under the ROC curve=0.996 (95% CI 0.991–0.998)], as well as all-cause jaundice [area under the ROC curve=0.75 (95% CI 0.72–0.77)].
First-day bilirubin measurement has been used to predict the development of significant hyperbilirubinemia in healthy-term newborns. Alpay et al.24 have prospectively followed term newborns over the first 5 days of life by measuring serum bilirubin levels daily. They concluded that serum bilirubin measurement and the use of the critical bilirubin level of 102μmol/L in the first 24h of life will predict nearly all healthy-term newborns who will have significant hyperbilirubinemia and determine all of those infants who will later require phototherapy treatment during the first days of life.
Those findings are confirmed by an even earlier serum bilirubin measurement (at 6h of life) reported by Sarici et al.25 This study has shown that even in the ABO blood group incompatibility, serum bilirubin levels could predict significant hyperbilirubinemia and the need for interventions. A serum bilirubin measurement and the use of the critical bilirubin levels of 68μmol/L and 102μmol/L at the sixth hour of life will predict nearly all newborns who will have significant hyperbilirubinemia and those who will develop severe hemolytic disease of the newborn.25
Practices on cord bilirubin evaluation vary across different institutions. Risemberg et al.26 suggest that cord blood should be sent for bilirubin estimation in all infants of group 0 mothers. If the cord bilirubin level is 68.4μmol/L or higher, blood grouping and DAT should also be performed without waiting for the onset of clinical jaundice. The level of 68.4μmol/L for cord bilirubin gave a reliable prediction of the severity for subsequent hyperbilirubinemia.
In our study, infants with positive DAT were found to have a 7.03 odds ratio (CI 3.15–15.7) of developing significant hyperbilirubinemia compared to DAT negative infants. These findings suggest that it could be more useful to perform umbilical cord DAT in all infants born to mothers with O blood type or Rh-negative, because DAT negative newborn infants have a significantly lower risk of developing hyperbilirubinemia in the first 24h. This is also suggested by Pradeep Kumar et al.,27 in whose study a positive DAT was associated with a higher risk of developing significant jaundice (>95th centile for the age), which in turn resulted in a greater need for interventions (phototherapy, NICU admissions, exchange transfusion and IVIG therapy). This study reemphasizes the need to screen babies at risk for blood group incompatibility with DAT soon after birth. By using cord blood for DAT, we can avoid subsequent painful blood sampling.
In our study, we investigated whether cord bilirubin levels could be used to predict early neonatal infection. Regarding the relationship between perinatal infection and hyperbilirubinemia, Özcan et al.9 concluded that bacterial infection was a significant cause of unexplained hyperbilirubinemia among jaundiced neonates. In our study, infection was reported in 16 patients who developed hyperbilirubinemia in the first 48h, but ROC curve analysis demonstrates that amongst all infants, uCB is a poor marker for predicting perinatal infection (AUC=0.59, 95% CI 0.57–0.63).
The results of the present study should be evaluated considering its strengths and limitations. Among its strengths is the selection of a large number of healthy newborn infants at risk for hemolysis. Among its limitations is the fact that this is a single-center, retrospective study. Also, in the analysis of uCB and prediction of perinatal infection, a small number of infants with perinatal infection resulted in a reduced sample, and this limits the findings.
In conclusion, this study reaffirms the concept that there is a positive correlation between umbilical cord bilirubin levels and the development of neonatal jaundice until 48h of life. Umbilical cord bilirubin analysis is a readily available, non-invasive method for predicting neonatal jaundice, especially in light of growing trends of very early discharge after vaginal delivery. However, since uCB is only a moderately good predictor for the development of hyperbilirubinemia in the first 48h of life, it should not be used as the sole indicator for the development of significant hyperbilirubinemia in the early neonatal period.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at University Hospital Osijek, School of Medicine, J. J. Strossmayer of University, Osijek, Croatia.