To describe the clinical characteristics, laboratory parameters, treatment, and predictors of an unfavorable outcome of critically ill children with SARS-CoV-2 infection.
MethodThis was a prospective observational study performed in a pediatric intensive care unit (PICU) of a tertiary care COVID referral hospital among critically ill children in the age group 1 month - 12 years admitted due to SARS-CoV-2 infection from June to December 2020. Demographic, clinical profile, pSOFA and PRISM III scores, laboratory parameters, treatment, and outcomes of the patients were recorded. Children who had a prolonged PICU stay (>14 days) or died were compared with those who were discharged from PICU within 14 days to assess predictors of unfavorable outcomes.
ResultsPICU admission rate among hospitalized SARS-CoV-2 infected children was 22.1% (92/416). Infants comprised the majority of the ICU population. Invasive mechanical ventilation and inotropic support were required for 28.3% and 37% of patients, respectively. Remdesivir, IVIg, and steroids were administered to 15.2%, 26.1%, and 54.3% of the subjects, respectively. The mortality rate was 7.6 %. MIS-C patients were older, less comorbid, and required less ventilator support but more inotrope support than acute severe COVID-19 patients. Predictors of unfavorable outcomes were age < 1 year, fever duration > 5 days, respiratory distress, shock, comorbidity, elevated CRP (> 50 mg/L), procalcitonin (> 6 ng/L), D-dimer (> 6 µg/L) and arterial lactate (> 2 mmol/L).
ConclusionCritically ill children with unfavorable outcomes were predominantly infants, comorbid, prolonged fever, respiratory distress, shock and elevated inflammatory markers, D-dimer and lactate. These factors may be useful for watchful monitoring and early intervention.
Coronavirus has taken the world by storm since its inception in Wuhan in December 2019.1 As evident from surveillance data from many countries, children have been less affected by the pandemic than adults accounting for 1-8% of the laboratory-confirmed cases.2–6 As per the COVID-NET, the cumulative COVID-19 hospitalization rates for children less than 18 years are much lower than adults (8/100,000 population vs. 164.5/100,000 population), but ICU requirements among hospitalized children (33.2%) are similar to that of adults (32%).7
Several studies have come up in the west demonstrating characteristics of critically ill children with COVID infection requiring ICU care.8,9 More cases of pediatric ARDS and septic shock syndromes among children with COVID infection have been reported over time.10 Currently, India stands amidst the second wave of the coronavirus pandemic, with 403,405 new cases reported on May 08, 2021.11 Also, with the detection of mutated strains (N440K and E484K) in some states of India, they have been found to be more transmissible than their predecessor strain12; there are concerns regarding increased disease severity and ICU requirements in children.
Sufficient data regarding characteristics of critically ill children with COVID infection and bad prognostic markers in resource-restricted settings are lacking. Through this study, the authors hope to provide an insight into the clinical characteristics, laboratory parameters, treatment, and outcomes and put forward specific predictors of an unfavorable outcome among the pediatric population infected by SARS-CoV-2 virus.
Materials and methodsThis prospective observational study was conducted from June to December 2020 at a tertiary care teaching hospital in Eastern India dedicated to COVID care. Data collection was done after obtaining approval from the Institutional Ethics Committee (Ref no. – MC/KOL/IEC/NON-SPON/781/06/20). Informed consent/ assent was obtained from the legal guardian/ child enrolled in the study in their own language.
Children between 1 month - 12 years with evidence of SARS-CoV-2 infection in their nasopharyngeal swab RT-PCR requiring critical care support were included in the study. Those who needed PICU care of fewer than 24 hours for stabilization or refused to furnish assent/consent were excluded.
Demographic characteristics like age, sex, weight and contact history were collected. Presenting signs and symptoms and the presence of comorbidities with details of comorbid conditions were taken into account. Multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C) was defined as per the WHO preliminary definition criteria.13 PRISM III scores14 were calculated within 24 hours of admission, multiorgan dysfunction was assessed by Pediatric SOFA(p-SOFA)15 score.
Laboratory parameters following admission and hospital stay, including hematological parameters, coagulation tests, D-dimer, creatinine, urea, liver function markers and presence of coinfections, were assessed for every patient. Inflammatory markers such as C reactive protein (CRP), procalcitonin, ferritin, and Il-6 levels were measured. In patients with myocarditis, NT-ProBNP assay was performed. During the PICU stay, these investigations were performed on more than one occasion as per clinical indications, but the laboratory reports obtained within the first 48 hours of admission were taken for statistical analysis. All critically ill patients admitted in PICU were screened for bacterial coinfection by culture from a suitable sample and serology for tropical fevers like malaria, dengue, enteric fever, scrub typhus and leptospira.
For identifying predictors of unfavorable outcome the cut off values were taken as - elevated CRP (> 50 mg/L), high procalcitonin (> 6 µg/L), hyperferritinemia (> 1000 µg/L), high IL-6 (≥ 7 ng/L), raised pro-BNP ≥ 1000 ng/L), elevated D-dimer (> 6 µg/L).16,17 The cut-off values for hematological were - anemia (Hb < 9 gm/dL), thrombocytopenia (platelet ≤ 150 × 109/L), leucopenia (WBC < 4.5 × 109/L), leucocytosis (WBC > 12 × 109/L), Lymphopenia (< age-appropriate normative value) hypoalbuminemia (< 2.5 gm/dL) and deranged INR (> 1.5).
Chest X-Ray, lung ultrasound, and echocardiographic findings were recorded. Echocardiography was performed in patients with features of shock, unexplained tachycardia, and symptoms suggestive of MIS-C. Myocardial dysfunction was defined by the presence of global or segmental contractility alterations, ventricular dilatation, ejection fraction less than 55% measured by modified Simpson's method. A coronary artery Z score greater than +2 standard deviation was considered significant coronary artery dilatation. Bedside lung USG was performed by Philips HD7 (Philips Healthcare, Netherlands) with the high-frequency linear probe (L 7-12 mHz), and the lung USG score was calculated in 12 zones for patients18,19 with pulmonary involvement within 24 hours of admission. Presence > 3 B lines at a particular lung field is considered abnormal findings.
Treatment received by the patients such as type of highest respiratory support with the duration of ventilator days in those requiring invasive ventilation, inotropes/vasopressor requirement, highest Vasoactive inotrope score (VIS),20 remdesivir, steroids, immunomodulators and additional supports like osmotherapy (3% NaCl) and renal replacement therapy were tabulated.
Septic shock, acute respiratory distress syndrome (ARDS) and acute kidney injury were defined and managed as per the Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock in children,21 Pediatric Acute Lung Injury Consensus Conference (PALIC) definition22 and KDIGO guidelines23 respectively.
To measure the outcomes, children were segregated into two groups - Group A, favorable outcome group, which consisted of the patients discharged from PICU within 14 days, and Group B, unfavorable outcome group, which comprised children with prolonged PICU stay (> 14 days) and death. The authors also analyzed the length of PICU stay and hospital stay.
Statistical analysisData were analyzed using SPSS statistical package (IBM SPSS Statistics, Version 25.0. Armonk, NY: IBM Corp). Results were presented as frequency and percentages for categorical data and as mean, median, standard deviation, and interquartile ranges for continuous data. Relations between categorical variables were tested using Chi-square and Fisher's exact tests, and that between continuous variables was tested using Mann Whitney U or unpaired t-test. Risk factors for unfavorable outcomes were analyzed by univariate analysis followed by backward multivariate analysis of statistically significant factors (p-value < 0.05) identified by univariate analysis.
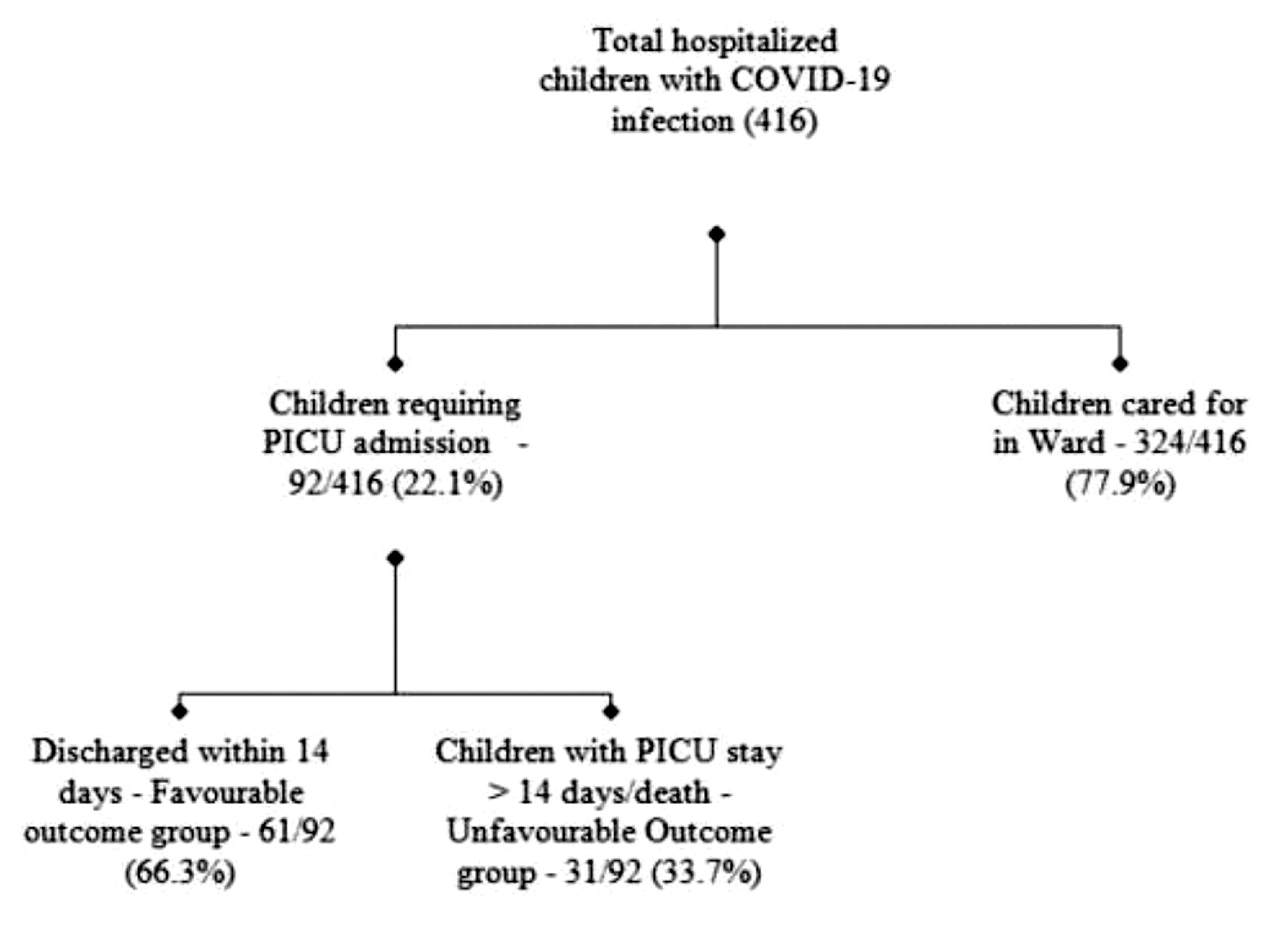
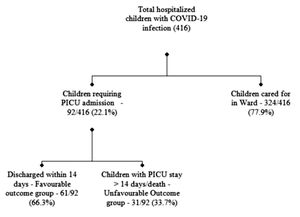
ResultsDuring the study period, out of 416 confirmed Covid-19 infected hospitalized children, 92 (22.1%) required PICU support (Figure 1). The majority were infants 39 (42.4%) (Supplementary Table 1). Pneumonia (42.4%), shock (37%) and refractory convulsion (23.9%) were the leading reasons for PICU admission. Twenty (21.7%) patients satisfied the WHO preliminary case definition for MIS-C, out of whom 14 had shock or MODS and 6 presented with Kawasaki disease phenotype. Half of the patients (54.3%) had underlying comorbidities, most common being neurological disability (21.7%), hematological malignancies (10.9%), and congenital heart diseases (8.7%). Coinfections were present in 25% of the subjects. Mean PRISM III scores on the day of admission were (12.44 ± 8.45) whereas the highest mean pSOFA score during the stay was (12.74 ± 6.20).
The trends of laboratory parameters in the study sample are exhibited in Supplementary Table 2. Hemoglobin level was low (median Hb levels: 9.2 gm/dL), neutrophilic predominance and neutrophil/lymphocyte ratio was noted to be 5.2 (3.7- 10.3). Overall, thrombocytopenia was observed with a median value of 145 × 109/L. Median CRP value was 48 mg/L (IQR - 6.7- 97.0). Other inflammatory markers like procalcitonin, IL-6, ferritin and NT pro-BNP showed higher than normal median values. Coagulation profile showed no significant variation from normal except D-dimer [4.7 µg/L (IQR 1.2 - 9.7)]. Forty-one (44.6%) of the children had abnormal infiltrates on chest radiography, predominantly of the diffuse type (22.8%). Lung USG exhibited the existence of abnormal findings in 54 subjects (58.7%), commonly B Lines (54.9%), pleural line abnormalities (48.3%), subpleural micro-consolidation (43.9%), and reduced lung sliding (34.06%).
Respiratory support in any form was needed for all critically ill patients. Among them, 26 (28.3%) required invasive mechanical ventilation (Supplementary Table 3). The mean duration of ventilator days was (7.8 ± 6.2) days. Proning was performed for 14/20 patients who met ARDS criteria. Inotrope/vasopressor support was needed in 37% of children, and the maximum VIS score was (54.7 ± 29.9). Remdesivir was administered to 14 (15.2%) children with severe disease and emergent or increasing need of oxygen within the first 10 days of illness. Intravenous immunoglobulin was employed in (26.1%) all patients of MIS-C and demyelinating lesions of the nervous system. Steroids were given to 50 (54.3 %) of the patients either with acute COVID-19 pneumonia/ARDS23 or MIS-C20 or other indications like SVC syndrome and HLH (07).
Thirty-one (33.7%) of the patients had a prolonged PICU course (PICU stay >14 days or death). The overall mortality rate was 7.6% among PICU admitted patients. All deceased patients previously had debilitating chronic illnesses: one had a Gaucher disease, two had a relapse of acute lymphoblastic leukemia and severe immunodeficiency, two had multiple congenital anomalies with severe neurodisability, and two patients of systemic lupus erythematosus with multiorgan failure and immunodeficiency.
Univariate analysis revealed infants were more frequent in the unfavorable outcome group (67.7% vs. 39.3%, p-value 0.01) (Table 1). The proportion of patients with under-nutrition, presence of comorbidity, coinfections were significantly higher in children with unfavorable outcomes (p-value, 0.04, 0.003 and 0.039, respectively). Fever duration was significantly longer among children in Group B (10.74 ± 8.96 vs 6.49 ± 4.04, p = 0.025). Clinical presentation like respiratory distress, shock, and GCS < 7 at admission was associated with worsened prognosis.
Comparison of clinical characteristics, laboratory parameters and severity between children with favorable outcomes vs. children with unfavorable outcomes.
Hemoglobin levels, lymphocyte counts, platelet counts, albumin levels were lower, and total leucocyte counts, neutrophilic predominance, and inflammatory markers like CRP, procalcitonin, IL-6, pro-BNP, d-dimer, and blood lactate were significantly elevated in the unfavorable outcome group. The mean duration of PICU stay was significantly longer in children with unfavorable outcomes (15.2 ± 5.2 vs. 6.5 ± 4.3, p = 0.002).
On binary logistic regression analysis, it was revealed that as predictors for unfavorable outcome, adjusted odds ratio for infant age (OR- 2.58, p = 0.031), comorbidity (OR - 4.67, p = 0.002) was statistically significant (Table 2). Clinical features like prolonged fever > 5 days (OR – 3.54, p = 0.005), respiratory distress at admission (OR – 8.70, p = < 0.001), presence of shock (OR 3.39, p = 0.036) and GCS < 7 was significantly associated with unfavorable outcome. Need of invasive mechanical ventilatory support of day 1 was an independent worse outcome predictor. Among laboratory parameters elevated CRP > 50 mg/L (OR- 7.83, p ≤ 0.001), procalcitonin > 6 ng/L (OR-4.52, p = 0.004), D-dimer > 6 ng/L (OR- 3.88, p = 0.01) and arterial lactate > 2 mmol/L (OR- 5.65, p = 0.002) was observed to be significant unfavorable outcome predictors.
Binary logistic regression analysis for predictors of unfavourable outcomes among the study population.
Patients with MIS-C (20/72) were older than acute COVID patients (7.9 ± 3.6 vs 5.4 ± 4.8, p = 0.02) (Table 3). The prevalence of comorbidities was significantly higher in the latter. Shock, gastrointestinal and mucocutaneous symptoms predominated in the MIS-C group (p-value 0.003, 0.04, and 0.001 respectively). Respiratory involvement was more commonly noted in the acute COVID group. Laboratory parameters showed lower platelet counts higher CRP and ferritin levels in the MIS-C group. Coronary dilatation (6/20 vs 0/72) and myocardial dysfunction (p = 0.001) were more commonly observed in this group. Most patients in the MIS-C group recovered with non-rebreathing mask oxygen. Inotrope requirement and median VIS were significantly higher. IVIG and steroid were administered to all the MIS-C patients. Duration of PICU stay was shorter (7.08 ± 4.85 vs. 9.11 ± 5.14, p = 0.04), and no mortality was reported in MIS-C group.
Comparison of patients with acute covid infection and MIS-C.
Characteristics of critically ill children with COVID-19 infection requiring PICU care have been described from western countries,8–10,24–26 but literature is scarce from resource-restricted Asian counties. The authors observed that the PICU admission rates among COVID-19 hospitalized children was 22.1%. Infants were the most prevalent age group. Pneumonia, shock and refractory convulsion were the leading causes that called for PICU admission. Two-thirds of the patients were successfully discharged from PICU within 14 days. The mortality rate was 7.6%. Infant age group, presence of comorbidity, clinical presentations like either respiratory distress or shock or GCS < 7, laboratory parameters like elevated CRP, procalcitonin, D-dimer and arterial lactate were independently associated with unfavorable outcomes. Mean of maximum VIS score, PRISM III scores on admission, and highest pSOFA scores were double in the unfavorable outcome group.
Previous reports from all over the world mentioned a wide range of PICU admission rates (1.3-39%) among COVID-19 infected hospitalized children.9,10,25,26 A systematic review of the early period of the pandemic, Castagnoli et al.24 reported only one critically ill child among 1065 children (0.1%) from Asia with confirmed COVID-19. In a cohort of 77 hospitalized children from New York, 31 (39%) were cared for in the PICU; 14 (18.1%) had organ dysfunction and the majority (97%) survived to discharge.25 Multicentre cohort study across 25 European countries, Götzinger et al. reported the PICU admission rate as 8%.26 So it is evident that as the pandemic unfolded, a greater number of children required critical care support.
The present study found the clinical course and outcome of COVID-19 to be more severe in critically ill children than in studies from the USA.8,10,25 The mortality rate was recorded as 7.6% in the present study, whereas mortality ranged from 1.3 to 4% in reports from the USA. Swann et al. observed a mortality rate of 5% (6 out of 118) in PICU admitted patients in a multicenter study from the UK.9 The higher mortality rate may be due to the fact that disease severity at presentation was higher in the study population as reflected in the p-SOFA score (12.74 ± 6.20) score compared to median p-SOFA score (3, IQR 2-7) among 14 PICU admitted children from New York.25 Considering the early reports of very low mortality, an unfavorable outcome was defined if the patient required continuous PICU care for more than 14 days or was deceased in the study study cohort.
Data related to the association between comorbidity and more severe course and the unfavorable outcome is inconsistent among previously published literature. Shekerdemian et al. observed comorbidities were prevalent in 80% of the 48 children hospitalized with serious illness from Covid-19,8 whereas Fisler et al. concluded that the presence of underlying comorbidity was not associated with need for PICU admission (p = 0.227) or organ dysfunction (p = 0.87).25 The authors found the presence of comorbidity was an independent predictor for unfavorable outcome. Question remains whether the severity of disease in these patients was solely due to comorbid condition (COVID as coincidental infection) or comorbid condition exacerbated by COVID-19 infection. Elezkurtaj et al. in autopsy series of adult COVID-19 patients, revealed that in the majority of decedents, causes of death were directly related to COVID-19, not an immediate result of pre-existing comorbidities.27
The present study revealed infants were the most common age group (42.4%) requiring PICU care and was independently associated with unfavorable outcome. Initial reports from China reflected similar findings where severe and critical cases were reported in 10.6% of the children aged less than one year.28 However, multiple reports from the USA found that older children (> 12 yrs) were the most vulnerable group for PICU admission.10,25 As the authors cater children up to 12 years of age at the study's centre it was not possible to draw a conclusion on adolescent age group.
Most common presenting symptoms across studies were pyrexia followed by symptoms of lower respiratory tract infection, reported in more than 70% of children.10,25 Hypoxia, hemodynamic instability and radiological changes suggestive of pneumonia or ARDS were the most common indications for PICU admission.25,26 The almost identical observation was noticed in the present study's cohort; pneumonia, shock and refractory convulsion were the leading causes for PICU admission.
Among a large PICU population, 20 (28.6%) patients required invasive mechanical ventilation, 7 (10%) prone positioning, 1 (1.4%) extracorporeal membrane oxygenation (ECMO) and 14 (20%) supported with vasopressor.25 Although being similar in a number of patients, inotrope/vasopressor support and mechanical ventilation were needed almost double in proportion in the present study. The mean duration of proning was 31.6 hours and the median ventilator stay was 6.7 days. The study centre doesn't have ECMO facility. Fourteen (15.2%) of the study's patients received remdesivir, particularly in those with severe disease and having respiratory involvement. Similar data were presented by Chao et al., remdesivir was administered in 18.3% of patients and the majority had ARDS.10 In view of the scant evidence available on the effectiveness of remdesivir therapy in children infected with SARS-CoV-2, further studies should be conducted to assess the efficacy.
Laboratory data from 8 severe pediatric cases showed normal or increased leukocyte count and high levels of C-reactive protein (CRP, procalcitonin, and LDH from China.28 Admission to an ICU and organ dysfunction was associated with higher WBC count, elevated CRP, procalcitonin, pro–BNP, and decreased platelet count.10,25 However, the authors found no association between total white blood cell count, absolute lymphocyte count, platelet count, and pro-BNP with unfavorable outcomes. The authors’ multivariate analysis revealed elevated CRP > 50 mg/L, procalcitonin > 6 ng/L, D-dimer > 6 ng/L and lactate > 2 mmol/L were predictors for unfavorable outcome.
Feldstein et al. compared patients with acute COVID-19 and MIS-C. MIS-C patients were younger and less likely to have a comorbidity.29 Higher CRP level and lower platelet count was observed among MIS-C group. The authors had similar observations except the older age group being more affected by MIS-C. This could be explained by the exclusion of adolescents more than 12yrs from the present study. Left ventricular dysfunction and coronary artery aneurysm were noted in 43.8% and 13.4% patients, respectively in the US study. As the authors included only PICU admitted patients, a higher LV dysfunction was found (80%) as well as coronary dilatation (30%) among MIS-C patients. Similar to the present study, García-Salido et al. found that patients in the MIS-C group were less likely to receive invasive ventilation (p = 0.005) but were more often treated with vasoactive drugs (p < 0.001).30
LimitationsThe authors’ observational data was from a single centre and may not reflect the whole pediatric population as there may be variation in the spectrum of clinical characteristics, severity, and outcome. Lack of data of children above 12 years, one of the most affected ranges of age in children with COVID-19, is another limitation of the present study. Contribution of comorbid conditions vs. SARS-CoV-2 infection leading to critical illness in children with pre-existing comorbidity could not be ascertained definitely.
ConclusionThe authors have been able to highlight that infant age group, presence of comorbidity, either respiratory distress or shock or poor GCS, laboratory parameters like elevated CRP, procalcitonin and arterial lactate were independent predictors of unfavorable outcome in Covid-19 infected children, which necessitate the need for watchful monitoring and early intervention. Observation of the present research may help in the management of such critically ill pediatric populations in resource-restricted settings.
Ethics approvalApproved by institutional Ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participateInformed consent and assent were obtained as applicable in understandable language.