To determine the frequency of nonalcoholic fatty liver disease using nuclear magnetic resonance as a noninvasive method.
MethodologyThis was a cross-sectional study conducted on 50 children and adolescents followed up at an outpatient obesity clinic. The subjects were submitted to physical examination, laboratory tests (transaminases, liver function tests, lipid profile, glycemia, and basal insulin) and abdominal nuclear magnetic resonance (calculation of hepatic, visceral, and subcutaneous fat).
ResultsNonalcoholic fatty liver disease was diagnosed in 14 (28%) participants, as a severe condition in eight (percent fat >18%), and as non-severe in four (percent fat from 9% to 18%). Fatty liver was associated with male gender, triglycerides, AST, ALT, AST/ALT ratio, and acanthosis nigricans. Homeostasis model assessment of insulin resistance and metabolic syndrome did not show an association with fatty liver.
ConclusionThe frequency of nonalcoholic fatty liver disease in the present population of children and adolescents was lower than that reported in the international literature. It is suggested that nuclear magnetic resonance is an imaging exam that can be applied to children and adolescents, thus representing an effective noninvasive tool for the diagnosis of nonalcoholic fatty liver disease in this age range. However, further national multicenter studies with longitudinal design are needed for a better analysis of the correlation between nonalcoholic fatty liver disease and its risk factors, as well as its consequences.
Determinar a frequência da doença hepática gordurosa não alcoólica utilizando ressonância magnética nuclear como um método não invasivo.
MetodologiaEste foi um estudo transversal realizado em 50 crianças e adolescentes acompanhadas em uma Clínica Ambulatorial de Obesidade. Os indivíduos foram submetidos a exame físico, testes de laboratório (transaminases, testes de função hepática, perfil lipídico, glicemia e insulina basal) e ressonância magnética nuclear abdominal (cálculo da gordura hepática, visceral e subcutânea).
ResultadosA doença hepática gordurosa não alcoólica foi diagnosticada em 14 (28%) participantes, como uma condição grave em oito (percentual de gordura > 18%) e não grave em quatro (percentual de gordura de 9 a 18%). Fígado gorduroso foi associado a sexo masculino, triglicerídeos, aspartato aminotransferase (AST), alanina aminotransferase (ALT), proporção de AST/ALT e acanthosis nigricans. O Modelo de Avaliação da Homeostase de Resistência à Insulina e a síndrome metabólica não mostraram associação com fígado gorduroso.
ConclusãoA frequência da doença hepática gordurosa não alcoólica na população atual de crianças e adolescentes foi inferior à relatada na literatura internacional. Sugerimos que a ressonância magnética nuclear seja um exame de imagem que pode ser aplicado em crianças e adolescentes, representando, assim, uma ferramenta não invasiva eficaz no diagnóstico de doença hepática gordurosa não alcoólica nessa faixa etária. Contudo, estudos multicêntricos nacionais adicionais de modelo longitudinal são necessários para uma melhor análise da correlação entre a doença hepática gordurosa não alcoólica e seus fatores de risco, bem como suas consequências.
Obesity is a chronic disease with high worldwide prevalence.1 Among its consequences is nonalcoholic fatty liver disease (NAFLD), considered to be the most common etiology of liver disease among children in developed countries. NAFLD may manifest as simple hepatic steatosis (HS) and steatohepatitis (NASH), and rarely as cirrhosis or hepatocellular carcinoma.2 NAFLD is frequently associated with obesity, insulin resistance, and hypertriglyceridemia. Known clinical risk factors for NASH include type 2 diabetes mellitus and panhypopituitarism, among others.3
The gold standard for the diagnosis of NAFLD is a liver biopsy; however, since this is an invasive method, other indirect methods such as imaging and laboratory exams in combination with medical history and clinical examination have been used in children and adolescents.4
Nuclear magnetic resonance (NMR) is the most sensitive and objective imaging exam for the detection and quantitation of HS in adults, including levels much lower than normal reference values, whose cut-off point is ≤9%.5 NMR differentiates focal HS from possible malignant lesions,6 evaluates the distribution of body adiposity, does not emit ionizing radiation, and is not operator-dependent.7
The prevalence of NAFLD among obese children and adolescents varies when diagnosed by a liver biopsy, NMR, ultrasound (US), or changes in transaminases, with respective percentages of 13–58.1%, 31.8–40%, 13.7–75%, and 14–55.8% having been found.1 This variation may also be influenced by various factors such as age, gender, ethnicity, geographic region, and degree of obesity, in addition to the method of assessment.8
In view of the high prevalence of obesity1 and, consequently, the high prevalence of hepatic steatosis, the aim of the present study is to determine the frequency of NAFLD by NMR, and to correlate this with clinical and laboratory parameters in obese children and adolescents followed at an outpatient obesity clinic.
MethodologyThis was a cross-sectional study conducted on obese children and adolescents followed up at a reference obesity outpatient clinic, during the period from 2014 to 2016, located in Ribeirão Preto, state of São Paulo, having a high human development index (HDI) of 0.80, 40th in the ranking of Brazil, with 24.3% children and adolescents, and a population composed of 73.8% whites and 26% non-whites.9
According to researchers, Ribeirão Preto has a prevalence of diabetes and glucose intolerance in adults of 12.1% and 7.7%, respectively,10 with overweight in 30.9% of the schoolchildren,11 thus characterizing a city with a large at-risk population for NAFLD.
Sample size was 50 patients according to a statistical calculation that considered 20% prevalence and 10% precision, i.e., an acceptable error percentage for the estimated prevalence of 20%.
Patients aged 4 to 16 years were invited to participate. Informed consent was obtained from the parent/guardian, and assent was obtained from adolescents when applicable. Exclusion criteria included presence of hepatic or genetic disease, immunodeficiency, medications that might alter transaminases, and infectious or inflammatory conditions.
Obesity was characterized by a body mass index (BMI) ≥ the 95th percentile according to the reference values of the Centers for Disease Control and Prevention (CDC).12 BMI was calculated as weight (kg)/height2 (m2). Weight was measured with an electronic platform scale, with the subject barefoot and standing on the center. Height was measured with a rigid stadiometer with the subject standing barefoot with joined heels, straight back, and arms extended along the body. For homogeneous analysis of gender and age, the z-score for BMI was calculated using AnthroPlus software (WHO, AnthroPlus, version 1.0.2). Subjects with a z-score between +2 and +3 and >3 were included in the obese and super obese groups, respectively.
Quantification of hepatic, abdominal, and subcutaneous fat fractions were determined by abdominal NMR13,14 using a high field instrument with 1.5Tesla magnitude (ACHIEVA model; Philips Medical Systems, Netherlands), which permitted the execution of T2 and T1 sequences: (a) weighted T2 sequence on the coronal plane, turbo-spin-echo (TSE) with suppressed respiration [TR (repetition time)=737msec, TE (echo time)=80msec, flip angle=90°, echo train length=121, section thickness=6mm, gap=8%, 30 sections within 22seconds of suppressed respiration] used as a localizer; (b) weighted T1 sequence on the axial plane, double echo in phase (TE=4.6msec) and out of phase (TE=2.3msec) with suppressed respiration, spoiled gradient echo (SGE) (TR=111msec, flip angle=80°, section thickness=6mm, gap=7%, 30 sections per echo during 29seconds of suppressed respiration), with acquisitions in the abdomen including the liver and umbilical region. For the analysis of visceral fat, an axial section obtained in the T1 sequence was used at the level of the umbilical scar, with manual segmentation of subcutaneous and visceral fat and calculation of the area in mm2. The sum of the areas of visceral and subcutaneous fat in the same section was considered to be the total area of abdominal fat. The quantity of fat in these areas was calculated by obtaining the number and size of the segmented voxels (mm). For the analysis of hepatic fat, the central region with the best positioning of the liver was selected, and four regions of interest (ROI) measuring 10mm2 were selected in the VI/VII, V/VIII, IV, and II/III segments, avoiding large intrahepatic vessels. The mean of the four measurements permitted the calculation of the hepatic fat fraction using the formula described by Fishbein et al.13 (IS in-phase – IS out-of-phase)/2 IS in-phase. Reference values higher than 18% and between 9% and 18% were considered to be severe and non-severe HS, respectively.5 No patients required sedation.
Dependent variables included the following: subject gender; z-score BMI; triglycerides, HDL, aspartate aminotransferase (AST), alanine transaminase (ALT), AST/ALT ratio (<1 suggests NAFLD and ≥ 1 suggests intense fibrosis and cirrhosis),15 gamma GT, total cholesterol, LDL, HOMA, acanthosis nigricans, metabolic syndrome, and blood pressure.
Data were analyzed statistically using the SAS/STAT software (SAS Institute Inc, software for Windows, version 2014, NC, USA).
As the quantitative data showed asymmetric distribution, non-parametric tests were used for analysis.
Mann–Whitney test was used to compare the dependent variables in relation to the percentage of hepatic fat, subcutaneous fat, and visceral fat. The Kruskal–Wallis test was used to compare systolic and diastolic arterial pressure in relation to the above variables. Fisher's exact test was used to compare proportions of the parameters studied. The level of significance was set at 5% in all analyses.
Spearman's correlation coefficient (ρ) was calculated to determine the correlation between percent hepatic fat, visceral fat, subcutaneous fat, and abdominal circumference, and a log-binomial regression model was used to estimate the prevalence ratio in the analysis of associated factors for HS.
The study was approved by the local research ethics committee, HCRP protocol No. 6984/2010.
ResultsOf the 50 subjects included in the study, 56% (28) were males, aged 4 to 16 years. Distributed according to age range, 44.0% (22) were classified as children aged 4 to 10 years (mean 8.3; SD ±1.8) and 56% (28) as adolescents aged 11 to 19 years (mean 12.6; SD ±1.6).
Clinical examination revealed an altered abdominal circumference measurement in 100% (50) of the subjects, systolic and diastolic hypertension in 20% (ten), a palpable liver in 10% (five), and acanthosis nigricans in 58% (29). No subjects had jaundice or a palpable spleen.
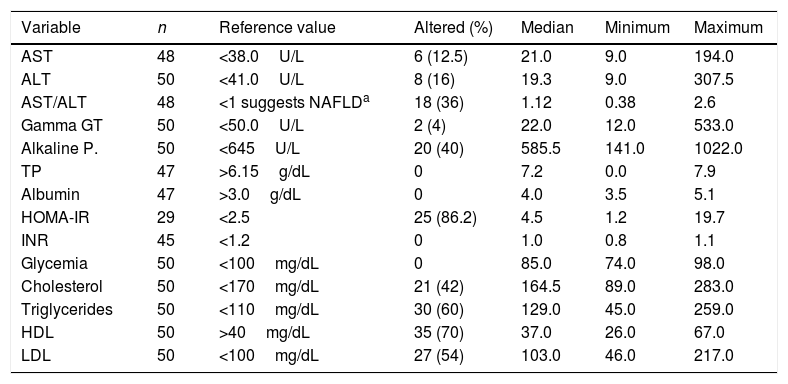
Although the frequency of an altered HOMA index was 86.2% (25/29), it was not possible to calculate this index in the entire sample. The results of the remaining laboratory exams are listed in Table 1.
Results of the biochemical tests conducted on 50 patients, followed-up at an outpatient obesity clinic, number of patients tested, reference value, number of patients with altered results, median, and minimum and maximum values.
| Variable | n | Reference value | Altered (%) | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| AST | 48 | <38.0U/L | 6 (12.5) | 21.0 | 9.0 | 194.0 |
| ALT | 50 | <41.0U/L | 8 (16) | 19.3 | 9.0 | 307.5 |
| AST/ALT | 48 | <1 suggests NAFLDa | 18 (36) | 1.12 | 0.38 | 2.6 |
| Gamma GT | 50 | <50.0U/L | 2 (4) | 22.0 | 12.0 | 533.0 |
| Alkaline P. | 50 | <645U/L | 20 (40) | 585.5 | 141.0 | 1022.0 |
| TP | 47 | >6.15g/dL | 0 | 7.2 | 0.0 | 7.9 |
| Albumin | 47 | >3.0g/dL | 0 | 4.0 | 3.5 | 5.1 |
| HOMA-IR | 29 | <2.5 | 25 (86.2) | 4.5 | 1.2 | 19.7 |
| INR | 45 | <1.2 | 0 | 1.0 | 0.8 | 1.1 |
| Glycemia | 50 | <100mg/dL | 0 | 85.0 | 74.0 | 98.0 |
| Cholesterol | 50 | <170mg/dL | 21 (42) | 164.5 | 89.0 | 283.0 |
| Triglycerides | 50 | <110mg/dL | 30 (60) | 129.0 | 45.0 | 259.0 |
| HDL | 50 | >40mg/dL | 35 (70) | 37.0 | 26.0 | 67.0 |
| LDL | 50 | <100mg/dL | 27 (54) | 103.0 | 46.0 | 217.0 |
AST, aspartate transaminase; ALT, alanine transaminase; gamma GT, gamma-glutamyl transpeptidase; Alkaline P., alkaline phosphatase; TP, total proteins; HOMA-IR, homeostasis model assessment of insulin resistance; INR, international normalized ratio; HDL, high-density lipoproteins; LDL, low-density lipoprotein.
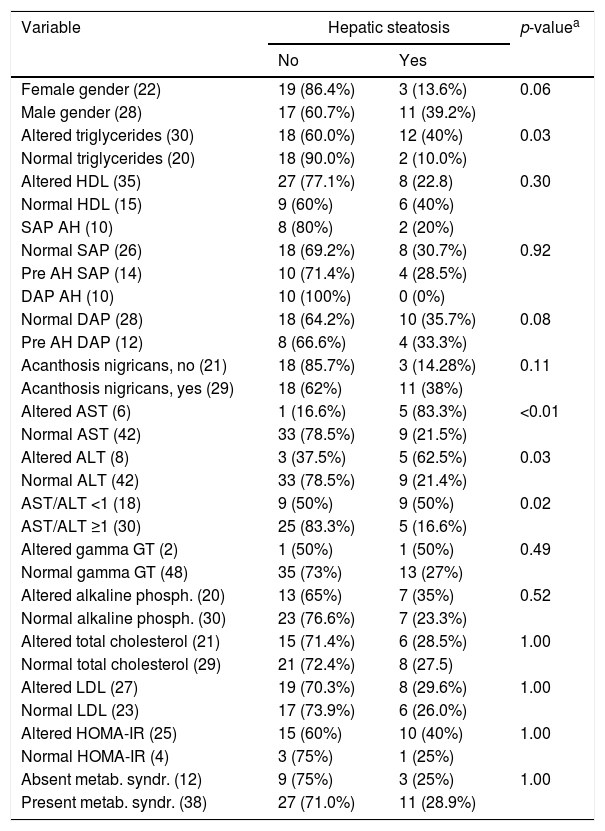
NAFLD was diagnosed in 28% of the subjects (14/50), with 57.1% (8/14) showing severe steatosis and 42.9% (6/14) non-severe steatosis. Table 2 lists the frequency of the diagnosis of HS according to the different variables analyzed.
Frequency of the variables studied regarding the diagnosis of hepatic steatosis in 50 patients followed up at an outpatient obesity clinic.
| Variable | Hepatic steatosis | p-valuea | |
|---|---|---|---|
| No | Yes | ||
| Female gender (22) | 19 (86.4%) | 3 (13.6%) | 0.06 |
| Male gender (28) | 17 (60.7%) | 11 (39.2%) | |
| Altered triglycerides (30) | 18 (60.0%) | 12 (40%) | 0.03 |
| Normal triglycerides (20) | 18 (90.0%) | 2 (10.0%) | |
| Altered HDL (35) | 27 (77.1%) | 8 (22.8) | 0.30 |
| Normal HDL (15) | 9 (60%) | 6 (40%) | |
| SAP AH (10) | 8 (80%) | 2 (20%) | |
| Normal SAP (26) | 18 (69.2%) | 8 (30.7%) | 0.92 |
| Pre AH SAP (14) | 10 (71.4%) | 4 (28.5%) | |
| DAP AH (10) | 10 (100%) | 0 (0%) | |
| Normal DAP (28) | 18 (64.2%) | 10 (35.7%) | 0.08 |
| Pre AH DAP (12) | 8 (66.6%) | 4 (33.3%) | |
| Acanthosis nigricans, no (21) | 18 (85.7%) | 3 (14.28%) | 0.11 |
| Acanthosis nigricans, yes (29) | 18 (62%) | 11 (38%) | |
| Altered AST (6) | 1 (16.6%) | 5 (83.3%) | <0.01 |
| Normal AST (42) | 33 (78.5%) | 9 (21.5%) | |
| Altered ALT (8) | 3 (37.5%) | 5 (62.5%) | 0.03 |
| Normal ALT (42) | 33 (78.5%) | 9 (21.4%) | |
| AST/ALT <1 (18) | 9 (50%) | 9 (50%) | 0.02 |
| AST/ALT ≥1 (30) | 25 (83.3%) | 5 (16.6%) | |
| Altered gamma GT (2) | 1 (50%) | 1 (50%) | 0.49 |
| Normal gamma GT (48) | 35 (73%) | 13 (27%) | |
| Altered alkaline phosph. (20) | 13 (65%) | 7 (35%) | 0.52 |
| Normal alkaline phosph. (30) | 23 (76.6%) | 7 (23.3%) | |
| Altered total cholesterol (21) | 15 (71.4%) | 6 (28.5%) | 1.00 |
| Normal total cholesterol (29) | 21 (72.4%) | 8 (27.5) | |
| Altered LDL (27) | 19 (70.3%) | 8 (29.6%) | 1.00 |
| Normal LDL (23) | 17 (73.9%) | 6 (26.0%) | |
| Altered HOMA-IR (25) | 15 (60%) | 10 (40%) | 1.00 |
| Normal HOMA-IR (4) | 3 (75%) | 1 (25%) | |
| Absent metab. syndr. (12) | 9 (75%) | 3 (25%) | 1.00 |
| Present metab. syndr. (38) | 27 (71.0%) | 11 (28.9%) | |
SAP, systolic arterial pressure; DAP, diastolic arterial pressure; AH, arterial hypertension; metab. syndr., metabolic syndrome; ALT, alanine transaminase; gamma GT, gamma-glutamyl transpeptidase; HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high-density lipoproteins; LDL, low-density lipoprotein.
Of the 14 participants with NAFLD, 78.6% (11) were males and 21.4% (3), females. There was a significant difference in comparison of percentage of hepatic fat, favoring boys (p=0.04); however visceral fat was prevalent in the female gender (p=0.04).
Regarding the degree of obesity, 34% (17/50) of the subjects were obese and 66% (33/50) were super obese. HS was observed in 23.5% (4/17) of the obese subjects and in 30.3% (10/33) of the super obese subjects. There was a statistically significant difference of percent visceral and subcutaneous fat between the two groups (p=0.03 and p=0.04, respectively), but not of percent hepatic fat.
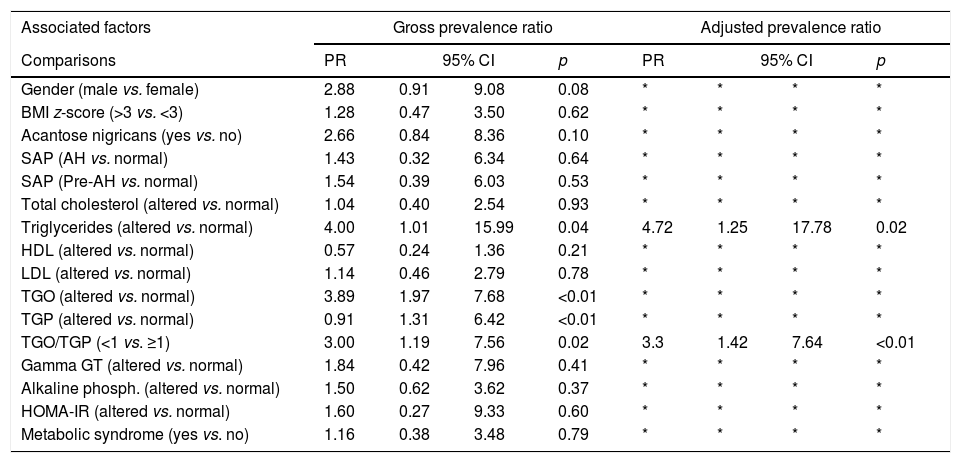
Among serum lipids, only altered triglycerides were associated with percent hepatic fat (prevalence ratio: 4; 95% CI: 1.01–15.99).
Acanthosis nigricans was detected in 58% (29/50) of the subjects, with 37.9% (11/29) of them having HS; however, significant difference was only observed for the percentage of hepatic fat (p=0.03).
Changes in AST were observed in 12.5% (6/50) of cases, 83.5% (5) having NAFLD. ALT was altered in 16% (8/50) of cases, 62.5% (5) having NAFLD. The transaminases were associated with the hepatic fat fraction. The prevalence ratio was 3.89 (95% CI: 1.97–7.68) for patients with altered AST and 2.91 (95% CI: 1.31–6.42) for those with altered ALT.
The AST/ALT ratio was <1 in 36% (18/50) of the population, with 50% (9/18) of the subjects with an altered ratio having HS. There was an association between the AST/ALT ratio and the hepatic fat fraction; the prevalence ratio for patients with an AST/ALT ratio <1 was equal to 3 (95% CI: 1.19–7.56). Table 3 lists the prevalence ratio for factors associated with hepatic steatosis.
Prevalence ratio estimated through adjustment of log-binomial regression models for factors associated with hepatic steatosis in 50 patients followed up at an outpatient obesity clinic.
| Associated factors | Gross prevalence ratio | Adjusted prevalence ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Comparisons | PR | 95% CI | p | PR | 95% CI | p | ||
| Gender (male vs. female) | 2.88 | 0.91 | 9.08 | 0.08 | * | * | * | * |
| BMI z-score (>3 vs. <3) | 1.28 | 0.47 | 3.50 | 0.62 | * | * | * | * |
| Acantose nigricans (yes vs. no) | 2.66 | 0.84 | 8.36 | 0.10 | * | * | * | * |
| SAP (AH vs. normal) | 1.43 | 0.32 | 6.34 | 0.64 | * | * | * | * |
| SAP (Pre-AH vs. normal) | 1.54 | 0.39 | 6.03 | 0.53 | * | * | * | * |
| Total cholesterol (altered vs. normal) | 1.04 | 0.40 | 2.54 | 0.93 | * | * | * | * |
| Triglycerides (altered vs. normal) | 4.00 | 1.01 | 15.99 | 0.04 | 4.72 | 1.25 | 17.78 | 0.02 |
| HDL (altered vs. normal) | 0.57 | 0.24 | 1.36 | 0.21 | * | * | * | * |
| LDL (altered vs. normal) | 1.14 | 0.46 | 2.79 | 0.78 | * | * | * | * |
| TGO (altered vs. normal) | 3.89 | 1.97 | 7.68 | <0.01 | * | * | * | * |
| TGP (altered vs. normal) | 0.91 | 1.31 | 6.42 | <0.01 | * | * | * | * |
| TGO/TGP (<1 vs. ≥1) | 3.00 | 1.19 | 7.56 | 0.02 | 3.3 | 1.42 | 7.64 | <0.01 |
| Gamma GT (altered vs. normal) | 1.84 | 0.42 | 7.96 | 0.41 | * | * | * | * |
| Alkaline phosph. (altered vs. normal) | 1.50 | 0.62 | 3.62 | 0.37 | * | * | * | * |
| HOMA-IR (altered vs. normal) | 1.60 | 0.27 | 9.33 | 0.60 | * | * | * | * |
| Metabolic syndrome (yes vs. no) | 1.16 | 0.38 | 3.48 | 0.79 | * | * | * | * |
SAP, systolic arterial pressure; AH, arterial hypertension; ALT, alanine transaminase; gamma GT, gamma-glutamyl transpeptidase; HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high-density lipoproteins; LDL, low-density lipoprotein; BMI, body mass index.
There was a low correlation between abdominal circumference and hepatic fat fraction (Spearman's coefficient: 0.31); a strong correlation for subcutaneous fat (Spearman's coefficient: 0.87) and a moderate correlation for visceral fat (Spearman's coefficient: 0.61).
Percent visceral and subcutaneous fat showed a low correlation with percent hepatic fat according to the Spearman's correlation coefficient, i.e., 0.3 and 0.36, respectively.
DiscussionAn exam that has been strongly emphasized in the literature over the last few years is NMR spectroscopy (NMRS) which, despite its high accuracy, is a time-consuming and difficult exam. For this reason, the present study used NMR, which involves minimal risks, is noninvasive, and can be performed faster and more easily. NMR yields more precise results than US, does not use ionizing radiation, and provides spatial coverage of the entire liver.16 Using this tool, the frequency of hepatic steatosis in the present study was 28%. It was lower than that detected in the world literature (Italian and American studies) when NMR was used, which ranges from 31.8% to 40% according to Padilha et al.1 This difference in prevalence may be due to differences in regions, ethnic groups, and age ranges. No Brazilian studies were found to compare with. The present study's population consisted of children with considerable racial mixing, with a little more than one-third self-declared white and the others, black. This fact may explain, in part, the lower prevalence, since the American literature reports that the proportion of NAFLD is lower among black children (1.5%) and higher in children of Hispanic origin (11.8%).17 The highest prevalence has been detected in Asia.8 Studies on the prevalence of disease related to racial/ethnic differences have several inherent limitations, i.e., data on race and ethnicity is self-reported and may not be collected reliably; differences between racial groups also may be difficult to interpret because there is difficulty in classifying multi-ethnic individuals. Finally, race and socioeconomic status are often highly correlated, complicating interpretation of the observed disparities in health outcomes.18
The gender ratio was 3.6:1 favoring boys, which corroborates with the literature, confirming the predominance of hepatic steatosis in male individuals.19 Another Brazilian study detected a 2.7:1 ratio.20 One explanation for higher rates of fatty liver in male than in female individuals is that male individuals are more likely to distribute excess body fat in the intra-abdominal compartment. Studies in adults demonstrate a relationship between the amount of visceral adipose tissue and the presence of hepatic steatosis. Another potential reason for a gender-based difference in fatty liver development is the influence of sex hormones. Sex hormones effect the distribution of both fat and muscle.19
Regarding age, among children younger than 10 years, 31.8% had HS, which was severe in four. These finding is different from those reported by Duarte & Silva.20 who, in a study of 77 obese children/adolescents, observed only mild steatosis in children, with the moderate form being detected only among the adolescents. Fatty liver prevalence increased with age, ranging from 0.7% for ages 2–4 years up to 17.3% for ages 15–19 years. The pediatric prevalence of NAFLD peaks at puberty; factors that can explain the higher rate of NAFLD in adolescents include sex hormones and insulin resistance in puberty, or their increased control over unhealthy food choices and sedentary lifestyle.21 It is probable that children in the present series were accessing unhealthy food earlier.
Regarding the lipid profile, there was statistical significance only between triglycerides and the hepatic fat fraction. According to Schwimmer et al.,22 children with HS confirmed by biopsy had significantly higher levels of triglycerides, total cholesterol, LDL, insulin, glucose, and blood pressure. Other authors have demonstrated a correlation between increased triglycerides and reduced HDL in patients with HS.23
Hepatic steatosis in combination with an increase in serum ALT is a marker used for a presumptive diagnosis of HS in the absence of histopathological evaluation.20 The present study detected increased levels of altered transaminases in 16% of the series. The proportions of altered transaminases vary considerably in the literature. In Brazil, Souza et al.24 detected only a 3% rate of abnormal ALT levels in overweight and obese children. An American population study25 of obese adolescent and other publications involving children followed at reference centers for the treatment of obesity in Italy26 and China27 detected an increase in ALT levels in 9.5%, 25%, and 24% of the subjects investigated, respectively. An explanation for these different values is that reference services usually serve a population of pathologically obese patients with more associated comorbidities, thus leading to a higher proportion of elevated transaminases.
It was observed that obese patients with HS showed greater transaminase changes. Five subjects (37.5%) with steatosis showed altered AST and the same percentage was observed for ALT. These proportions were higher than those reported by Duarte & Silva,20 who detected changes in 9.1% (3/33) of their group with HS. The present study detected a significant difference in transaminase levels when compared to percent hepatic fat. According to Pacifico et al.,28 the concentration of transaminases is positively correlated with an increase in the hepatic fat fraction, as is also the case for insulin and insulin resistance. Fishbein et al.5 showed that the increase in transaminase levels was significantly higher in patients with a severe increase in hepatic fat. Souza et al.24 reported that changes in ALT may already be related to some degree of hepatic inflammation, characterizing a more advanced stage of NAFLD.
AST/ALT ratio, which suggests the presence of NAFLD when less than 1,15 was altered in 18 (36%) patients, only one of whom had HS. The ratio was correlated with the hepatic fat fraction, with a prevalence ratio equal to 3.
Metabolic syndrome was detected in 76% patients, with 28.9% of them having HS, and it was not statistically correlated with hepatic, visceral, or subcutaneous fat. However, the literature shows that metabolic syndrome, a highly atherogenic condition, is related to the presence of NAFLD in adults.3,29
Acanthosis nigricans was present in 58% participants, 11 of them with HS. This proportion was intermediate in relation to that reported by Schwimmer et al.,30i.e., 49% of 43 children submitted to a liver biopsy for the diagnosis of NAFLD, and to that reported by Duarte & Silva,20 who identified acanthosis nigricans in 81.8% of their patients. Thus, this finding is an important signal that should be appreciated by the pediatrician since it reflects hyperinsulinemia, and, indirectly, HS. The present study detected a statistically significant difference for percent hepatic fat and acanthosis nigricans.
The world literature shows a relationship between NAFLD and insulin resistance.24 It has also been proposed that visceral fat may be the main factor contributing to HS in conditions of insulin resistance.23 The present study did not demonstrate a correlation between HOMA and percent hepatic, visceral, or subcutaneous fat.
Considering obese and superobese groups, no statistically significant difference in percent hepatic fat between groups was detected, with a difference observed only for visceral and subcutaneous fat.
This study did not find a difference in mean abdominal circumference between obese subjects with and without HS. These findings are different from those reported by Duarte & Silva,20 who found a significant difference in this result in a study of obese children/adolescents. There was a positive correlation for subcutaneous fat, a moderate correlation for visceral fat, and none for percent hepatic fat.
Awai et al.,31 in a systematic review, observed that the presence of hepatic fat determined by US in a little more than half the children (48–56%) was confirmed by NMR, and observed that the resonance findings were strongly correlated (r=0.88) with the histological grade of steatosis in children with NAFLD.
This study had some limitations, such as small sampling, and it was performed in a reference center for obesity, included a large proportion of super-obese individuals, thus representing a high-risk cohort for NAFLD, which could be a selection bias. A national multicenter study would be needed for external validation for the Brazilian population.
In conclusion, the results observed show that the 28% frequency of NAFLD determined by NMR as the diagnostic tool was lower than the values reported in the international literature. Among the parameters studied, the best factors that may have been associated with HS were triglycerides, AST, ALT, AST/ALT ratio, male gender, acanthosis nigricans, and elevated z-score (higher than 3) for BMI. The authors wish to call more attention to the value of these parameters of physical and laboratory examination when assessing obese patients, since they can help identify HS, thus permitting an early diagnosis. This study confirmed that NMR is an imaging exam that can be applied to children and adolescents, thus representing an effective noninvasive tool for the diagnosis of HS in this age range. However, further national multicenter studies with longitudinal design are needed for a better analysis of the correlation between HS and its risk factors, as well as its consequences.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Benetolo PO, Fernandes MI, Ciampo IR, Elias-Junior J, Sawamura R. Evaluation of nonalcoholic fatty liver disease using magnetic resonance in obese children and adolescents. J Pediatr (Rio J). 2019;95:34–40.