Premature newborns are considered at risk for motor development deficits, leading to the need for monitoring in early life. The aim of this study was to systematically review the literature about gross motor development of preterm infants, assessed by the Alberta Infant Motor Scale (AIMS) to identify the main outcomes in development.
Data sourceSystematic review of studies published from 2006 to 2015, indexed in Pubmed, Scielo, Lilacs, and Medline databases in English and Portuguese. The search strategy included the keywords: Alberta Infant Motor Scale, prematurity, preterm, motor development, postural control, and follow-up.
Data summaryA total of 101 articles were identified and 23 were selected, according to the inclusion criteria. The ages of the children assessed in the studies varied, including the first 6 months up to 15 or 18 months of corrected age. The percentage variation in motor delay was identified in the motor outcome descriptions of ten studies, ranging from 4% to 53%, depending on the age when the infant was assessed. The studies show significant differences in the motor development of preterm and full-term infants, with a description of lower gross scores in the AIMS results of preterm infants.
ConclusionsIt is essential that the follow-up services of at-risk infants have assessment strategies and monitoring of gross motor development of preterm infants; AIMS is an assessment tool indicated to identify atypical motor development in this population.
Recém-nascidos prematuros são considerados de risco para déficits no desenvolvimento motor, ocasionando a necessidade de acompanhamento nos primeiros anos de vida. O objetivo do presente estudo é revisar de forma sistemática as publicações que abordam o desenvolvimento motor amplo de crianças nascidas prematuras, avaliadas por meio da Alberta Infant Motor Scale (AIMS), de modo à apontar os principais desfechos motores.
Fontes dos dadosRevisão sistemática das publicações do período de 2006 a 2015, indexadas nas bases de dados Pubmed, Scielo, Lilacs e Medline, nos idiomas inglês e português. A estratégia de busca incluiu palavras-chaves: prematuro, pré-termo, prematuridade, desenvolvimento motor, controle postural, seguimento, Alberta Infant Motor Scale, prematurity, pre-term, motor development, postural control and follow-up.
Síntese dos dadosForam identificados 101 artigos e selecionados 23, conforme critérios de inclusão. As idades das crianças avaliadas nos estudos incluíram os primeiros 6 meses até os 15 ou 18 meses de idade corrigida. Variado percentual de atraso motor foi identificado na descrição dos desfechos motores de 10 estudos, indo de 4 a 53%, dependendo da idade em que o bebê foi avaliado. Os estudos apontam diferenças significativas no desenvolvimento motor de prematuros e crianças nascidas a termo, com descrição de escores brutos mais baixos nos resultados da AIMS de crianças prematuras.
ConclusõesÉ fundamental que os serviços de follow-up de bebês de risco apresentem estratégias de avaliação e acompanhamento do desenvolvimento motor amplo de prematuros, sendo a AIMS uma ferramenta de avaliação indicada para identificar comportamentos motores atípicos nessa população.
Advances in clinical management, including the use of pediatric mechanical ventilators, surfactants, and prenatal corticosteroids, are factors that have greatly contributed to improve survival of preterm and at-risk babies in recent decades.1 Although the mortality rate has dramatically improved over the past decades, preterm newborns remain vulnerable to many complications, including neurological insult and long-term growth and development deficits,2 resulting in the necessity for a much stricter monitoring than in the past.3
As birth weight and gestational age decrease, and in cases where there is an association of adverse biological conditions, such as grade III and IV peri-intraventricular hemorrhage, periventricular leukomalacia, prolonged mechanical ventilation, stage III retinopathy of prematurity or bronchopulmonary dysplasia, the risk of neurodevelopmental abnormalities increases.4 Particularly, infants born at less than 32 weeks of gestational age and weighing less than 1500g have a high biological risk condition for development.5
Although transient neurological abnormalities occur in 40–80% of cases, disappearing in the second year of life, severe and definitive neurosensory sequelae, such as visual and auditory deficiency and cerebral palsy, are detected in 4–20% of extremely low-weight preterm infants.5–7 Significant developmental delays are also evident in 16% of the cases,7 demonstrating a significant correlation between developmental delay and preterm birth.8
In this sense, carrying out periodic evaluations of each child's motor development (MD) progress is essential for the identification of deficits, thus facilitating referral to early intervention programs.3,8 Although there is no homogeneity among the several studies regarding the best method for evaluating development, the importance of early identification, i.e., within the child's first year of life, is a consensus.5,9 Among the assessment tools used to monitor alterations in MD and differentiate atypical motor behaviors, the Alberta Infant Motor Scale (AIMS) is highlighted as a valid and reliable tool for evaluating at-risk infants,10 demonstrating unique characteristics regarding preterm infants’ quality of movement at an early age.11,12 In contrast with the traditional neurological examination, the scale emphasizes functional capacities and the quality of movement,13 offering up-to-date normative reference values.14 AIMS was validated for the Brazilian pediatric population, resulting in a Brazilian Portuguese version,15 and new standards were established to best represent this.16 It has high sensitivity, specificity, and accuracy to detect motor deficits, being indicated in the follow-up of preterm children's MD in the first 18 months of life.17
No systematic reviews that addressed MD outcomes in preterm infants evaluated by AIMS, establishing a comparative analysis with children born at term, were retrieved. Considering the importance of the diagnosis and early intervention of abnormalities for the development of this at-risk population, this article aimed to systematically review the publications that address the gross motor development of premature infants, evaluated through AIMS, to identify the main motor outcomes in relation to children born at term, aged 0–18 months of corrected age (CoA).
MethodSource of dataA systematic review of articles published in the last 10 years and available in the following databases: US National Library of Medicine National Institutes of Health (PubMed), Scientific Electronic Library Online (SciELO), Latin American and Caribbean Health Sciences (LILACS), and National Library of Medicine United States (MEDLINE) was carried out. The search strategy included the combination of the following keywords in Portuguese: prematuro, pré-termo, prematuridade, desenvolvimento motor amplo, controle postural, seguimento. It also included the following words in English: preterm, prematurity, gross motor development, postural control, Alberta Infant Motor Scale, follow-up. The words were always combined using the term AND. A similar search was performed in all databases. The keywords were selected based on the search for Decs/MeSH terms (LILACS and SciELO).
Selection criteriaStudies were included when they met the following criteria: (1) Original articles involving the observational study of MD of preterm infants, aged 0–18 months of CoA, published in the last ten years (January 01, 2006 to December 31, 2015). The CoA represents the adjustment of the chronological age according to the degree of prematurity, that is, the weeks that were lacking for the gestational age to reach 40 weeks are subtracted from the preterm infant's chronological age.6 (2) Studies with one of the following designs: cohort study (prospective or retrospective), cross-sectional study, control–case study; (3) studies that used AIMS as a tool for assessing motor development; (4) studies published only in Portuguese or English.
In the present study, prematurity was defined according to the Shapiro–Mendoza and Lackritz classification18: late prematurity (34 weeks completed to 36 weeks and 6 days of gestational age), moderate prematurity (32 weeks to 33 weeks and 6 days of gestational age), and extreme prematurity (23:31 weeks and 6 days of gestational age).
All studies that met the inclusion criteria were submitted to data extraction and critical evaluation process. The main characteristics were summarized following a data extraction model consisting of: author/local; method, and sample; gestational age and age at the evaluations; main results; associated risk factors; and strengths and limitations.
Data synthesis/analysisThe search strategy resulted in a total of 101 listed titles, of which 23 were selected for the review. After reading the title and the abstract, 78 articles were excluded, based on the inclusion criteria. The 23 selected articles were included in the review and the results were descriptively analyzed.
Results and discussionThe characteristics of the studies are described in Table 1.
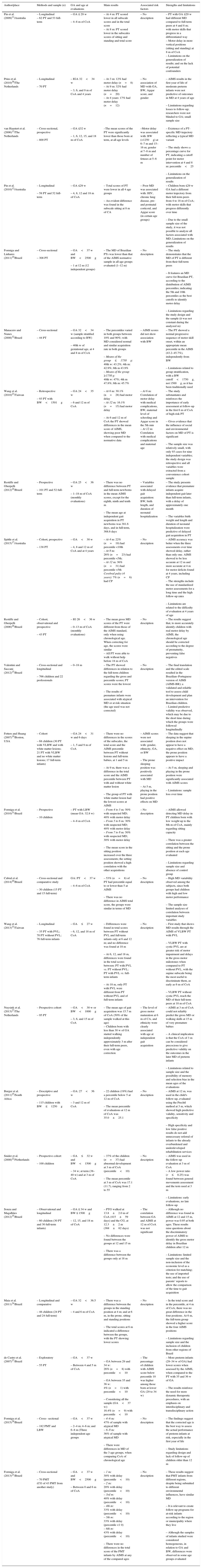
Characteristics of studies involving the motor development of at-risk preterm infants assessed by the AIMS.
| Author/place | Methods and sample (n) | GA and age at evaluations | Main results | Associated risk factors | Strengths and limitations |
|---|---|---|---|---|---|
| Pin et al. (2009)11/Australia | – Longitudinal – 62 PT and 53 full-term | – GA ≤ 29 w – 4–8 m of CoA | – At 4 m: PT scored lower in all subscale scores and in the total score – At 8 m: PT scored lower in the subscales scores of sitting and standing and total score | – No description | – PT with GA ≤29 w had different MD compared to full-term peers at 4 and 8 m; with motor skills that progress in a differentiated way – Motor delay in more vertical positions (sitting and standing) at 8 m of CoA – Limitations on the generalization of results; and on the lack of potential confounders |
| Prins et al. (2010)36/The Netherlands | – Longitudinal – 70 PT | – IGA 32<34 w – 3, 6, and 9 m of CoA and 4 years | – At 3 m: 12% had motor delay (n=8) – At 9 m: 32% had motor delay (n=20) – At 4 years: 17% had motor delay (n=12) | – No association of MD with GA, BW, Apgar score, and gender | – AIMS results in the first year of life of moderate preterm infants were not predictive of outcomes in MD at 4 years of age – Limitations regarding losses to follow-up; researchers were not blinded to GA; small sample size |
| van Haastert et al. (2006)13/The Netherlands | – Cross-sectional, prospective – 800 PT | – GA ≤32 w – 1, 6, 12, 15, and 18 m of CoA | –The mean scores of the PT were significantly lower than those born at term, at all age levels | –Motor delay was associated with: BW (<1250g) at 6–7 m and 15–16 m; gender at 7–8 m and number of fetuses at 5–6 m | – Existence of a PT-specific MD trajectory reflecting a typical MD variant – The study shows a percentage curve for PT, indicating a cutoff point for motor intervention at 4 and 8 m: percentile<25 – Limitations on the generalization of results |
| Pin et al. (2010)12/Australia | – Longitudinal – 58 PT and 52 full-term | – GA ≤29 w – 4, 8, 12 and 18 m of CoA | – Total scores of PT were lower in all 4 age groups – An evident difference was found in the subscale sitting at 8 m of CA | – Poor MD was associated with: HIV, chronic lung disease, pre- and postnatal corticoid, and Apgar score (in certain age groups) | – Children born ≤29 w GA had a different motor trajectory from their full-term peers from 4 to 18 m of CoA; with motor skills that progress differently over time – Due to the small sample size of the study, it was not possible to analyze all factors associated with MD; Limitations on the generalization of results |
| Formiga and Linhares (2011)10/Brazil | – Cross-sectional – 308 PT | – GA<37 w and BW<2500g – 1 at 12 m (12 independent groups) | – The MD of Brazilian PTs was lower than that of the AIMS normative sample in all age groups evaluated (1–12 m) | – No description | – The study demonstrates that the MD of PT is different from their full-term peers – It features an MD curve for Brazilian PT, according to the distribution of AIMS percentiles; indicating the 5th and 10th percentiles as the best cutoffs to identify motor delay – Limitations regarding the study design and the sample (it was not constant during the analyzed m) |
| Manacero and Nunes (2008)32/Brazil | – Cross-sectional – 44 PT | – GA 32<34 w (sample stratified according to BW) – 40th w of gestational age, at 4 and 8 m of CoA | – The percentiles varied in both groups between 10% and 90%; with MD considered normal and similar acquisition rate in both groups – Means of the group≤1750g: 40th w: 43.2%; 4th m: 42.9%; 8th m 43.9% – Means of the group ≥1750 g: 40th w: 47%; 4th m 47.8%; 8th m: 45.7% | – AIMS scores did not show association with BW | – The PT showed a normal progressive sequence of motor skill onset, within an appropriate mean percentile in the AIMS (43.2–45.7%), independently from BW – Limitations related to group stratification, with a BW cutoff<1750g, not 1500g, as it has been traditionally used |
| Wang et al. (2010)23/Taiwan | – Retrospective – 93 PT with BW<1501g | – GA 24<35 w – 6 and 12 m of CoA | – At 6 m: 30.1% (n=28) had motor delay – At 12 m: 16.1% (n=15) had motor delay – At 6 and 12 m of CoA the PT showed differences in the mean score of AIMS, showing poor MD when compared to the normative data | – At 6 m: Correlation of motor delay with medical complications, BW, maternal level of schooling and Apgar score in the 5th min – At 12 m: Correlation with medical complications and maternal age | – The study substantiates and reinforces the importance of early assessment at follow-up in the first 6 m of CoA of high-risk PT – Gives evidence that the influence of social and environmental factors on MD of PT is significant – The sample size was relatively small, with only 93 cases for nine independent variables; the study design was retrospective and all variables were extracted from a convenience cohort sample |
| Restiffe and Gherpelli (2012)24/Brazil | – Prospective – 101 PT and 52 full-term | – GA 25<36 w – 1–18 m of CoA (monthly evaluations) | – There was no difference between PT and full-term newborns in the mean AIMS scores, except for the eighth, ninth and tenth m – The mean age at independent gait acquisition in PT newborns was 381.6 days, and in full-term, 368.6 days | – Variables associated with delayed gait acquisition: BW, birth length, and duration of neonatal hospitalization | – The study presents evidence that premature infants acquire independent gait later than full-term infants, with a delay of approximately one month – The variables birth weight and length and duration of neonatal hospitalization were predictive of delayed gait acquisition in PT |
| Spittle et al. (2015)17/Australia | – Cohort, prospective – 138 PT | – GA<30 w – 4, 8 and 12 m of CoA and at 4 years | – At 4 m: 22% (n=19) had percentile <10th – At 8 m: 26% (n=23) had percentile <5th; – At 12 m: 36% (n=31) had percentile <5th Cerebral palsy (4 years): 7% (n=6) had CP | – No description | – AIMS accuracy was better when the three assessments over time showed delay, rather than only one. AIMS showed to be less accurate at 12 m and more accurate at 4 m for motor deficits found at 4 years, including CP – The strengths include the use of standardized motor assessments for a long time and the high follow-up rates – Limitations are related to the difficulty of evaluation at 4 years of age |
| Restiffe and Gherpelli (2006)26/Brazil | – Cohort, observational and prospective – 43 PT | – IG 26<36 w – 0–13 m of CoA (monthly evaluations) | – The mean gross MD scores of the PT were different from those of the AIMS standard, only when using chronological age. When correcting for age, the scores were similar – All PT were able to walk without help before 18 m of CoA | – No description | – The results suggest that, to more accurately identify children with real motor delay by AIMS, the chronological age should be corrected according to the degree of prematurity, preventing false negatives |
| Valentini and Saccani (2012)15/Brazil | – Cross-sectional and longitudinal – 766 children and 22 professionals | – 0–18 m | – The PT showed differences in relation to the full-term children regarding the gross and percentile scores; PT scores were the lowest – The results of premature infants were associated with atypical MD or at risk situation (the age used was not corrected) | – No description | – The final translation and the edited scale resulted in the Brazilian–Portuguese version of AIMS (AIMS-BR); a validated and reliable tool to assess child development and plan an intervention for Brazilian children. – Limited predictive validity was observed, which may be due to the short time during which the groups were followed longitudinally |
| Fetters and Huang (2007)19/Boston, USA | – Cohort – 68 children (30 PT with VLBW and with white matter lesions; 21 PT with VLBW and no white matter lesions; 17 full-term infants) | – GA 24<31 w and 6 days – 1, 5 and 9 m of CoA | – There were no differences in the scores of the subscales, the total score and the AIMS percentile between PT without lesions and full-term babies, at 1 and 5 m – At 9 m, there was a difference in the total score and the AIMS percentile between PT with and without white matter lesion – The group of PT with white matter lesion had the lowest scores at AIMS | – AIMS scores were not associated with: gender, ethnicity, GA, or BW – The prone sleeping position was positively associated with MD – At 5 m, playing in the prone position had positive effects on MD | – The data suggest that sleeping in the supine position does not appear to have a negative effect on MD; the prone position appears to have a positive impact – At 5 m, sleeping and playing in the prone position were significantly associated with AIMS scores – Limitations: sample loss over time |
| Formiga et al. (2010)31/Brazil | – Prospective – 10 children | – PT with LBW (mean GA: 32.8 w) – 4–8 m of CoA | – From 4 to 5 m: 50% with suspected MD; 40% with motor delay – From 5 to 6 m: 30% with suspected MD; 40% with motor delay – From 7 to 8 m: 50% with suspected MD; 30% with motor delay – The mean score in the sitting position increased over the three assessments; the setting position showed a high correlation with the other acquisitions | – No description | – AIMS allowed detecting MD delay in PT children born with low weight up to the 8th m of CoA, mainly regarding sitting capacity – There was a greater correlation between the sitting and the prone position at each age evaluated – Limitations regarding sample size and absence of control group |
| Cabral et al. (2014)20/Brazil | – Cross-sectional and comparative study – 30 children (15 PT and 15 full-term) | GA: PT<37 w – 4–6 m of CoA | – 53% (n=8) of PT had percentile equal to or lower than 5 at AIMS – There was no difference in AIMS total score, the groups were similar in terms of MD | – No description | – High MD variability observed among the subjects, since both groups had children with high and low motor performance – The sample size limited analyses of correlation between important study variables |
| Wang et al. (2013)33/Taiwan | – Longitudinal – 35 PT with PVL; 70 PT without PVL; 76 full-term infants | – GA≤27 w – 6, 12, and 18 m of CoA | – Differences were found in total scores between PT without PVL and full-term infants only at 6 and 12 m; and no difference was found at 18 m – At 6, 12, and 18 m, differences were found in the total scores between: PT with PVL vs. PT without PVL; PT with PVL vs. full-term infants – At 18 m, only PT with PVL were different from PT without PVL and of full-term infants | – No description | – First study that shows MD results through the AIMS of VLBW PT with PVL – VLBW PT with cystic PVL are at greater risk of motor impairment and delays in the gross motor milestones when compared to PT without PVL, with the supine subscale being the most useful to discriminate them, as early as 6 m of CoA – VLBW PT without cystic PVL reach the MD of their full-term peers at 18 m of CoA |
| Nuysink et al. (2013)27/The Netherlands | – Prospective cohort – 95 PT | – GA<30 w or BW<1000g – 3, 6, and 15 m of CoA | – The mean age of gait acquisition was 15.7 m of CoA (50% of the sample walked at this age) – Children born with less than 30 w of GA started walking independently approximately 3 m after their full-term peers, even with age correction | – The level of gross motor maturation at 6 m of CA, and ethnicity were clearly associated with age at independent gait acquisition | – AIMS at 3 m of CoA could not reliably predict the gross MD or walking skills at 15 m of very premature babies – A clinical implication is that the CoA of 3 m can be considered precocious to give predictive validity on the outcomes in the later MD of preterm infants – Limitations related to sample size and the possibility of memory and selection bias in the mean ages of the evaluations |
| Burger et al. (2011)25/South Africa | – Descriptive and prospective – 115 children with BW≤1250g | – GA: 27<36 w – 3 and 12 m of CoA | – 22 children (19%) had a percentile below 5 at 12 m of CoA – The mean percentile of evaluations at 12 m of CoA was 35.0±25.1 | – No description | – AIMS at 12 m, was used in the child's follow-up, evaluated using the Prechtl method at 3 m, which showed high predictive validity, sensitivity and specificity – High specificity and low false positive results do not add unnecessary referral of infants to the already overburdened and underdeveloped rehabilitation services |
| Snider et al. (2008)22/Netherlands | – Prospective cohort – 100 children | – GA≤32 w and BW<1500g – 34 w; at term (38–40 w) and at 3 m of CoA | – 37% of the children (n=37) had abnormal development at 3 m of CoA (percentile<10) – The mean percentile at 3 m of CoA was 17.1 (11.7), ranging from 2 to 55 | – No description | – AIMS was used in the follow-up evaluation at 3 m of CoA – A low power ratio (r≤0.25) was found between general movements assessment and the tests used at 3 m – Limitations: early evaluations, no late follow-up |
| Souza and Magalhães (2012)35/Brazil | – Observational and longitudinal – 60 children (30 PT and 30 full-term infants) | – GA ≤ 34 w and BW ≤ 1500g – 12, 15, and 18 m of CoA | – PTG walked at 13.8±2.0 m of CoA (415±59 days) and the CG, at 12.3±2 m (368±62 days) – No differences were found between the groups at 12 and 15 m – There was a difference between the groups only at 18 m | – The correlation between GA and AIMS at 12 m of CoA was not significant | – Although no difference was found in AIMS at 12 and 15 m, power was 0.95 at both ages. These results raise questions about the discriminatory power of AIMS to identify the gross motor delay in Brazilian children after 12 m – Limitations: limited sample size and the non-inclusion of the economic level as a criterion for matching; the use of imported tests; and the use of parents’ reports to allow the comparison of the time to gait acquisition |
| Maia et al. (2011)34/Brazil | – Longitudinal and comparative – 48 children (24 PT and 24 full-term) | – GA 32<36.5 w – 4 and 6 m of CoA | – There was a difference between the groups in the standing position at 4 m; and at 6 m, in the prone, sitting and standing positions – The total scores at 6 m indicated a difference between the groups, with the PT showing lower scores | – No description | – In the total score and in the percentile, at 4 m of CoA, there was no great difference in the four positions. At 6 m, the full-term group showed a higher score in the four AIMS positions – Limitations regarding sample size and the inclusion of children from other regions of Brazil |
| de Castro et al. (2007)37/Brazil | – Exploratory – 55 PT | – GA<37 w – Between 4 and 5 m of CoA | – GA between 29 and 34 w: 26% (n=8) with percentile<10 – GA between 35 and 36 w: 4% (n=1) with percentile<10 – Considering all the sample (GA<37 w): 16.4% (n=9) with percentile<10 | – The percentage of children with AIMS score below percentile 10 was higher among those born at lower GA (29 to 34 w) | – More preterm infants (29–34 w of GA) had lower scores when assessed by the AIMS, when compared to the PT with 35 and 36 w of GA – The results reinforce the need for more dynamic therapeutic procedures, with an emphasis on interdisciplinary and transdisciplinary action |
| Formiga et al. (2015)38/Brazil | – Cross– sectional – 182 PMT and LBW | – GA<37 w – 2–4 m; 4–6 m; and 6–8 m (Three independent age groups | – 4–6 m: 47% of sample with atypical MD – 6–8 m: 36% of sample with atypical MD – There were differences in MD of the 3 age groups, when comparing CoA of chronological age | – No description | – The findings suggest that the corrected age is the best way to assess the actual performance of preterm infants at risk, especially in the first year of life – Study limitations regarding design and lack of follow-up of children older than 12 m |
| Formiga et al. (2013)21/Brazil | – Cross-sectional – 70 PMT (CG of 43 PMT from another study) | – GA<37 w and BW<2500g – Between 0 and 6 m of CoA | – 1 m: 30% with delay (percentile<10) – 2 m: 20% with delay (percentile<10) – 3rd m: 40% with delay (percentile<10) – 4th m: 33% with delay (percentile<10) – 5th m: 33% with delay (percentile <1 0) – 6th m: 43% with delay (percentile<10) – There were no differences in the total score of the PMT infants by AIMS at any of the compared ages | – No description | – These results suggest that PMT infants from different regions, despite being submitted to different environmental influences, have similar MD – It is relevant to create follow-up programs for at-risk infants according to the region or municipality where they live – Although the samples of infants studied were considered homogeneous, in relation to GA and BW, differences were observed in some age groups evaluated |
AIMS, Alberta Infant Motor Scale; w, weeks; m, months; GA, gestational age; BW, birth weight; PT, preterm infant; MD, motor development; CoA, corrected age; CP, cerebral palsy; VLBW, very low birth weight; LBW, low birth weight; PVL, periventricular leukomalacia; CG, control group; PTG, preterm group.
It can be observed that the period of 2010–2015 had the highest number of publications on the subject, except for the year 2014, in which no article was published with this approach. In the analysis of study locations, Brazil was the most prevalent, with 12 publications. The Netherlands and Australia came in second place, with seven studies.
It can be observed that children's ages varied in the studies. Of the 23 selected articles, six addressed the evaluation of MD in age groups involving the first 6 months to 15 or 18 months, whereas four involved the first 6 months up to 12 or 13 months of CoA. A single study addressed the analysis after 12 months of CoA up to 18 months. Only two articles included the long-term study of MD, with a follow-up until the age of 4 years, applying an appropriate scale for this age group. Conversely, the lack of follow-up in children after 12 months of CoA was observed in some studies.19–22 One of them involved evaluations at 3 months of CoA; four included evaluations up to 5 or 6 months, and five until 8 or 9 months of CoA.11,19–22
Regarding the gestational ages involved in the studies, it was observed that almost half of the publications (n=11) comprised a sample of preterm infants, both moderate and extreme, of which eight were of extreme premature infants only. Conversely, nine studies included heterogeneous samples regarding the classification of prematurity, since they involved the three types in the same sample (late, moderate, and extreme).21,23–25 One study did not clarify the GA range of the evaluated children.15
As for the designs, it was observed that most studies had a prospective cohort, totaling 14 articles; followed by the cross-sectional design, with eight publications. Most of the analyzed studies (n=15) had a sample consisting only of preterm infants, and did not include a control group comprising infants born at term. For comparative purposes, five of these studies used the AIMS normative sample to identify differences in the gross motor development between the groups.10,13,23,26,27 The normative data are based on a population of 2200 infants born at term, aged 0–18 months, from Alberta, Canada.28 Recently, the original AIMS data, collected 20 years ago, were compared to data from a contemporary sample of 650 Canadian children. The current normative values remain appropriate to interpret the total AIMS score, and the original percentiles continue to reflect the contemporary order and age at onset of infant motor skills represented in the AIMS.14
Therefore, AIMS normative data have been widely used nationally and internationally as a measure of clinical outcome and research,10,12,13,17,21,29 although there is concern that AIMS Canadian standards would be inadequate for children of different cultures.29,30 In this sense, the authors of the normative value reassessment study affirmed that, given the stability of the results over a 20-year period and the increase in the ethnic diversity of the contemporary sample, it may not be necessary to investigate international differences.14
In turn, in the Brazilian scenario, the lower percentiles of the Brazilian sample, as described by Saccani and Valentini,29 reinforce the need to use national normative values to categorize children's motor performance. The differences between Brazilian, Canadian, and Greek children, found in that comparative study of three population samples, prevailed up to 15 months of age; a representative portion of the Brazilian sample (34.6%) had lower motor performance than the expected. According to the authors,29 the results may indicate a different trajectory in MD, possibly influenced by sociocultural factors pertinent to child care.
Regarding the main results related to MD when assessed by AIMS, there is a certain heterogeneity in the description of motor outcomes. Only two studies11,12 discussed the differences observed in motor acquisitions on each subscale (prone, supine, sitting, and standing), showing the percentage of preterm children versus children born at term that scored the assessed acquisition. Pin et al.11 described this data in their study of MD of preterm infants born at a gestational age ≤29 weeks and infants born at term, assessed at 4 and 8 months of CoA. At the age of 4 months, all full-term infants were able to play with their hands on the midline, in comparison with 81% of preterm infants; at 8 months of CoA, preterm infants did not progress as much as expected, since many were not able to sit independently (25% vs. 90%).
Pin et al.12 followed-up this cohort until 18 months of CoA, and discussed the differences of each evaluated acquisition. At 12 months of CoA, more full-term infants than preterm infants reached the total score in the sitting subscale (94% vs. 68%); moreover, a larger number of children in the control group were able to perform lateral gait along a piece of furniture in the standing subscale (90% vs. 70%). At 18 months of CoA, almost all full-term children reached the total AIMS score; however, 17 premature children were unable to do so (37% vs. 2%).
Several studies have compared the MD of preterm and full-term children in the first 2 years of life and demonstrated that the former had an inferior motor performance.10–13,23,31 Of the 23 selected articles, 14 observed significant differences in motor performance between preterm and full-term infants; however, the age when the differences are identified varied.
Conversely, two studies20,26 failed to observe a significant difference in the MD of preterm infants versus that of full-term infants when CoA was considered. Restiffe and Gherpelli26 demonstrated that the means of the gross scores of 43 low-risk preterm newborns were similar, after age correction, to the AIMS standards in the different age groups over the 13-month of CoA period. Cabral et al.20 also stated that, when comparing a group of preterm (n=15) with a group of full-term infants (n=15) at 4 and 6 months, there was no significant difference in AIMS total score, as well as in the prone and sitting subscale scores. In these two studies, the methodological characteristics, such as the small sample size20 and the inclusion of preterm infants with low risk for neurological lesion and neuromotor disorders,26 may have influenced the described findings.
Corroborating these findings, Manacero and Nunes32 stated that the motor performance of preterm infants without neurological disorders, evaluated at the 40th week of gestational age, in the fourth and eighth months of CoA, was normal by the AIMS scale. The preterm infants showed a normal progressive sequence of motor skill onset in all assessed postures (prone, supine, sitting, and standing), expressed by the mean percentile of 43.2–45.7%, considered adequate in the AIMS.32
Among the studies that highlighted significant differences in motor performance between preterm and full-term infants, a critical analysis was performed based on the age at the assessments. At 4 months of CoA, three studies showed differences.10–12 However, only two of these established this comparative analysis involving extreme preterm infants, with a control group of full-term infants.
Pin et al.11 described lower scores in all subscales and in the total scale score, noting that preterm infants with gestational age ≤29 weeks have motor skills that progress differently from their full-term peers in the four postures assessed at 4 months of CoA. Pin et al.12 also reported lower total AIMS scores for preterm infants in this age group when compared with the control group, demonstrating lower scores in the supine, prone, and sitting subscales.
Differences in the MD of preterm and full-term infants were also described at 6 months of CoA. Five studies showed that preterm infants had lower total AIMS scores in this age group when compared to those born at term.10,13,23,33,34 In the study by Wang et al.,33 preterm infants scored significantly lower than the control group in all subscales. However, although they showed lower scores at 6 months of CoA, they reached the MD of their full-term peers over the 18 months of CoA. Maia et al.34 also described lower scores in the group of preterm infants in the four positions assessed by the AIMS.
The differences found between preterm and full-term infants at 4 and 6 months of CoA support and reinforce the importance of early assessment in the follow-up as early as in the first 6 months of CoA, especially in high-risk preterm infants.23 At 8 months of CoA, four studies found differences in the AIMS total score between preterm infants and their full-term peers.10–12,24 Of these, two studies, by Pin et al.11,12 involved the evaluation of the motor performance of extreme preterm infants (those with gestational age ≤29 weeks). The authors11,12 described significantly lower total scores than those observed for the controls, as well as lower scores in the sitting and standing subscales, demonstrating a motor delay in more vertical postures in this age group.
Five studies found differences in MD at 12 months of CoA, indicating lower total scores in preterm children.10,12,13,23,33 In three of these studies, extreme prematurity was the assessed group, indicating there is a specific trajectory of MD that reflects a variant of the typical MD in this population.12,13,33 Pin et al.12 found lower scores in the prone, sitting, and standing subscales. Wang et al.33 also stated that preterm infants differed from their full-term peers in the standing subscale in this age group.
At 18 months of CoA, three studies described differences in motor performance between preterm infants and their peers.12,13,35 Pin et al.12 indicated significantly lower scores in the extreme preterm group, also in this age group, in the prone, sitting and standing subscales. For the authors,12 the results described at 12 and 18 months of CoA reflect the lack of mature trunk control in extreme preterm infants, which affects the range of more complex motor skills, not only in the position of four support points and in the acquisition of reciprocal crawling, but also in the sitting and standing positions.
Regarding independent gait, three studies presented evidence that preterm infants acquire this ability at a later age than those full-term.24,27,35 Restiffe and Gherpelli24 and Souza and Magalhães35 reported that there is a delay of approximately one month, with a mean of 13.8 months of CoA for preterm infants and 12.3 months for the control group. In turn, Nuysink et al.27 reported that children born at less than 30 weeks of gestational age start walking independently approximately three months after their full-term peers, even with age correction. In this study,27 the mean age of independent gait acquisition for preterm infants was 15.7 months of CoA.
For Pin et al.,11,12 the significant differences in motor performance between preterm and full-term infants over the 18 months of CoA appear to be related to the delay in motor skill development in the more vertical positions, such as sitting and standing, positions that require greater muscle strength and antigravity motor control. Signs of trunk dystonia or imbalance between flexor and extensor forces were found more frequently in preterm infants and over time, possibly because the demand for postural control made the inadequate flexor control in the trunk more apparent, leading to a delay in the capacity to maintain sitting and standing postures, affecting the ability to walk independently.
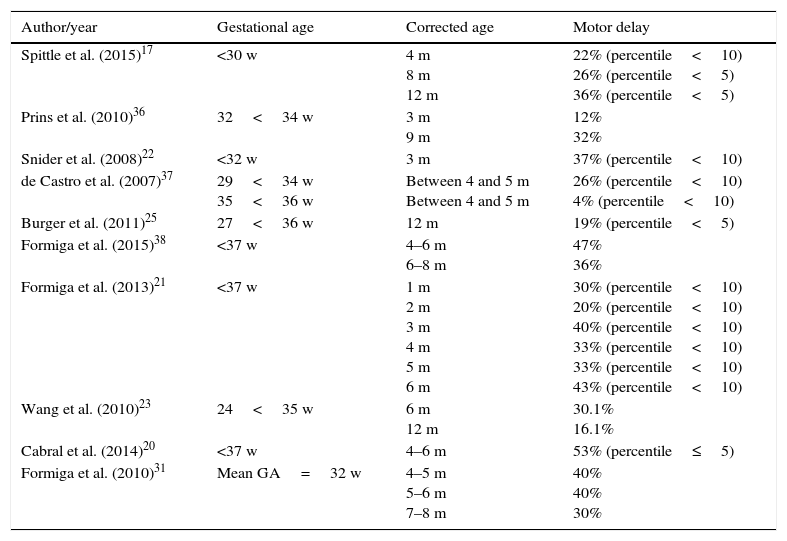
Although it is important to identify differences in the motor performance of preterm infants in different age groups, it is also important to identify the motor delay percentage in this population. Therefore, some studies17,20–23,25,31,36–38 presented the motor outcomes based on the AIMS percentile, describing the percentage of delay found in preterm infants (Table 2).
Motor delay percentage in preterm children assessed by AIMS.
| Author/year | Gestational age | Corrected age | Motor delay |
|---|---|---|---|
| Spittle et al. (2015)17 | <30 w | 4 m 8 m 12 m | 22% (percentile<10) 26% (percentile<5) 36% (percentile<5) |
| Prins et al. (2010)36 | 32<34 w | 3 m 9 m | 12% 32% |
| Snider et al. (2008)22 | <32 w | 3 m | 37% (percentile<10) |
| de Castro et al. (2007)37 | 29<34 w 35<36 w | Between 4 and 5 m Between 4 and 5 m | 26% (percentile<10) 4% (percentile<10) |
| Burger et al. (2011)25 | 27<36 w | 12 m | 19% (percentile<5) |
| Formiga et al. (2015)38 | <37 w | 4–6 m 6–8 m | 47% 36% |
| Formiga et al. (2013)21 | <37 w | 1 m 2 m 3 m 4 m 5 m 6 m | 30% (percentile<10) 20% (percentile<10) 40% (percentile<10) 33% (percentile<10) 33% (percentile<10) 43% (percentile<10) |
| Wang et al. (2010)23 | 24<35 w | 6 m 12 m | 30.1% 16.1% |
| Cabral et al. (2014)20 | <37 w | 4–6 m | 53% (percentile≤5) |
| Formiga et al. (2010)31 | Mean GA=32 w | 4–5 m 5–6 m 7–8 m | 40% 40% 30% |
AIMS, Alberta Infant Motor Scale; w, weeks; m, months; GA, gestational age.
Ten studies that used this approach were retrieved, showing a varied percentage of evaluated motor delay, ranging from 4% to 53%, depending at what age the baby was evaluated in the first year of life. It is observed that four studies defined the gestational age of the sample to include only moderate and/or extreme premature infants17,22,36,37; the others involved the outcome analysis, considering a broader range of gestational age. Extreme preterm infants had a 22–37% delay at the corrected ages of 3–4 months,17,22 and 26–36% at the ages of 8 and 12 months.17 Moderately preterm infants appear to have better outcomes at 3 months, with a 12% motor delay; however, this fact does not seem to be confirmed when the age of 9 months is assessed (32%).36
Among the variables associated with motor delay, it was observed that lower birth weight was associated with lower gross AIMS score in three studies.13,23,24 In turn, some studies did not find this association.19,32,36 Other variables associated with poor motor outcome refer to PIVH (peri-intraventricular hemorrhage), chronic lung disease, pre- and post-natal corticosteroids, and lower Apgar score.12 Ethnicity, low maternal schooling, and young maternal age are social and environmental factors that also have a significant influence on preterm MD.23,27
Among the strengths of the assessed studies, the evidence that the performance of preterm infants in AIMS is different from that of their full-term peers, being significantly lower at certain ages during the first 18 months of life, is noteworthy.10,12,13,15,23,26,33 The limitations observed reflect the difficulties of the studies regarding the generalization of the results, the losses to follow-up, and the limited sample sizes. The lack of methodological quality assessment of the included studies was also a limitation of the present review. For future studies, a systematic review of publications involving standardized assessments of the motor performance of preterm infants in the pre-school and school years is suggested, as there is a concern that premature infants may be more vulnerable when entering school age.
Motor delay, as well as the differences in MD in preterm infants, are associated with the biological factors involved, such as gestational age, birth weight, cerebral white matter lesion,39 and associated morbidities.40 Additionally, adverse sociocultural conditions can aggravate the children's risk,29 with a poor prognosis for their development. Preterm birth challenges motor control development, as the child starts the extrauterine life with immature and more vulnerable central and sensory-motor systems. As a result, one of the most frequent sequelae is the lack of adequate postural control during motor activities.41 Thus, healthcare professionals must be attentive to the different risk factors and the MD of the preterm infant, in order to detect deficits early, referring the child and the family for early intervention.
ConclusionMost of the analyzed studies sought to identify differences in gross motor development through the AIMS between preterm and full-term infants at different ages of evaluation. The studies indicate an inferior motor performance of preterm infants in the first 18 months of CoA, either through the comparative analysis with the Canadian AIMS data or with the data obtained from a control group, consisting of children born at term. Depending on the age of the assessment and the sample characteristics, a variable percentage of motor delay was identified in preterm infants. Low maternal schooling and young maternal age, as well as factors related to prematurity, such as lower birth weight, PIVH, and chronic lung disease, were associated with atypical motor outcome in the AIMS.
Thus, children born prematurely and in unfavorable environmental and social conditions may be more vulnerable to motor problems at a very early age. Therefore, it is crucial that the follow-up services of at-risk infants have strategies for the evaluation and follow-up of the gross motor development of preterm infants, from the discharge from the neonatal ICU to the first 2 years of the child's life; AIMS is a tool indicated to identify atypical motor behaviors in this population.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Fuentefria RN, Silveira RC, Procianoy RS. Motor development of preterm infants assessed by the Alberta Infant Motor Scale: systematic review article. J Pediatr (Rio J). 2017;93:328–42.