To compare the short-term efficacy of surfactant administration by laryngeal mask airway versus endotracheal tube.
MethodsPreterm infants (28–35 weeks of gestational age), weighing 1kg or more, with respiratory distress syndrome, requiring nasal continuous positive airway pressure, with increased respiratory effort and/or fraction of inspired oxygen (FiO2)≥0.40 to maintain oxygen saturation 91–95%, were randomized to receive surfactant by LMA following nCPAP or by ETT following mechanical ventilation (MV). The primary outcome was a clinical response defined as FiO2≤0.30 three hours after surfactant. Secondary outcomes for LMA group were: need of surfactant retreatment during the first 24h, MV requirement, and presence of surfactant in gastric content.
ResultsForty-eight patients were randomized; 26 in the LMA group and 22 in the ETT group. Six of 26 patients (23%) in the LMA group and five of 22 patients (22.7%) in the ETT group did not meet the primary outcome (p=0.977). Fourteen (53.8%) of the LMA patients were not intubated nor ventilated; 12 (46.1%) were ventilated: for surfactant failure (23%), for nCPAP failure (11.5%), and for late complications (11.5%). Groups were similar regarding prenatal status, birth conditions, and adverse events. No significant gastric content was found in 61.5% of the LMA patients. Oxygen and second dose surfactant requirements, arterial/alveolar ratio, and morbidities were similar among groups.
ConclusionsSurfactant administration by LMA showed short-term efficacy, with similar supplementary oxygen need compared to surfactant by ETT, and lower MV requirement. Further studies with larger sample size are necessary to confirm these results.
Comparar a eficácia de curto prazo da administração de surfactante por máscara laríngea em comparação ao tubo endotraqueal.
MétodosNeonatos prematuros (28–35 semanas de idade gestacional), pesando 1kg ou mais, com Síndrome do Desconforto Respiratório, necessitando pressão positiva nasal contínua nas vias aéreas, com aumento do esforço respiratório e/ou fração de oxigênio inspirado (FiO2)≥0,40 para manter a saturação de oxigênio 91–95%, foram randomizados para receber surfactante por ML seguido por nCPAP ou por TE seguido por ventilação mecânica (VM). O resultado clinico primário foi definido como FiO2 ≤ 0,30 três horas após o surfactante. Os resultados secundários do grupo de ML foram: necessidade de segunda dose de surfactante nas primeiras 24 horas, necessidade de VM e presença de surfactante no conteúdo gástrico.
ResultadosQuarenta e oito pacientes foram randomizados; 26 no grupo de ML e 22 no grupo de TE. Seis dentre os 26 pacientes (23%) do grupo de ML e cinco dentre 22 pacientes (22,7%) do grupo de TE não apresentaram o resultado primário (p=0,977). Quatorze (53,8%) dos pacientes do grupo de ML não foram intubados nem ventilados; 12 (46,1%) foram submetidos a VM: por falha do surfactante (23%), por falha da nCPAP (11,5%) e por complicações tardias (11,5%). Os grupos foram semelhantes em relação às condições pré-natais e de nascimento e a ocorrência de eventos adversos. Não foi encontrado conteúdo gástrico significativo em 61,5% dos pacientes do grupo de ML. As necessidades de oxigênio e da segunda dose de surfactante, o índice arterial/alveolar e as morbidades foram semelhantes entre os grupos.
ConclusõesA administração de surfactante por ML mostrou eficácia de curto prazo com necessidade complementar de oxigênio semelhante ao surfactante por TE e menor necessidade de VM. Serão necessários estudos adicionais com tamanho da amostra maior para confirmar esses resultados.
Surfactant treatment in neonatology practice has substantially reduced mortality and improved the prognosis of respiratory distress syndrome (RDS).1,2 Traditionally, surfactant has been given by endotracheal tube (ETT), however, superimposed lung injury from facemask-bag or ETT-bag positive pressure ventilation (PPV) followed by mechanical ventilation (MV) may impair surfactant function and trigger an inflammatory response in the lung, leading to bronchopulmonary dysplasia (BPD).1,3 In the era of noninvasive respiratory support, neonatologists began to search for new techniques that might allow nasal continuous positive airway pressure (nCPAP) and surfactant at the same time, without ETT and MV.4–6
The laryngeal mask airway (LMA) is a supraglottic airway device designed to maintain a seal around the laryngeal inlet to deliver PPV in situations of difficult airway management and anesthesia practice.7–9 For newborns, LMA has shown its potential in a variety of circumstances, mainly in neonatal resuscitation and drug administration.6–10 A few observational studies and a small randomized controlled trial (RCT) have reported the administration of surfactant through LMA.3,6,10 Until now, there has been no published prospective RCT comparing surfactant administration by LMA vs. ETT and MV.
The aim of the present study was to assess if surfactant administration through LMA followed by nCPAP has the same short-term clinical efficacy for RDS treatment as the conventional treatment by ETT followed by MV.
MethodsStudy design and patientsThis prospective single-center RCT (CAAE 00160287000-10 and NCT01173237) was conducted in the neonatal intensive care unit (NICU) of Maternidade UNIMED-BH, in Belo Horizonte, Brazil, between July 2011 and May 2014. The ethics committee of the institution approved the study and written informed consent was obtained from the patient's parents/guardians. Preterm infants were assessed for eligibility and enrolled for randomization if they had the following characteristics: gestational age (GA) at birth between 28–35 weeks and birth weight ≥1kg; <8h of age; requirement of nCPAP, Silverman-Anderson (SA) score greater than four and/or respiratory frequency >60bpm and/or fraction of inspired oxygen (FiO2)≥0.40 to maintain oxygen saturation (SpO2) 91–95%; clinical diagnosis of RDS; typical RDS chest X-ray.11 The exclusion criteria were: GA>35 weeks, major congenital anomalies; previous ETT; Apgar score <3 at 5min; chorioamnionitis, fever and/or rupture of membranes >18h.
Randomization and maskingBy using a table of random numbers, 60 patients were randomized to one of two treatment arms before data collection. Attending neonatologists identified eligible patients according to the inclusion and exclusion criteria after clinical evaluation, umbilical vein catheterization, chest X-ray, arterial blood gas analysis, and assignment of surfactant treatment. After eligibility and enrollment, only the same investigator checked the sequential randomization, performed the LMA or ETT insertion, administered surfactant, and followed all patients for the next six hours. Those responsible for the data analysis were not masked to the treatment group.
ProceduresPrenatal, perinatal, and postnatal patient conditions and laboratory findings (vital signs, SA score, arterial blood gas analysis, Neonatal Infant Pain Scale (NIPS),12 and arterial/alveolar (a/A) ratio) were registered before the procedures. All patients received the same surfactant preparation (poractant-α, 120mg/1.5mL) and the same dose (200mg/kg), by bolus instillation within the first 8h of age. The chest X-ray findings were evaluated before and six hours after surfactant.
Patients randomized to the oral ETT group received premedication (remifentanil and midazolam bolus) and the Viby–Mogensen score was used to evaluate intubation quality.13 Tube placement was verified by chest X-ray before surfactant instillation, which was followed by conventional MV. Patients were extubated as soon as possible (inspiratory pressure less than 20cmH2O, respiratory frequency 30 or less, and FiO2<0.40).
Patients randomized to the LMA group were on nCPAP 5–6cmH2O and received surfactant through a ProSeal™ (Teleflex®, NC, USA) size one LMA (PLMA; Intavent Orthofix – Maidenhead, United Kingdom), inserted by the classical technique described by Brain.7,8 Gastric contents were aspirated and the orogastric tube was removed before LMA insertion. Lidocaine gel was utilized around the LMA cuff to lubricate and to prevent discomfort. After insertion, the proximal end of the airway device was connected to a self-inflated bag for PPV, during one or two minutes, time enough to achieve SpO2>88% and heart rate (HR)>100 beats/min. Afterward, a thin silicone size 6-F catheter (shortened previously to be approximately 1cm bigger than the airway device length) was introduced through the LMA airway tube as a conduit for surfactant administration in two to four aliquots, according to patient tolerance and surfactant reflux, followed by LMA-bag PPV during one or two minutes to obtain SpO2 and HR improvement.14 When full dose of surfactant was administered, LMA was removed and nCPAP restarted. A thin silicone orogastric tube was replaced after LMA withdrawal to measure gastric contents. Any gastric volume aspirated was assumed to be surfactant because patients had not been fed prior to enrollment.
Cardio-respiratory adverse events during both procedures were registered. Just after the procedures, NIPS was recorded again. Likewise, cardio-respiratory signs, nCPAP pressure, and ventilator settings were monitored and registered soon after procedures and at five, 20, 35, and 60min after surfactant and then every 30min until six hours afterwards. Arterial blood gas analysis and a/A ratio were obtained three hours after surfactant. Respiratory support and FiO2 were progressively reduced in the ETT group during the first six hours, based on HR, blood pressure, respiratory effort, SpO2, and blood gas analysis. The CPAP pressure of LMA patients was maintained at 5–6cmH2O and FiO2 was reduced according to the same cardio-respiratory parameters. After the first six hours of study, attending neonatologists made decisions for both groups regarding the need for retreatment and/or MV. A second dose of surfactant was administered six to 33h after the first dose if any infant presented the following: increasing respiratory effort, hemodynamic instability, frequent apneas (≥two/hour), pH<7.20, partial arterial pressure of carbon dioxide (PaCO2)>65mmHg, partial arterial pressure oxygen (PaO2)<50mmHg, SpO2<91%, or FiO2≥0.50.11 LMA patients received a second dose by ETT followed by MV.
OutcomesFiO2≤0.30 three hours after surfactant was the primary outcome since oxygen need reduction results from surfactant response as gas exchange improves. It was also based on current therapy protocols and manufacturer's low-threshold criteria for retreatment that indicate an additional surfactant administration when the FiO2 needed is >0.30 after the first dose.15,16 The sample size was calculated as 30 patients per group to obtain a power of 85% in detecting significant differences (alpha=0.05) in FiO2 between groups.17 Furthermore, an interim analysis was performed when 48 patients were enrolled and randomized. This analysis showed that both groups were equivalent. Therefore, due to ethical reasons, it was decided to interrupt the study since the primary outcome was achieved with 26 patients in LMA group. Indeed, a post hoc analysis showed that 20 patients in each group were sufficient to detect a minimum difference of FiO2 between groups as small as 8% (above or even below; two-tailed) for a beta error of 20% and an alpha error of 5%.17 Secondary outcomes for LMA group were: need of second surfactant dose within 24h of life, MV requirement at any time, and gastric residual volume after surfactant administration >1.5mL. This cut-off point for gastric volume was defined since this volume was more than half the dose for a patient of 1kg. For both groups, other secondary outcomes were: number of surfactant doses, cardio-respiratory events during procedures, mortality, duration of oxygen, nCPAP, MV and hospitalization, the incidence of intraventricular hemorrhage (IVH), pneumothorax, BPD, retinopathy of prematurity (ROP), and early and/or late onset sepsis.
Surfactant treatment failure by the LMA was diagnosed when a second dose was indicated and administered by ETT at any time. In such a case, as insufficient surfactant should have been delivered to the lungs by LMA, the study design established for patient security and higher chance of response, that the second dose should be administered by ETT. CPAP failure was diagnosed in LMA patients treated for RDS when they did not need surfactant retreatment according to radiological and blood gas analysis findings, but MV was indicated due to respiratory worsening.
Statistical analysisData were stored in Microsoft Excel (Microsoft®, WA, USA) spreadsheet and analyzed using Minitab version 16 (Minitab®, PA, USA). For categorical variables, the chi-squared test or Fisher's exact test was used. For quantitative variables, the Ryan–Joiner test was used to check for data normality, followed by parametric Student's t-test or nonparametric Mann–Whitney.17 In all statistical tests, the level of significance was set at p<0.05.
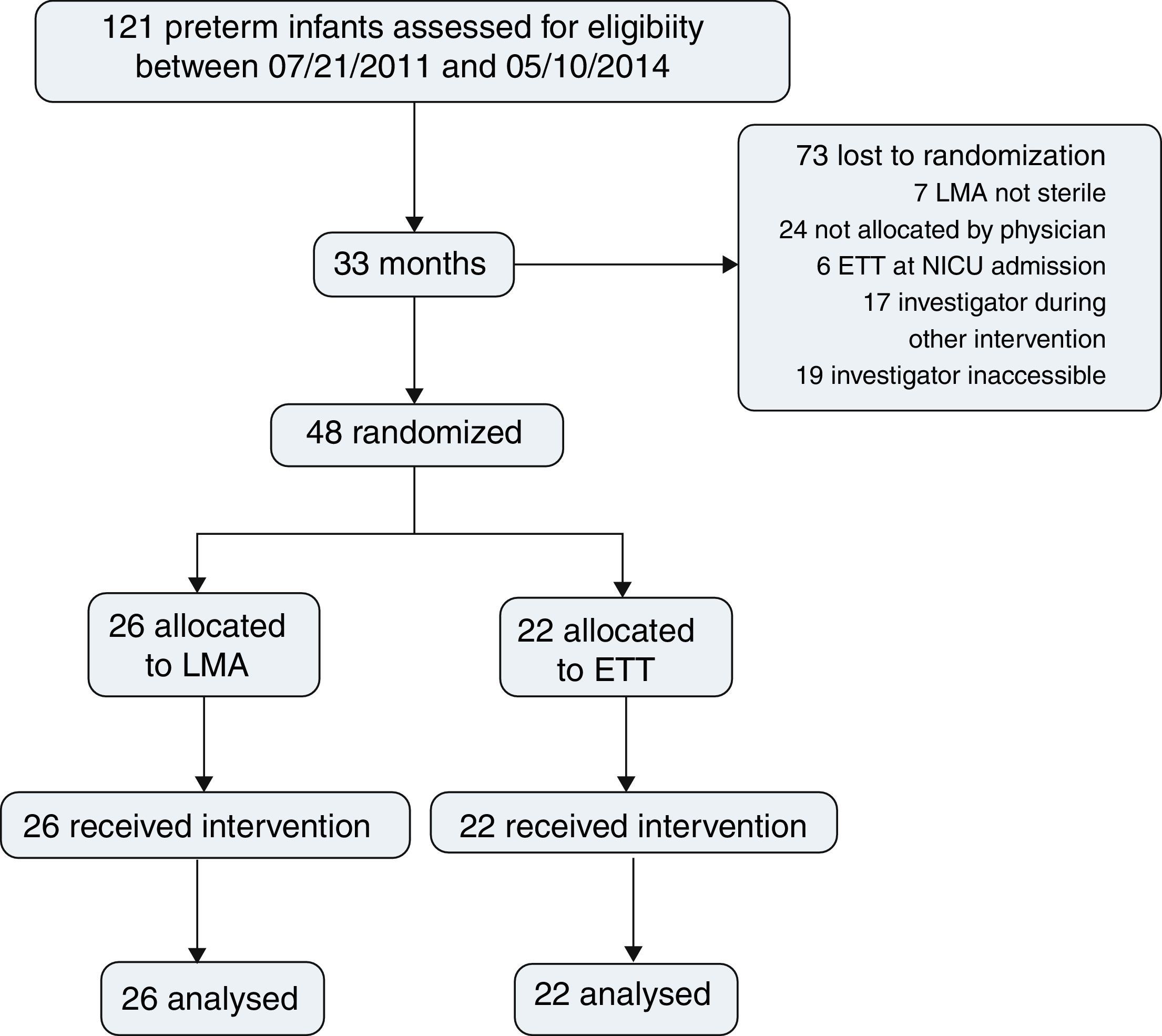
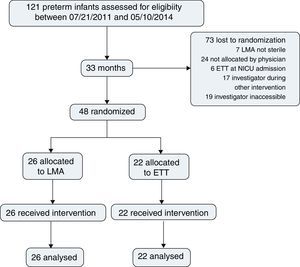
ResultsDuring the study period, 121 preterm infants were born and assessed for eligibility. Surfactant treatment for RDS was indicated for all patients on nCPAP; 48 patients were randomized, 26 for LMA insertion and nCPAP and 22 for ETT and MV; 73 were lost prior to randomization (Fig. 1).
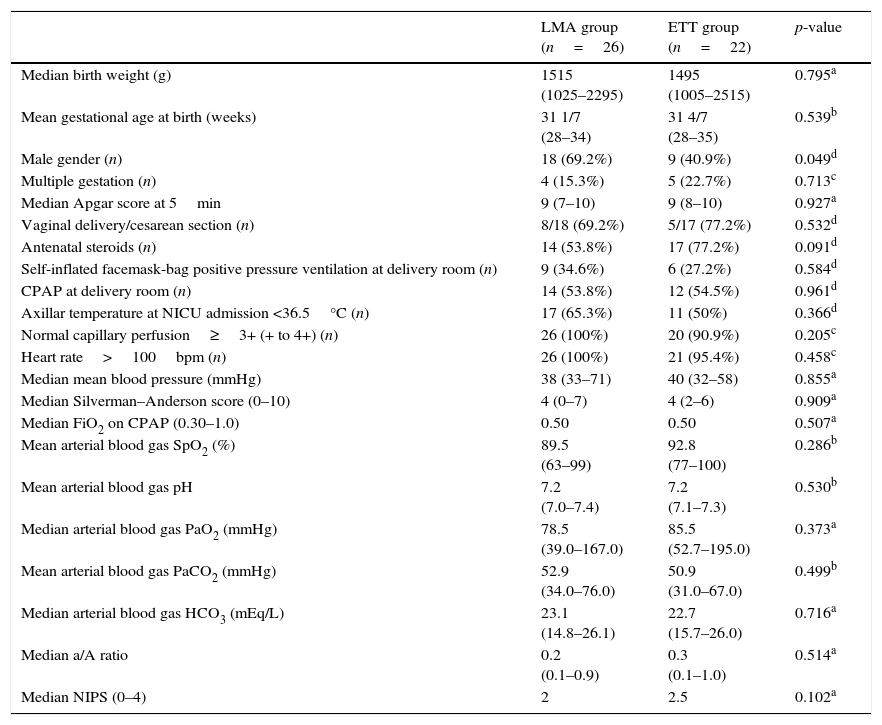
Table 1 presents demographic and clinical characteristics at birth, in addition to pain, cardiovascular, and respiratory support data before LMA insertion or ETT, which were not significantly different.
Demographic and clinical characteristics at birth of patients from the LMA and ETT groups. Pain, cardiovascular, and respiratory support data before LMA insertion or endotracheal intubation.
| LMA group (n=26) | ETT group (n=22) | p-value | |
|---|---|---|---|
| Median birth weight (g) | 1515 (1025–2295) | 1495 (1005–2515) | 0.795a |
| Mean gestational age at birth (weeks) | 31 1/7 (28–34) | 31 4/7 (28–35) | 0.539b |
| Male gender (n) | 18 (69.2%) | 9 (40.9%) | 0.049d |
| Multiple gestation (n) | 4 (15.3%) | 5 (22.7%) | 0.713c |
| Median Apgar score at 5min | 9 (7–10) | 9 (8–10) | 0.927a |
| Vaginal delivery/cesarean section (n) | 8/18 (69.2%) | 5/17 (77.2%) | 0.532d |
| Antenatal steroids (n) | 14 (53.8%) | 17 (77.2%) | 0.091d |
| Self-inflated facemask-bag positive pressure ventilation at delivery room (n) | 9 (34.6%) | 6 (27.2%) | 0.584d |
| CPAP at delivery room (n) | 14 (53.8%) | 12 (54.5%) | 0.961d |
| Axillar temperature at NICU admission <36.5°C (n) | 17 (65.3%) | 11 (50%) | 0.366d |
| Normal capillary perfusion≥3+ (+ to 4+) (n) | 26 (100%) | 20 (90.9%) | 0.205c |
| Heart rate>100bpm (n) | 26 (100%) | 21 (95.4%) | 0.458c |
| Median mean blood pressure (mmHg) | 38 (33–71) | 40 (32–58) | 0.855a |
| Median Silverman–Anderson score (0–10) | 4 (0–7) | 4 (2–6) | 0.909a |
| Median FiO2 on CPAP (0.30–1.0) | 0.50 | 0.50 | 0.507a |
| Mean arterial blood gas SpO2 (%) | 89.5 (63–99) | 92.8 (77–100) | 0.286b |
| Mean arterial blood gas pH | 7.2 (7.0–7.4) | 7.2 (7.1–7.3) | 0.530b |
| Median arterial blood gas PaO2 (mmHg) | 78.5 (39.0–167.0) | 85.5 (52.7–195.0) | 0.373a |
| Mean arterial blood gas PaCO2 (mmHg) | 52.9 (34.0–76.0) | 50.9 (31.0–67.0) | 0.499b |
| Median arterial blood gas HCO3 (mEq/L) | 23.1 (14.8–26.1) | 22.7 (15.7–26.0) | 0.716a |
| Median a/A ratio | 0.2 (0.1–0.9) | 0.3 (0.1–1.0) | 0.514a |
| Median NIPS (0–4) | 2 | 2.5 | 0.102a |
LMA, laryngeal mask airway; ETT, endotracheal tube; NICU, neonatal intensive care unit; CPAP, continuous positive airway pressure; bpm, beats per minute; FiO2, fraction of inspired oxygen; SpO2, oxygen saturation; PaO2, partial arterial pressure of oxygen; PaCO2, partial arterial pressure of carbon dioxide; HCO3, bicarbonate; a/A, arterial/alveolar ratio; NIPS, neonatal infant pain scale.
Soon after LMA and laryngoscope removal, NIPS evaluation showed a significantly lower score for intubated patients who had just received premedication (p=0.0001). There were no serious adverse events associated with ETT or LMA insertion and surfactant administration. During the procedures, patients of both groups equally presented transitory cardiovascular events (HR<100beats/min and/or SpO2<80%) managed by PPV with immediate recovery (p=0.594).
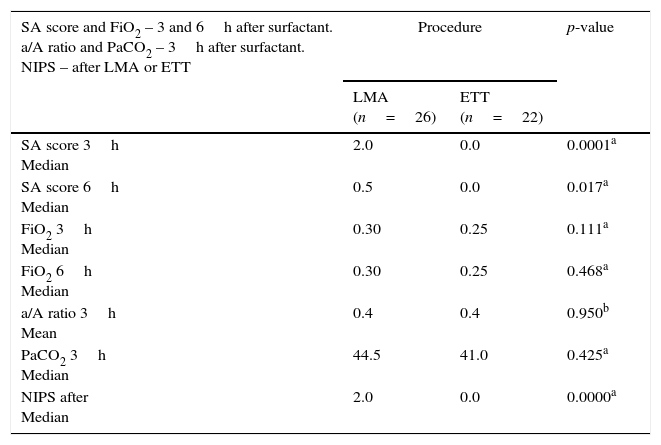
The respiratory effort measured by the SA score three and six hours after surfactant were significantly higher in LMA patients, but the a/A ratio, FiO2 on nCPAP or MV, and PaCO2 values were equivalent in both groups (Table 2).
Silverman–Anderson score and FiO2 3 and 6h after surfactant in patients from the LMA and ETT groups; a/A ratio and PaCO2 3h after surfactant in both groups; NIPS after LMA insertion and endotracheal intubation.
| SA score and FiO2 – 3 and 6h after surfactant. a/A ratio and PaCO2 – 3h after surfactant. NIPS – after LMA or ETT | Procedure | p-value | |
|---|---|---|---|
| LMA (n=26) | ETT (n=22) | ||
| SA score 3h Median | 2.0 | 0.0 | 0.0001a |
| SA score 6h Median | 0.5 | 0.0 | 0.017a |
| FiO2 3h Median | 0.30 | 0.25 | 0.111a |
| FiO2 6h Median | 0.30 | 0.25 | 0.468a |
| a/A ratio 3h Mean | 0.4 | 0.4 | 0.950b |
| PaCO2 3h Median | 44.5 | 41.0 | 0.425a |
| NIPS after Median | 2.0 | 0.0 | 0.0000a |
FiO2, fraction of inspired oxygen; LMA, laryngeal mask airway; ETT, endotracheal tube; a/A, ratio arterial/Alveolar ratio; PaCO2, partial arterial pressure carbon dioxide; NIPS, neonatal infant pain scale; SA score, Silverman–Anderson score.
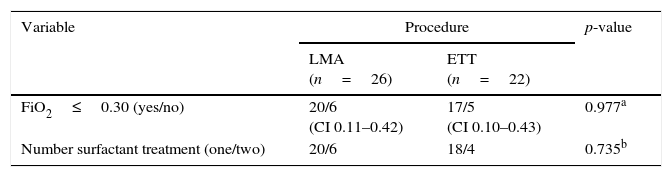
Eleven out 48 patients (22.9%) did not meet the primary outcome: FiO2 was>0.30 three hours after surfactant; six (23.0%) in the LMA group vs. five (22.7%) in the ETT group. Ten out 48 patients (20.8%) received a second dose of surfactant: six (23%) previously treated by LMA and four (18.1%) by ETT (Table 3). Four LMA patients received a second dose of surfactant in the first day of life, six to 12h after the first one; two received retreatment during the second day of life because they were intubated later on, due to increasing respiratory effort.
Primary outcome and number of surfactant doses in preterm patients diagnosed with RDS from LMA and ETT groups.
| Variable | Procedure | p-value | |
|---|---|---|---|
| LMA (n=26) | ETT (n=22) | ||
| FiO2≤0.30 (yes/no) | 20/6 (CI 0.11–0.42) | 17/5 (CI 0.10–0.43) | 0.977a |
| Number surfactant treatment (one/two) | 20/6 | 18/4 | 0.735b |
RDS, respiratory distress syndrome; LMA, laryngeal mask airway; ETT, endotracheal tube; FiO2, fraction of inspired oxygen; CI, confidence interval.
Regarding the six LMA patients who did not meet the primary outcome, three received retreatment within 12h of age, while, due to progressive respiratory effort without retreatment, intubation was performed in two of them. One patient was never intubated nor ventilated and remained on nCPAP.
Among 20 patients of LMA group who met the primary outcome, seven (35%) were intubated afterward: three for retreatment (one before and two after 24h of life); the remaining four were intubated due to respiratory effort or apnea and sepsis after 24h.
Overall, twelve (46.1%) LMA patients were intubated and mechanically ventilated, but only six patients (23%) experienced treatment failure requiring another dose of surfactant. CPAP failure was characterized in three patients (11.5%) who required MV without retreatment; another three patients (11.5%) were intubated due to late complications (pleural effusion and sepsis). Moreover, 14 LMA patients (53.8%) were never intubated or ventilated, independent of birth weight.
The residual gastric content was measured in 23 of 26 of LMA patients and gastric contents >1.5mL occurred in four. Only one patient with a gastric content of 3mL needed ETT.
MV duration was significantly higher in LMA patients who were intubated in comparison to ETT group (p=0.043). Only one patient from the LMA group (3.8%) presented IVH grade III and later pneumothorax. In both groups, eight patients had IVH grade I, and 11 had ROP grade I. Two patients from the ETT group died (4.1%) from sepsis. There was no difference between groups regarding duration of supplemental oxygen and hospitalization, mortality, the incidence of BPD, and early or late sepsis.
DiscussionSince 1994, a gentler approach to MV in RDS treatment has been sought. Initially, the strategy of intubation surfactant extubation (INSURE) was introduced, but it requires laryngoscopy, ETT, and PPV and may cause pain and lung injury.18 Several trials demonstrated that INSURE did not decreased rates of mortality or BPD and was not superior to early nCPAP.4 Besides, the experience of being intubated is unpleasant and painful; the adverse effects of laryngoscopy and ETT include laryngospasm, bronchospasm, hypoxia, bradycardia, pulmonary and systemic hypertension, raised intracranial pressure, and increased risk of IVH. The use of premedication may attenuate such effects, but it may cause respiratory depression and delay extubation.18–21 The assumption that infants did not feel pain has been proven to be untrue; reducing pain is a human and ethical obligation.20,21 In 2010, the American Academy of Pediatrics recommended premedication to be used for all intubations in neonates, except during resuscitation.21
In 2004, Kattwinkel et al. described a method of nasopharyngeal surfactant instillation soon after birth. However, no RCTs have proven its efficacy.22 Göpel et al. and Kanmaz et al. demonstrated the effectiveness of surfactant delivery for spontaneously breathing preterm infants through a thin catheter.2,23,24 It could be an alternative to INSURE, but the method still requires laryngoscopy, usually without analgesia.1 A small RCT by Attridge et al. in 2013 showed an abrupt decrease in supplemental oxygen requirements following surfactant through LMA when compared to the control group on nCPAP without surfactant.3 Recently, Pinheiro et al., in a non-blinded trial, compared (with a similar sample to the current study) somewhat early or late (4–48h of life) surfactant treatment by LMA after atropine to INSURE after atropine and morphine. They found a substantially higher rate of early failure (within 1h of treatment) to avoid mechanical ventilation in the INSURE group, suggesting that respiratory depression was responsible for the superiority of the LMA strategy. Enrollment was stopped because there were important differences between groups.25
The present study compared a less invasive RDS surfactant treatment (first 8h) by LMA to the conventional treatment. It was demonstrated that surfactant by LMA had the same short-time clinical efficacy of ETT treatment, because it was followed by similar supplemental oxygen need and lower MV requirements. The authors sought an efficient, safe, and less invasive method for RDS treatment that prevents premedication, laryngoscopy, ETT, and MV. Because of all the adverse effects of laryngoscopy and ETT, the authors did not compare LMA to INSURE. Rather, a new less invasive method of surfactant administration was compared with the worldwide-validated, ethical, invasive method of RDS treatment.
Since it was not possible to blind the procedures, in order to avoid the methodological bias of randomization, only one investigator had access to the sequential randomization after enrollment. To prevent bias of different individuals’ abilities and to standardize techniques, the same investigator was the only one responsible for LMA insertion and for all procedures and data collection during the first six hours after surfactant. The observed 60.3% loss of eligible patients was attributed to initial resistance or misinformation of the attending staff about the methodology, which was dependent on only one investigator's availability. The sample size would have been larger, especially if the study design had permitted the attending neonatologists to be also responsible for the procedures and interventions.
LMA insertion appeared to be easy and safe, as previously described. Additionally, Attridge et al. and Trevisanuto did not find serious adverse events associated with LMA surfactant administration.3,7 Sedative and analgesic drugs were also not required for LMA insertion in observational studies conducted by Trevisanuto et al. and in Brimacombe's or in Attrigde's trials.3,6,10 Although some transient bradycardia and desaturation occurred during the LMA insertion and/or surfactant instillation, the obstruction-like symptoms were judged to be similar to those frequently encountered in treatment by ETT, as did Attridge et al.3,7,26 Pinheiro et al. also did not find adverse effects related to LMA insertion, but they used a vagolytic agent before the procedure.25
In the current study, higher SA score 3 and 6h after surfactant by LMA suggested slower clinical response, which may be due to smaller concentrations reaching the lungs. However, the reduced respiratory effort found in the control group may also be due to a residual effect of the premedication.
In the COIN trial, >50% of the preterm infants failed on CPAP in the first three days of life. In the CURPAP and SUPPORT trials, 48.5% and 67.1%, respectively, were intubated to receive surfactant.1,2,11,15,16,27–29 Even though with less immature patients, the present study found nCPAP failure in 11.5% and surfactant failure in 23% of LMA patients. These respiratory outcomes might have been associated with insufficient surfactant reaching the lungs, or perhaps with more severe RDS.
Surfactant response is clinically measurable in regards to ventilation (pCO2), oxygenation (SpO2 and a/A ratio), and supplemental oxygen need (FiO2). During the first six hours after surfactant administration, the investigator's goal for both groups was to manage respiratory and oxygen support to reduce them and to achieve FiO2≤0.3 and SpO2 91–95% with progressive respiratory effort reduction, within three hours after surfactant. Other authors also observed oxygenation and FiO2 responses in LMA and INSURE treatment groups25 or LMA-treated patients and non-surfactant nCPAP groups.3
Considering the frequency and volume of gastric content measured after surfactant administration through LMA, oxygen requirements, and clinical outcomes, it seems that sufficient amounts of the drug reached the lungs. Brimacombe estimated that at least half of the surfactant by LMA did.10 Attridge et al. provided objective evidence that surfactant delivered by LMA reaches the lungs because it resulted in an immediate decrease in the FiO2 requirement.3 Pinheiro et al. also found small post-surfactant gastric aspirate volumes.25
Maintenance for MV was significantly higher in the LMA group, especially due to the outcomes of four patients; three presented severe RDS and one sepsis. Possibly, when surfactant administered by LMA did not sufficiently reached the lungs, ventilation/oxygenation improvement was delayed, with impact on disease severity and MV duration.
The sample size was not large enough for a definitive conclusion regarding morbidities and secondary outcomes. A major limitation of LMA surfactant delivery is that the smallest device available is too large for the infants most likely to have RDS, less than 1kg and 28 weeks at birth.6,10 The limitations of this study were: a single center, variable GA and birth weight range, one investigator responsible for all procedures, no blinding for data analysis, and lack of specific protocol for post-LMA surfactant and of follow up after NICU discharge.
In this study, the treatment groups were statistically equivalent in their supplemental oxygen needs three hours after surfactant. Fourteen infants (53.8%) from the LMA group were never intubated or ventilated. The management of infants after LMA surfactant treatment will require larger multicenter samples and a learning curve regarding unknown features, including target population, best treatment timing, instillation method and surfactant dose, analgesia need, and respiratory and special radiological outcomes.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Barbosa RF, Simões e Silva AC, Silva YP. A randomized controlled trial of the laryngeal mask airway for surfactant administration in neonates. J Pediatr (Rio J). 2017;93:343–50.
Study conducted at Universidade Federal de Minas Gerais (UFMG), Faculdade de Medicina, Belo Horizonte, MG, Brazil.