Urinary tract infection (UTI) caused by resistant strains of bacteria is increasingly prevalent in children. The aim of this study was to investigate the clinical characteristics and risk factors for UTI caused by community-acquired extended-spectrum β-lactamase (CA-ESBL)-producing bacteria in infants.
MethodsThis was a retrospective study performed over 5 years in a single Korean center. Hospitalized infants with febrile UTI were enrolled and divided into two groups (CA-ESBL vs. CA non-ESBL UTI). The yearly prevalence was calculated. Baseline characteristics and clinical course such as fever duration, laboratory and radiological findings were compared between the two groups. Risk factors associated with the CA-ESBL UTI were investigated.
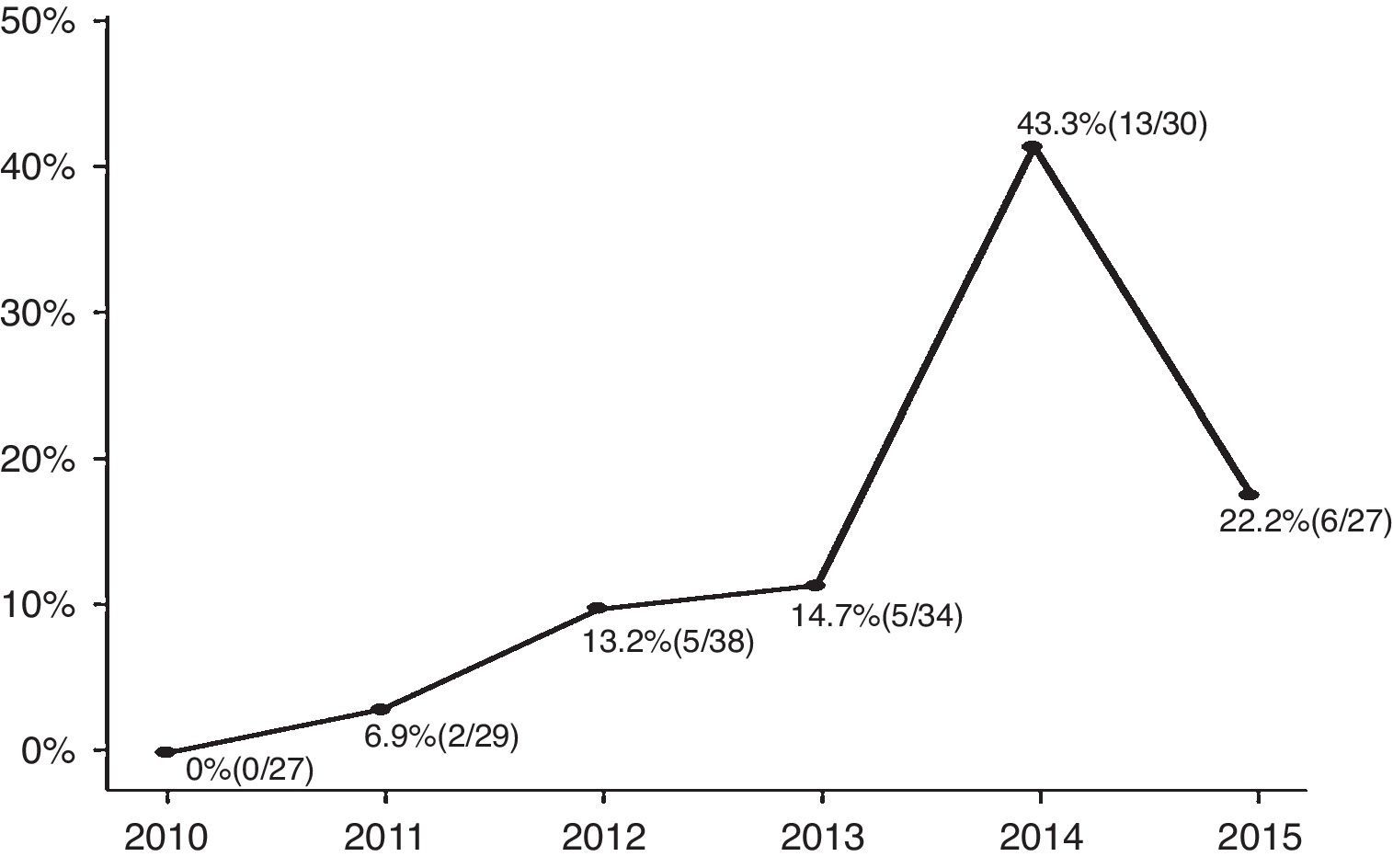
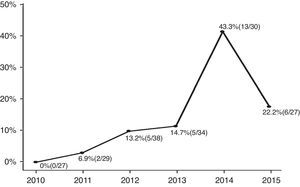
ResultsAmong the enrolled infants (n=185), 31 (17%) had CA-ESBL UTI. The yearly prevalence of ESBL of CA-ESBL UTI increased during the study (0% in 2010, 22.2% in 2015). Infants with CA-ESBL UTI had a longer duration of fever after initiating antibiotics (2.0±1.1 vs. 1.5±0.6 days, p=0.020). Cortical defects on renal scan and early treatment failure were more frequent in CA-ESBL (64.5 vs. 42.2%, p=0.023; 22.6 vs. 4.5%, p=0.001). A logistic regression analysis revealed that urinary tract abnormalities and previous UTI were independent risk factors for CA-EBSL UTI (odds ratio, 2.7; p=0.025; 10.3; p=0.022).
ConclusionThe incidence of UTI caused by ESBL-producing bacteria has increased in Korean infants. Recognition of the clinical course and risk factors for ESLB-producing UTI may help to determine appropriate guidelines for its management.
A infecção do trato urinário (ITU) causada por cepas de bactérias resistentes está cada vez mais prevalente em crianças. O objetivo deste estudo foi investigar as características clínicas e os fatores de risco de ITU causada por bactérias produtoras de β-lactamases de espectro ampliado adquiridas na comunidade (ESBL CA) em neonatos.
MétodosEste foi um estudo retrospectivo realizado por mais de 5 anos em um único centro coreano. Neonatos internados com ITU febril foram inscritos e divididos em dois grupos (ITU por ESBL CA em comparação a não ESBL CA). A prevalência anual foi calculada. As características básicas e o curso clínico, como duração da febre e achados laboratoriais e radiológicos, foram comparados entre os dois grupos. Os fatores de risco associados à ITU por ESBL CA foram investigados.
ResultadosEntre os neonatos inscritos (n=185), 31 (17%) apresentaram ITU por ESBL CA. A prevalência anual de ESBL em ITU por ESBL CA aumentou durante o estudo (0% em 2010, 22,2% em 2015). Os neonatos com ITU por ESBL CA apresentaram maior duração de febre após o início dos antibióticos (2,0±1,1 em comparação a 1,5±0,6 dias, p=0,020). Os defeitos corticais no exame renal e a falha precoce no tratamento foram mais frequentes em ESBL CA (64,5 em comparação a 42,2%, p=0,023; 22,6 em comparação a 4,5%, p=0,001). Uma análise de regressão logística revelou que as anomalias do trato urinário e a ITU anterior eram fatores de risco independentes de ITU por ESBL CA (razão de chance: 2,7; p=0,025; 10,3; p=0,022).
ConclusãoA incidência de ITU causada por bactérias produtoras de ESBL aumentou em neonatos coreanos. O reconhecimento do curso clínico e dos fatores de risco de ITU por ESBL poderá ajudar a determinar as diretrizes adequadas de manejo.
Urinary tract infection (UTI) is a common cause of bacterial infections among infants and young children with fever without a source. In general, initial antibiotic therapy for UTI is empirically supported until the culture and sensitivity results are available. However, due to the frequent use of antibiotics, resistant strains are emerging. The resistance of Gram-negative bacteria is usually due to plasmid-mediated enzymes called extended-spectrum β-lactamases (ESBLs).1 These are frequently produced by Escherichia coli and Klebsiella species, which are the most common UTI-causing pathogens.2 ESBL-producing bacteria were first reported in the 1983,3 and are now widespread worldwide.4 ESBLs confer not only resistance to β-lactam antibiotics but, oftentimes, cross-resistance to other antibiotics such as aminoglycosides, trimethoprim-sulfamethoxazole (TMP-SMX), and quinolones.5
Infections caused by these bacteria are thought to occur mainly in hospitals and nursing homes.6 More recently, ESBL-producing bacteria have started to disseminate within the wider community; indeed, in many countries, the incidence of community-onset infections has increased.7–10 Whereas several studies have analyzed risk factors for UTI caused by ESBL-producing bacteria in adults7,10 and children,8,9 only limited data are available on community-acquired (CA) UTIs caused by ESBL-producing bacteria in infants. The aim of this study, therefore, was to investigate the clinical characteristics and risk factors for CA UTI caused by ESBL-producing bacteria in infants.
MethodsStudy design and patient selectionThis retrospective study was conducted at single center in Korea between January 2010 and June 2015. The criteria for enrollment were as follows: (1) febrile UTI caused by E. coli and Klebsiella spp.; (2) hospitalized patients; (3) age under 12 months. Patients who had been hospitalized within the past week and those with urine cultures obtained more than 48h after admission were excluded. UTI was defined as abnormal urinalysis results and a positive urine culture (≥5×104colony forming units/mL) according to the American Academy of Pediatrics guidelines.11 Early treatment failure was defined as fever persisting for more than three days despite antibiotic treatment. Bacteremic UTI was defined as clinical symptoms of UTI with the same bacteria isolated from urine and blood cultures at the same time. The patient's medical records were reviewed regarding data such as age, gender, records of UTI episodes and urine culture results, past medical history, previous hospitalization, and laboratory and radiological findings. Possible risk factors for ESBL production were age, gender, urine microorganism, previous UTI, use of antibiotics, previous hospitalization in the previous three months, and underlying neurological, cardiac, respiratory, and renal diseases. The CA-ESBL and CA non-ESBL groups were compared regarding the possible risk factors. The study was approved by the Institutional Review Board of this hospital and was in agreement with the Declaration of Helsinki.
Imaging studiesRenal abnormalities, including vesicoureteral-reflux, hydronephrosis, and dysplastic kidney, were investigated. Renal ultrasound (US) and technetium-99m dimercaptosuccinic acid (DMSA) scan were performed to determine any anatomical abnormalities and the presence of acute pyelonephritis, respectively. The US results were considered pathological in case of abnormal kidney morphology and parenchymal echogenicity, any grade of dilatation of the collecting system (renal pelvis, calyces, or distal ureters), or bladder mucosal thickening. An abnormal DMSA result was defined by the presence of focal or diffuse areas of reduced radionuclide uptake without evidence of cortical loss or by the presence of diffusely decreased uptake in an enlarged kidney.12 DMSA was repeated after six months to detect any renal cortical scarring. Voiding cystourethrography was performed for patients with abnormal imaging results. The vesicoureteral reflux (VUR) was graded according to the International Reflux Study Committee classification.13
Identification of ESBLsAntimicrobial susceptibility testing was performed by the disk diffusion method as recommended by the Clinical and Laboratory Standards Institute guidelines.14 ESBL production was determined by the double-disk synergy test, also according to the Clinical and Laboratory Standards Institute guidelines.14 A minimum 5-mm increase in the zone tested in combination with clavulanic acid vs. the zone tested alone was accepted as an indication of ESBL production.15
Statistical analysisDescriptive statistics were reported as mean with standard deviation or percentages where appropriate. Continuous variables were analyzed using t-tests for independent samples, and categorical variables were analyzed using Fisher's exact or the chi-squared test. Linear regression was used to assess the trends of the yearly prevalence of CA-ESBL UTI. Logistic regression was used to determine the risk factors for ESBL-producing bacteria. The results were evaluated with 95% confidence intervals. A p-value<0.05 was considered significant. All statistical analyses were performed using SPSS version 21.0 (SPSS Inc., IL, United States).
ResultsA total of 185 infants were enrolled and divided into two groups (CA-ESBL vs. CA non-ESBL UTI). Thirty-one (16.7%) of the patients had UTIs caused by ESBL-producing bacteria, while the remaining 154 patients had non-ESBL-producing bacteria in their urine cultures. The proportions of ESBL-producing bacteria causing UTIs were 0% in 2010, 6.9% in 2011, 13.2% in 2012, 14.7% in 2013, 43.3% in 2014, and 22.2% in 2015. The yearly incidence rates of CA-ESBL UTI increased during the study period, and the p-value for the trend was significant (p<0.001; Fig. 1).
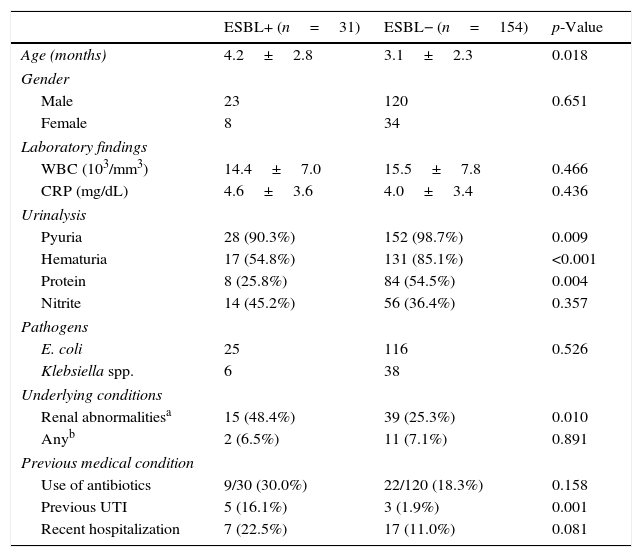
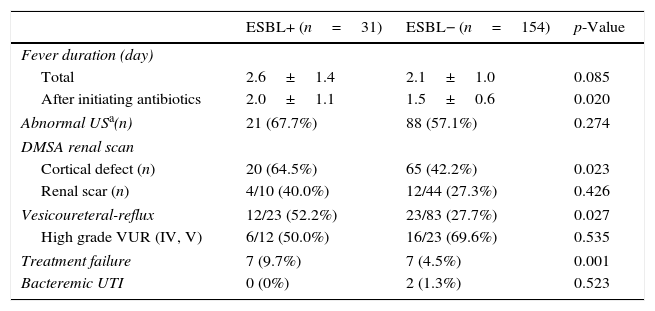
The CA-EBSL and CA non-ESBL UTI groups consisted of 31 (17%) and 154 (83%) patients, respectively. The mean age was higher in the CA-ESBL UTI group than in the non-ESBL UTI group (4.2±2.8 vs. 3.1±2.3 months, p=0.018). E. coli was the dominant pathogen in both groups: the ESBL-production proportion between E. coli and Klebsiella spp. did not differ (Table 1). No differences in sex or laboratory findings were observed between the two groups. Defervescence after treatment initiation was longer in CA-ESBL UTI than in CA non-ESBL UTI (2.0±1.1 vs. 1.5±0.6 days, p=0.020; Table 1). US abnormalities did not differ between the groups. Cortical defects on DMSA scan were more frequent in the CA-ESBL than in the CA non-ESBL UTI group (64.5 vs. 43.5%, p=0.023); however, presence of renal scars did not significantly differ (40.0 vs. 27.3%, p=0.426; Table 2). Of the 23 patients with CA-ESBL UTI, 12 (52.2%) were diagnosed with VUR, whose rate was significantly higher than that in the CA-non-ESBL UTI group (p=0.027). Bacteremic UTI was observed in two patients (1.3%) with CA non-ESBL UTI. Early treatment failure was more frequent in the CA-ESBL UTI group than in the CA non-ESBL UTI group (22.6 vs. 4.5%, p=0.001).
Baseline characteristics of patients.
| ESBL+ (n=31) | ESBL− (n=154) | p-Value | |
|---|---|---|---|
| Age (months) | 4.2±2.8 | 3.1±2.3 | 0.018 |
| Gender | |||
| Male | 23 | 120 | 0.651 |
| Female | 8 | 34 | |
| Laboratory findings | |||
| WBC (103/mm3) | 14.4±7.0 | 15.5±7.8 | 0.466 |
| CRP (mg/dL) | 4.6±3.6 | 4.0±3.4 | 0.436 |
| Urinalysis | |||
| Pyuria | 28 (90.3%) | 152 (98.7%) | 0.009 |
| Hematuria | 17 (54.8%) | 131 (85.1%) | <0.001 |
| Protein | 8 (25.8%) | 84 (54.5%) | 0.004 |
| Nitrite | 14 (45.2%) | 56 (36.4%) | 0.357 |
| Pathogens | |||
| E. coli | 25 | 116 | 0.526 |
| Klebsiella spp. | 6 | 38 | |
| Underlying conditions | |||
| Renal abnormalitiesa | 15 (48.4%) | 39 (25.3%) | 0.010 |
| Anyb | 2 (6.5%) | 11 (7.1%) | 0.891 |
| Previous medical condition | |||
| Use of antibiotics | 9/30 (30.0%) | 22/120 (18.3%) | 0.158 |
| Previous UTI | 5 (16.1%) | 3 (1.9%) | 0.001 |
| Recent hospitalization | 7 (22.5%) | 17 (11.0%) | 0.081 |
WBC, white blood count; CRP, C-reactive protein; ESBL, extended-spectrum β-lactamase; UTI, urinary tract infection.
Clinical course of CA-ESBL UTI and CA non-ESBL UTI.
| ESBL+ (n=31) | ESBL− (n=154) | p-Value | |
|---|---|---|---|
| Fever duration (day) | |||
| Total | 2.6±1.4 | 2.1±1.0 | 0.085 |
| After initiating antibiotics | 2.0±1.1 | 1.5±0.6 | 0.020 |
| Abnormal USa(n) | 21 (67.7%) | 88 (57.1%) | 0.274 |
| DMSA renal scan | |||
| Cortical defect (n) | 20 (64.5%) | 65 (42.2%) | 0.023 |
| Renal scar (n) | 4/10 (40.0%) | 12/44 (27.3%) | 0.426 |
| Vesicoureteral-reflux | 12/23 (52.2%) | 23/83 (27.7%) | 0.027 |
| High grade VUR (IV, V) | 6/12 (50.0%) | 16/23 (69.6%) | 0.535 |
| Treatment failure | 7 (9.7%) | 7 (4.5%) | 0.001 |
| Bacteremic UTI | 0 (0%) | 2 (1.3%) | 0.523 |
ESBL, extended-spectrum β-lactamase; UTI, urinary tract infection; US, ultrasonography; DMSA, dimercaptosuccinic acid; VUR, vesicoureteral-reflux.
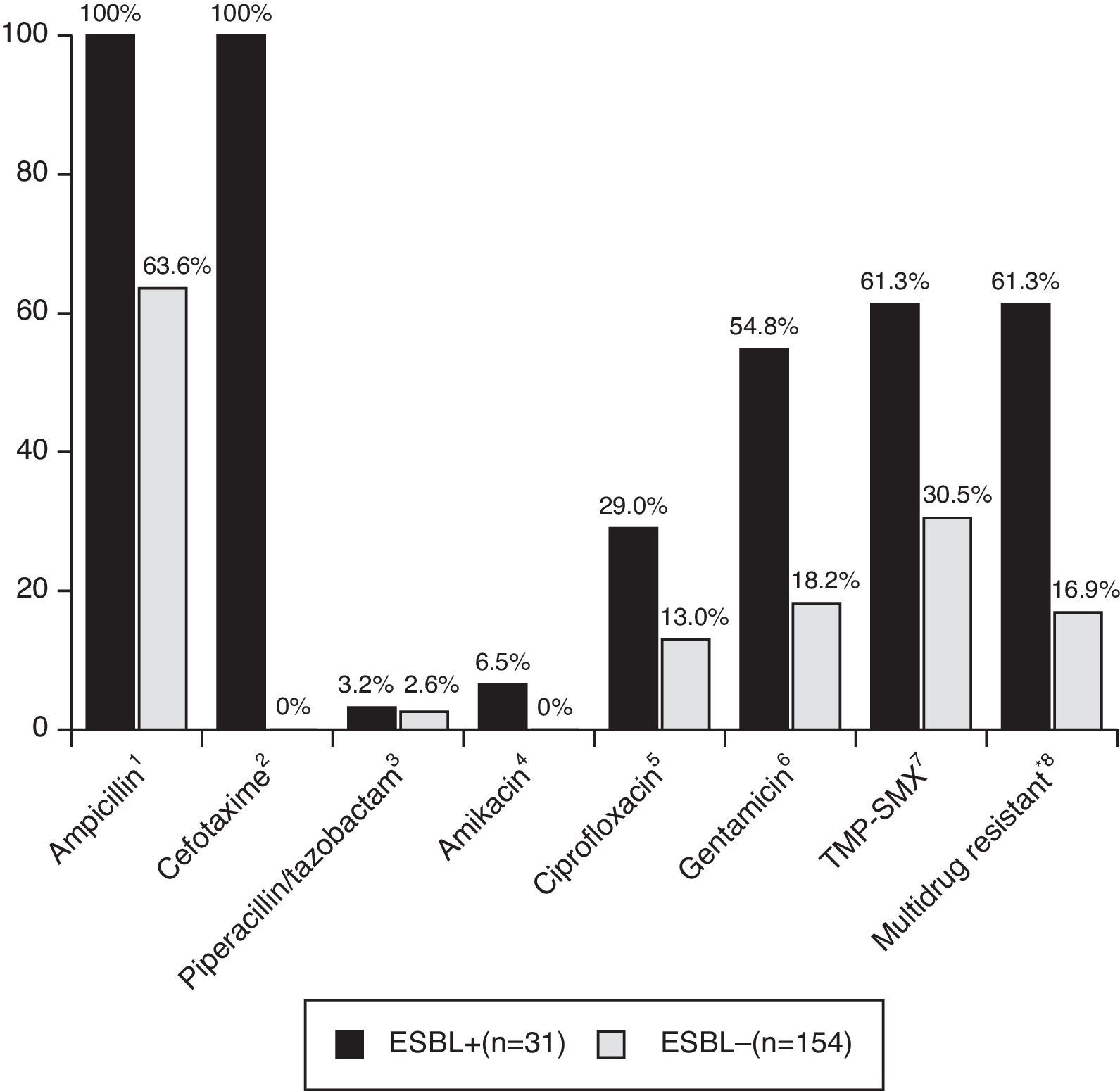
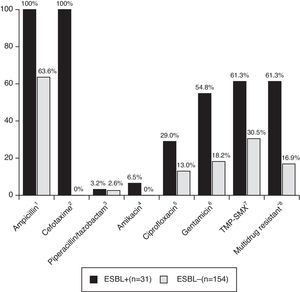
Patients with CA-non-ESBL UTI showed a rate of 63.6% of antimicrobial resistance to ampicillin. The resistance rates in the CA-ESBL UTI group were 61.3% for TMP-SMX, 29.0% for ciprofloxacin, and 54.8% for gentamicin; in the CA non-ESBL UTI group, in turn, these rates were 30.5% for TMP-SMX, 13.0% for ciprofloxacin, and 18.2% for gentamicin (Fig. 2). Patients with CA-ESBL UTI had a significantly higher rate of resistance to non-β-lactam antimicrobial agents: 19 of the 31 ESBL isolates (61.3%) were resistant to at least two groups of non-β-lactam antibiotic agents.
Antibiotic resistance of bacterial isolates causing UTI. UTI, urinary tract infection; TMP-SMX, trimethoprim/sulfamethoxazole. *Multidrug resistant, resistant to more than two non-β-lactam antibiotics. Significance calculated by Fisher's exact test: 1p<0.001; 2p<0.001; 3p=1.000; 4p=0.002; 5p=0.005; 6p<0.001; 7p<0.001; 8p<0.001.
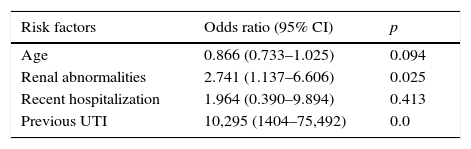
Regarding possible risk factors, age, renal abnormalities, previous UTI, and recent hospitalization were associated with CA-ESBL UTI (Table 1). The logistic regression analysis identified urinary tract abnormality and previous UTI history as independent risk factors, which were found to increase the risk by 2.7- and 10.3-fold, respectively (Table 3).
Logistic regression of risk factors associated with CA-ESBL UTI in infants.
| Risk factors | Odds ratio (95% CI) | p |
|---|---|---|
| Age | 0.866 (0.733–1.025) | 0.094 |
| Renal abnormalities | 2.741 (1.137–6.606) | 0.025 |
| Recent hospitalization | 1.964 (0.390–9.894) | 0.413 |
| Previous UTI | 10,295 (1404–75,492) | 0.0 |
ESBL, extended-spectrum β-lactamase; UTI, urinary tract infection.
The aim of this study was to highlight the emergence of CA-ESBL UTIs in Korean infants and to assess the clinical characteristics and possible risk factors for CA-ESBL UTI in infants. An increasing incidence of multidrug-resistant bacteria has been reported not only in hospital-acquired infections but also CA infections, mostly UTIs.8 Thus, the choice of appropriate antibiotics has become both more complicated and may delay appropriate therapy.16,17 This study analyzed 185 cases of infantile CA UTI, and showed an increasing trend in the occurrence of CA-ESBL UTI. Significant differences were observed in defervescence time and cortical defect on DMSA scans in CA-ESBL UTI. In the CA-ESBL UTI cases, urinary tract abnormalities, including VUR, were more frequent, and the rates of resistance to non-β-lactam antibiotics were relatively high. Urinary tract abnormalities and UTI history were found to be independent risk factors.
Over the past two decades, there has been wide use of extended broad-spectrum antibiotics to counter the increasing rates of ESBL-producing bacteria in patients with UTI worldwide.7,18E. coli and Klebsiella spp., the most common UTI pathogens, have various antibiotic resistance mechanisms, including enhanced drug efflux, alterations of the drug target, and production of plasmid-mediated β-lactamases.19 Resistance is usually due to β-lactamase enzymes called ESBLs. The patterns of antibiotic resistance differ temporally and regionally.18,20,21 The rates of ESBL-producing E. coli range from 2.2% in a 2011 Swiss study18 to 55.0% in a 2009 Chinese study.20 Several factors are involved in the rapid spread of ESBL-producing bacteria in countries with low use of antibiotics, including acquisition from food,22 person-to-person transmission from fecal carriers,23 dissemination of ESBL-producing bacteria in the environment,24 and the existence of reservoirs, such as long-term care centers.25
Most studies have determined that the risks factors for CA-ESBL-producing bacterial infections generally are recent hospitalization, previous UTI episodes, urinary tract abnormalities, underlying disease, UTI with Klebsiella spp., and use of cephalosporin UTI antimicrobial prophylaxis.7–9 The use of antimicrobial prophylaxis is an important risk factor to increase antibiotic resistance; TMP-SMX minimally decreased antimicrobial susceptibilities compared to cephalosporin.26 As only two patients in this study received the antimicrobial prophylaxis, it was not possible assess the risk of antimicrobial prophylaxis. In the present study, urinary tract abnormalities and UTI history were found to be independent risk factors for future CA-ESBL UTI in infants. However, a high number of CA-ESBL-producing bacterial infections occur in patients without obvious risk factors.27 In fact, in the present study, approximately 60% of patients with CA-ESBL UTI had no risk factors. This result might be related to the constant increase in the number of healthy carriers colonized with ESBL-producing bacteria.23
A major concern regarding ESBL-producing bacteria is the high rate of cross-resistance to non-β-lactam antibiotics such as ciprofloxacin, aminoglycosides, nitrofurantoin, and TMP-SMX. In this study, 61% of patients showed antibiotic resistance to at least two suitable non-β-lactam antibiotics used to treat UTI. Similar results have been reported by other investigators.28 The increasing incidence of multi-drug resistance by ESBL-producing bacteria could become a problem for the treatment of UTI.
Many studies have reported that infection by these bacteria might lead to a delay in appropriate therapy and a concomitant increase in the rates of treatment failure and comorbidity.21,29 Proposing an appropriate empirical antibiotic treatment for CA-ESBL UTI is difficult, and initial empirical antibiotic therapy is often unsuccessful. Additionally, when ESBL-producing bacteria are isolated, there are almost no options for oral therapy to improve patient symptoms. In the present study, treatment failure was more frequent in the CA-ESBL UTI group; however, there was no significant inter-group difference in clinical manifestations such as bacteremic UTI or renal scarring. Resistant strains were not associated with worsening of renal outcome in the present study, but previous studies indicated that ESBL-producing bacterial infection increase the rates of treatment failure and death.21,27,29
Several studies have shown increasing recognition of ESBL-producing bacteria as a cause of CA infection. Most data on CA-ESBL infections are based on adult populations, and relatively few pediatric studies are available.8,9,30 Among them, infantile data on clinical characteristics of CA-ESBL UTI and possible risk factors are scarce. However, as the incidence of ESBL-producing bacteria in infants with UTIs is increasing, it is important to understand the relevant epidemiological changes in infants.
This study has some limitations. First, this was retrospective study; therefore, some data were missing from the medical records. Second, this study entailed a single-center analysis with a restricted number of patients. The sample size of the CA-ESBL UTI group was small, thus imposing limitations on statistical power. Third, VCUG was performed only when image study findings were abnormal. In order to achieve a better understanding of the clinical characteristics and determine the risk factors for UTIs caused by ESBL-producing bacteria in infants, additional, cohort prospective studies based on a large population are required.
In conclusion, the incidence of UTI caused by ESBL-producing bacteria has increased gradually in Korean infants. Urinary tract abnormalities and previous UTI infection were found to be significant independent risk factors associated with CA-EBSL UTI. For such patients, recognition of the risk factors for ESLB-producing UTI may help to determine appropriate guidelines for its management and more attention to the choice of empirical antibiotic therapy is needed.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by a grant (HCRI 16916-1) Chonnam National University Hwasun Hospital Institute for Biomedical Sciencel.
Please cite this article as: Kim YH, Yang EM, Kim CJ. Urinary tract infection caused by community-acquired extended-spectrum β-lactamase-producing bacteria in infants. J Pediatr (Rio J). 2017;93:260–6.