This study aimed to survey children with celiac disease (CD) for psychiatric disorders, determine the possible factors that predict psychopathology, and analyze health-related quality of life and possible factors that could affect the quality of life.
MethodsIn this study, all children completed the Schedule for Affective Disorders and Schizophrenia for School Age Children – Present and Lifetime Version – Turkish Version (K-SADS-PL-T), as well as the Pediatric Quality of Life Inventory (PedsQL) for the 8–12 age group, and a sentence completion test. A face-to-face interview was performed with the parents of the participants to inform them about the study.
ResultsThis study included 52 children with celiac disease in the age range of 8–12 years, and 40 healthy children. The mean age of the study group was 10.36±0.36 years, and 31 (59%) of them were females. The mean age of the control group was 10.35±0.46 years and 24 (60%) of them were females. The mean subscale scores of the Pediatric Quality of Life Inventory were significantly lower in children with celiac disease when compared to the control group (p<0.05). There was at least one psychiatric disorder in the 26 (50%) children with celiac disease.
ConclusionsThis study has shown once more that celiac disease is associated with some psychiatric signs/diagnoses, and that it decreased quality of life. Further studies are needed to determine the factors that could reduce the psychiatric signs. It is apparent that those studies would contribute new approaches to improve diagnosis, treatment, and quality of life.
Neste estudo, foram avaliadas crianças com doença celíaca (DC) para verificar a existência de transtornos psiquiátricos, determinar os possíveis fatores que predizem psicopatologia e analisar a qualidade de vida relacionada à saúde e possíveis fatores que podem afetá-la.
MétodosNeste estudo, todas as crianças responderam à Entrevista para Transtornos Afetivos e Esquizofrenia em Crianças em Idade Escolar – Versão Presente e ao Longo da Vida – Versão Turca (K-SADS-PL-T), bem como ao Inventário Pediátrico de Qualidade de Vida (PedsQL) da faixa etária de 8-12 anos e ao teste de completar sentenças. Uma entrevista presencial foi realizada com os pais dos participantes para informá-los sobre o estudo.
ResultadosEste estudo incluiu 52 crianças com DC entre as idades de 8 a 12 anos e 40 crianças saudáveis. A idade média do grupo de estudo era de 10,36±0,36 anos, e 31 deles (59%) eram do sexo feminino. A idade média do grupo de controle era de 10,35±0,46 anos, e 24 deles (60%) eram do sexo feminino. Os escores médios das subescalas do PedsQL foram significativamente menores em crianças com DC quando comparados com o grupo de controle (p<0,05). Havia pelo menos um transtorno psiquiátrico em 26 (50%) crianças com DC.
ConclusõesEste estudo mostrou mais uma vez que a DC está associada a alguns sintomas/diagnósticos psiquiátricos e reduziu a qualidade de vida. São necessários estudos adicionais para determinar os fatores que podem reduzir os sintomas psiquiátricos. Está claro que esses estudos contribuiriam com novas abordagens para melhorar o diagnóstico, o tratamento e a qualidade de vida.
Celiac disease (CD) is a chronic, inflammatory, immune-mediated disease characterized by persistent intolerance of the small intestines to gliadin.1 Its prevalence is approximately 1% in most countries of the world. The manifestations of CD can be divided into gastrointestinal symptoms and extraintestinal symptoms. The diagnosis depends on gluten-dependent symptoms, CD-specific antibody levels, the presence of human leukocyte antigen (HLA-DQ2) and/or HLA-DQ8, and characteristic histological changes in the duodenal biopsy. In the presence of high antibody levels, the diagnosis of CD may be based on a combination of symptoms, antibodies, and HLA, thus omitting the duodenal biopsy. Compliance with a gluten-free diet (GFD) is monitored by endomysium antibody (EMA) status.2 The treatment consists of removing gluten from the diet throughout life.3 Just like other chronic diseases, CD affects physical, mental, and social life, as well as the health-related quality of life of children.4
A number of studies have investigated the effect of CD on quality of life and psychopathology. Although the prevalence of major depressive disorder, dysthymic disorders, and adjustment disorders varies between 10 and 80%, they are the most common psychiatric disorders (PD) in adults and children with CD.5 The basic factor focused on in those studies was the quality and degree of adherence to the GFD. It is known that lifelong GFD increases perception of well-being and positive feelings in some domains, and decreases those perceptions in some other domains.6 In conclusion, children with CD trying to adapt GFD are at risk for psychological disorders.
Most of the studies in the literature that investigated psychopathology and quality of life were conducted on adults, and the data on children are scarce.7 The present study aimed to screen children with CD for PD, determine possible factors that predict psychopathology, and analyze health-related quality of life and the possible factors that affected the quality of life in those children.
MethodsSamplesThis cross-sectional study was carried out with fifty-two patients who were formerly diagnosed with CD recruited from the Department of Pediatric Gastroenterology, Emel-Mehmet Tarman Children Hospital in Kayseri, Turkey, between January and March 2016. The diagnosis of CD was based on the criteria outlined by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.8 Biopsies of the small intestine were performed for all patients with a positive serum EMA test and all biopsy specimens were evaluated according to the modified Marsh criteria. All celiac patients had Type III-c enteropathy according to Marsh's criteria. Exclusion criteria included any child less than 8 years or more than 12 years of age; those who did not have a comorbid disease such as an IgA deficiency, diabetes mellitus type 1, or Down syndrome; and those on GFD for less than six months. The study sample was chosen among children between the ages of 8–12 years, which represented the period that children begin school and leave the Oedipal stage. Thus, they experience and try to handle social and academic stresses. In order to determine the effect of compliance to GFD, the celiac group was subdivided into a dietary compliant group and a dietary noncompliant group according to serum EMA levels. Forty age- and sex-matched healthy children who did not have any gastrointestinal disorder or PD and admitted to the Emel-Mehmet Tarman Children Hospital for various reasons were included as controls. All of controls had a negative serum EMA test. All characteristics including age, gender, symptoms, weight, height, body mass index (BMI), serum EMA level, and family characteristics (number of household members, socioeconomic status, PD in the history) were evaluated.
In the second step of the study, all patients included in the study were examined by the child psychiatrist. All participants were given the Schedule for Affective Disorders and Schizophrenia for School Age Children – Present and Lifetime Version – Turkish Version (K-SADS-PL-T), a semi-structured diagnostic tool. They were also given the Pediatric Quality of Life Inventory (PedsQL) 8–12 age group and a sentence completion test, and the parents were interviewed to obtain information about their children. In addition, the authors completed the sociodemographic data form. The study was approved by the Ethics Committee for Non-invasive Clinical Research of Kayseri Training and Education Hospital, under No. 2016/49. All participants provided their written informed consent, and participated in the study voluntarily.
Data collection toolsK-SADS-PLThis is a semi-structured scale developed by Kauffman et al. to screen psychopathology in children and adolescents between the ages of 6 and 18 years.9 In this scale, psychopathology is investigated by combining the data obtained from the parents and the child. The psychopathologies included in the scale include mood, psychosis, anxiety, disruptive behavior disorder, elimination disorders, eating and tic disorders, and alcohol and substance abuse. Reliability and validity of the K-SADS-PL in Turkish was verified by Gökler et al.10
PedsQLThis is a general quality of life tool used in 2–18 year-old children and adolescents.11 Emotional functioning score (EFS), social functioning score (SoFS), school functioning score (ScFS), total physical health score (TPhHS), total psychosocial health score (TPsHS), and total scale score (TSS) are used in the scale. A 5-point, Likert-type scoring scale is used in the scale (0=never, 1=almost never, 2=sometimes, 3=often, 4=almost always). The scores obtained from the items are linearly transformed into a value between 0 and 100 (0=100, 1=75, 2=50, 3=25, 4=0). The quality of life increases as the scores increases from 0 to 100.12 The reliability and validity of the PedsQL for ages 8–12 and for ages 13–18 in Turkish was studied by Cakin Memik et al., and 8–12 year-old form was used in this study.13
Beier sentence completion testBeing one of the projection techniques, this test has a wide usage area. It has two forms; Form A, which is suitable for children, can be applied to ages 8–16. Via this test the individuals project their interests, feelings, behaviors, wishes, sadness, and the other important personality features against an unclear stimulant. Thus, conscious and unconscious emotions and ideas can be obtained from the individual. With this test, problems seen frequently in environments like school and the home can be detected.
Sociodemographic data formVia this data form, prepared by the authors, the name (optional), age, sex, and the number and the identity of household members were identified by asking the participant or their family. In this form, the socioeconomic level was determined in accordance to income of the family. An income three-fold or more of minimum wage was considered as a good, an income between minimum wage and three-fold of minimum wage was considered as a moderate, and an income at the level of minimum wage or less was considered as low socioeconomic level. Their history was assessed regarding any previous diagnosis of PD, the presence of PD in family, and any information related CD such as date of diagnosis, body weight, height, primary gastrointestinal symptoms, and the compliance with the GFD.
Statistical analysisThe data were analyzed with SPSS version 16.0 (SPSS Inc. Released 2007. SPSS for Windows, version 16.0. IL, USA). Descriptive statistics were presented as mean, standard deviation, and percent. Student's t-test for independent samples was used to determine the significance of the intergroup differences for quantitative variables with normal distributions. Qualitative variables were compared with the chi-squared and Fisher's exact tests. The normality of the distribution of data was analyzed with the Shapiro–Wilk test. It was determined that the subscale scores of PedsQL were not distributed normally, and Spearman's correlation analysis, Mann–Whitney, chi-squared, and Kruskal–Wallis tests were used to compared the data without normal distributions. A p-value of less than 0.05 was considered significant.
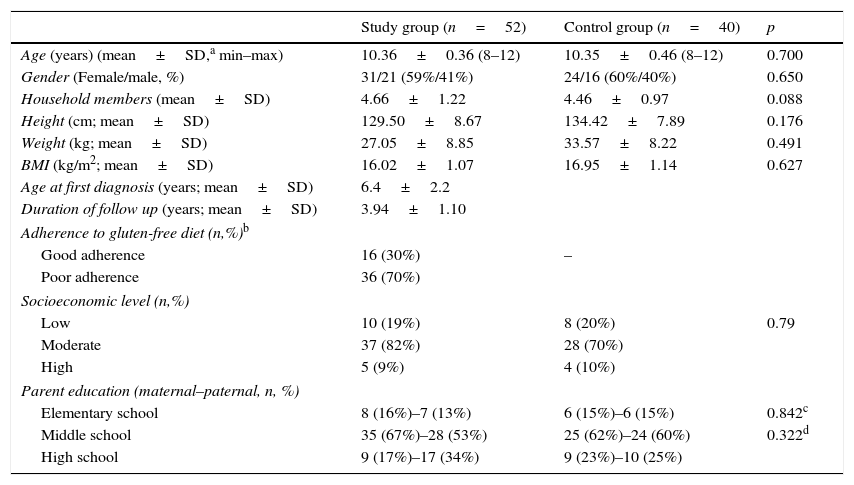
ResultsThis study included 52 children with CD between the ages of 8–12 years, and 40 healthy children in the same age group. The mean age of the celiac group was 10.36±0.36 years, and 31 (59%) were females. The mean age of the control group was 10.35±0.46 years and 24 (60%) were females. CD was diagnosed at a mean age of 6.4±2.2 years. At the diagnosis, the most frequent sign was chronic gastrointestinal symptoms, which included chronic diarrhea and abdominal pain in 36 (69%), followed by failure thrive in 16 (30%). Sixteen of the patients had a decreased BMI at diagnosis (BMI z-score <−1). The mean duration of follow up was 3.94±1.10 years. Nine (17%) patients with a decreased BMI at diagnosis increased their BMI after adherence to a GFD, in addition to 22 (42%) patients who had worse compliance with a GFD (non-strict diet or some gluten), who still suffered chronic gastrointestinal symptoms. The mean serum EMA level was 140RU/mL (0–760) in children with CD, and 16 (30%) of them adhered to GFD well; on the other hand, all of the controls had a negative serum EMA test. PD were identified in six (11%) families in celiac group and in three (7%) families in the control group, and there was no significant difference between the celiac and control groups (p>0.05). The mean number of household members (4.66±1.22 vs. 4.46±0.97, p>0.05) was not significantly different between celiac group and controls. As for parents, fathers were an average of 36.6 years and the mothers of 32.3 years, and it was also observed that 35 (67%) of the children's mothers and 28 (53%) of their fathers had graduated middle school in the celiac group. Table 1 shows that there were no significant differences between celiac group and controls for age, gender, height, BMI, and family characteristics (p>0.05).
Sociodemographic- clinic characteristics of the study and the control groups.
| Study group (n=52) | Control group (n=40) | p | |
|---|---|---|---|
| Age (years) (mean±SD,a min–max) | 10.36±0.36 (8–12) | 10.35±0.46 (8–12) | 0.700 |
| Gender (Female/male, %) | 31/21 (59%/41%) | 24/16 (60%/40%) | 0.650 |
| Household members (mean±SD) | 4.66±1.22 | 4.46±0.97 | 0.088 |
| Height (cm; mean±SD) | 129.50±8.67 | 134.42±7.89 | 0.176 |
| Weight (kg; mean±SD) | 27.05±8.85 | 33.57±8.22 | 0.491 |
| BMI (kg/m2; mean±SD) | 16.02±1.07 | 16.95±1.14 | 0.627 |
| Age at first diagnosis (years; mean±SD) | 6.4±2.2 | ||
| Duration of follow up (years; mean±SD) | 3.94±1.10 | ||
| Adherence to gluten-free diet (n,%)b | |||
| Good adherence | 16 (30%) | – | |
| Poor adherence | 36 (70%) | ||
| Socioeconomic level (n,%) | |||
| Low | 10 (19%) | 8 (20%) | 0.79 |
| Moderate | 37 (82%) | 28 (70%) | |
| High | 5 (9%) | 4 (10%) | |
| Parent education (maternal–paternal, n, %) | |||
| Elementary school | 8 (16%)–7 (13%) | 6 (15%)–6 (15%) | 0.842c |
| Middle school | 35 (67%)–28 (53%) | 25 (62%)–24 (60%) | 0.322d |
| High school | 9 (17%)–17 (34%) | 9 (23%)–10 (25%) | |
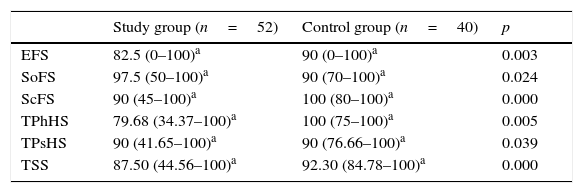
When the mean subscale scores of PEDSQL were taken into consideration in children with CD, it was observed that TPhHS was 79.68, EFS was 82.50, SoFS was 97.50, ScFS was 90, TPsHS was 90, and TSS was 87.50, while those scores were 100, 90, 90, 100, 90, and 92.30 in the control group, respectively. The PedsQL subscale scores (Table 2) were significantly lower in children with CD (p<0.05).
Mean Pediatric Quality of Life Inventory (PedsQL) subscale scores for the study and control groups.
| Study group (n=52) | Control group (n=40) | p | |
|---|---|---|---|
| EFS | 82.5 (0–100)a | 90 (0–100)a | 0.003 |
| SoFS | 97.5 (50–100)a | 90 (70–100)a | 0.024 |
| ScFS | 90 (45–100)a | 100 (80–100)a | 0.000 |
| TPhHS | 79.68 (34.37–100)a | 100 (75–100)a | 0.005 |
| TPsHS | 90 (41.65–100)a | 90 (76.66–100)a | 0.039 |
| TSS | 87.50 (44.56–100)a | 92.30 (84.78–100)a | 0.000 |
EFS, emotional functioning score; SoFS, social functioning score; ScFS, school functioning score; TPhHS, total physical health score; TPsHS, total psychosocial health score; TSS, total scale score.
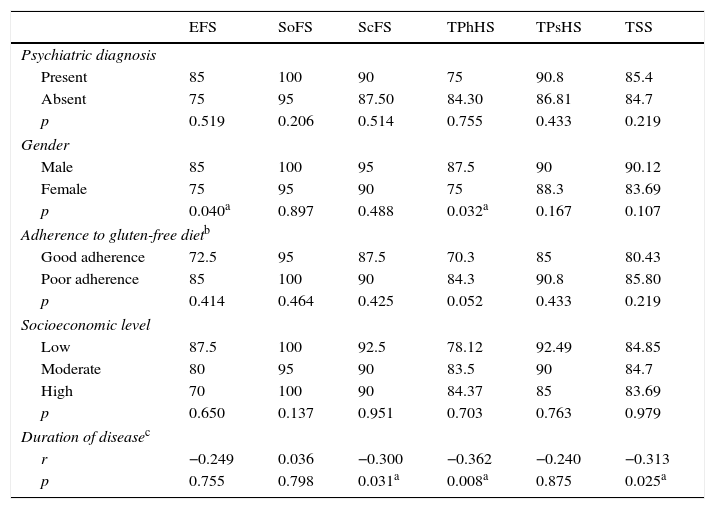
The effects of sociodemographic variables on subscale scores of PedsQL were analyzed. Mean TPhHS and EFS scores were significantly lower in girls with CD compared to boys with CD; however, there were no differences for mean SoFS, ScFS, TPsHS, or TSS scores. The children with CD were divided into three groups according to their income levels (low – medium – high), and they did not show any significant differences for PedsQL subscale scores (p>0.05). The children with CD were divided into two groups: those adhering well to GFD, and those adhering poorly to GFD; no significant differences were found between those two groups for mean PedsQL subscale scores (p>0.05). The correlation analysis performed to analyze the correlation of the duration of the disease with PedsQL subscale scores revealed insignificant negative correlations of disease duration with TPhHS, ScFS, and TSS (r: −0.362, p=0.08; r: −0.300, p=0.031; and r: −0.313, p=0.025, respectively). Table 3 shows the children with a PD; those without a PD did not show any significant differences for mean PedsQL subscale scores when the patients with CD were taken into consideration (p>0.05).
Effects of sociodemographic-clinical variables on the Pediatric Quality of Life Inventory (PedsQL) subscale scores in children diagnosed with celiac disease.
| EFS | SoFS | ScFS | TPhHS | TPsHS | TSS | |
|---|---|---|---|---|---|---|
| Psychiatric diagnosis | ||||||
| Present | 85 | 100 | 90 | 75 | 90.8 | 85.4 |
| Absent | 75 | 95 | 87.50 | 84.30 | 86.81 | 84.7 |
| p | 0.519 | 0.206 | 0.514 | 0.755 | 0.433 | 0.219 |
| Gender | ||||||
| Male | 85 | 100 | 95 | 87.5 | 90 | 90.12 |
| Female | 75 | 95 | 90 | 75 | 88.3 | 83.69 |
| p | 0.040a | 0.897 | 0.488 | 0.032a | 0.167 | 0.107 |
| Adherence to gluten-free dietb | ||||||
| Good adherence | 72.5 | 95 | 87.5 | 70.3 | 85 | 80.43 |
| Poor adherence | 85 | 100 | 90 | 84.3 | 90.8 | 85.80 |
| p | 0.414 | 0.464 | 0.425 | 0.052 | 0.433 | 0.219 |
| Socioeconomic level | ||||||
| Low | 87.5 | 100 | 92.5 | 78.12 | 92.49 | 84.85 |
| Moderate | 80 | 95 | 90 | 83.5 | 90 | 84.7 |
| High | 70 | 100 | 90 | 84.37 | 85 | 83.69 |
| p | 0.650 | 0.137 | 0.951 | 0.703 | 0.763 | 0.979 |
| Duration of diseasec | ||||||
| r | −0.249 | 0.036 | −0.300 | −0.362 | −0.240 | −0.313 |
| p | 0.755 | 0.798 | 0.031a | 0.008a | 0.875 | 0.025a |
EFS, emotional functioning score; SoFS, social functioning score; ScFS, school functioning score; TPhHS, total physical health score; TPsHS, total psychosocial health score; TSS, total scale score.
There were no PD in 26 (50%) of the children with CD. On the other hand, ten (19%) had depression, six had (12%) anxiety disorder, five had (9%) adjustment disorder, two (4%) had concomitant depression and anxiety disorder, and three (6%) had concomitant adjustment disorder and anxiety disorder.
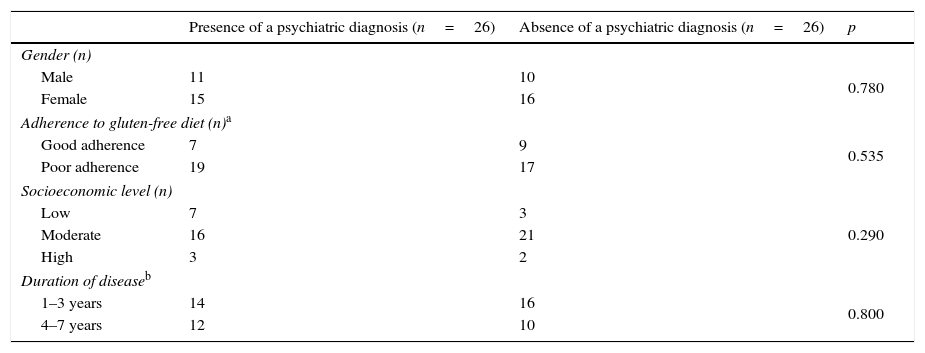
The effects of sociodemographic variables on presence of psychopathology were analyzed in Table 4. There were no significant differences for sociodemographic variables between patients with a psychiatric diagnosis and those without a psychiatric diagnosis (p>0.05).
Effects of sociodemographic-clinical variables on the presence of psychopathology in children diagnosed with celiac disease.
| Presence of a psychiatric diagnosis (n=26) | Absence of a psychiatric diagnosis (n=26) | p | |
|---|---|---|---|
| Gender (n) | |||
| Male | 11 | 10 | 0.780 |
| Female | 15 | 16 | |
| Adherence to gluten-free diet (n)a | |||
| Good adherence | 7 | 9 | 0.535 |
| Poor adherence | 19 | 17 | |
| Socioeconomic level (n) | |||
| Low | 7 | 3 | 0.290 |
| Moderate | 16 | 21 | |
| High | 3 | 2 | |
| Duration of diseaseb | |||
| 1–3 years | 14 | 16 | 0.800 |
| 4–7 years | 12 | 10 | |
In this study, the authors investigated quality of life, psychopathology, and the probable factors associated with both concepts, and found that all subscale scores of PedsQL we significantly lower in the study group. In this regard, it was concluded that CD impaired quality of life by decreasing functionality in social relations, emotional life, and physical health. It was also found that at least one psychiatric diagnosis was present in half of the cases.
Interesting results were reported in the literature regarding quality of life and psychopathology in children with CD. Peters et al. reported that exposure to gluten resulted in depressive symptoms even though it did not result in gastrointestinal symptoms in patients with non-celiac gluten sensitivity, which might explain why the patients with non-celiac gluten sensitivity felt better with a GFD.14 A study followed up nine adolescents with CD between the ages of 12 and 16 years for six months on a monthly basis with the K-SADS-PL and a child behavior control list, and measured serum amino acid and antibody levels before and after GFD. The authors diagnosed three adolescents with major depressive disorder, two adolescents with disruptive behavior disorder, and one adolescent with a learning difficulty; however, they did not find any PD in four (44%) patients. In the same study, it was reported that the tryptophan levels of the adolescents were low before GFD, and psychiatric symptoms decreased and serum tryptophan levels increased three months after starting the diet.15 Those results by Pynnönen et al. indicate that the most important factor for the high psychiatric diagnosis prevalence in the present study was the low adherence rate to GFD. Wagner et al. performed a study to investigate the effects of adherence to GFD on quality of life on 283 adolescents with CD and 82 healthy controls. The authors did not find any difference between the adolescents that adhered to the diet and the healthy controls for quality of life and well-being; however, the adolescents who did not adhere diet had worse quality of life; they felt sick, had more psychiatric problems, and had more problems in school. In the same study, it was emphasized that adherence to the diet was the main factor for an optimal quality of life, and those with low adherence to the diet should have psychological support.16 Those data show the importance of adherence to GFD in order to decrease psychiatric diagnosis/symptoms. Although the low compliance with GFD and the high prevalence of psychiatric diagnoses are notable in the present study, the degree of the compliance with GFD was not directly correlated with the presence or absence of a psychiatric diagnosis. Even when possible variants (sex, duration of disease) were examined, a direct correlation could not be shown. Therefore, to evaluate the potential effect of low GFD compliance on the appearance of psychopathology, a larger study sample may be needed.
The most frequently diagnosed disease was depression (23%) in this study; however, the rates of anxiety (22%) and adjustment (15%) disorders were also high when the comorbid diagnoses were taken into consideration. From another point of view, low rate of adherence to GFD may mean more exposure to gluten, and hence more psychiatric signs/diagnoses. Although the etiopathogenesis of psychiatric signs/diagnoses observed in CD is not clear, tryptophan deficiency due to malabsorption in patients with poor adherence to diet may cause a hyposerotonergic state in the central nervous system.17–20 Another important factor is psychosocial stress related to CD and GFD.21–23 Not being able to dine out, difficulty to find gluten-free foods, and inconvenience in school and social life result in isolation and stigmatization. Those may cause low self-esteem, and constitute risk factors for psychopathology.24,25 In accordance with the literature and in conjunction with the high prevalence of psychopathology seen in the present study, the statements that indicated low self-esteem in sentence completion test were as follows: “unfortunately I am a sick person,” “I wish I was not sick,” “I don’t understand why I am sick,” and “I never forget that I am sick.”
An interesting point in this study is that emotional functionality and physical health were affected more in girls. Supporting those data, all patients diagnosed with depression were girls in the study that investigated nine adolescents with CD.15 Various studies have reported higher anxiety levels in women diagnosed with CD26–28 and some others indicated that emotional well-being significantly improved with GFD in women.22 Studies conducted on adults showed that duration of disease was another factor that could affect the quality of life. Barratt et al. did not show a correlation between duration of disease and quality of life.29 On the other hand, Roos et al. did not find any difference between patients with CD who had been treated for ten years and the control group.30 Contradictory results may be explained by the differences in adherence to GFD. It is clear that patients with low adherence to GFD will become worse, both regarding physical and psychological well-being. In the present study, the rate of adherence to diet was low, and physical health, school life, and emotional functionality scores decreased as the duration of the disease increased.
This study has some limitations. First, it was cross-sectional, and there was no prospective follow-up. Therefore, there are no data concerning how the therapeutic interventions of the child psychiatrist affected adherence to diet, quality of life, and frequency of psychopathology. In addition, the small number of the patients included prevents generalization of the results. No relation was found between high diagnosis prevalence and the investigated variants, and this may be due to the small size of the study sample.
In conclusion, this study showed impaired quality of life and increased rate of psychopathology in children with CD. When the possible factors that cause these results were investigated, it was found that these outcomes are related with being female and the decrease in some parts of quality of life due to the duration of the disease. On the other hand, according to these findings, both impaired quality of life and increased psychopathologies were not related with worse compliance with a GFD. From this point, further prospective studies with larger sample size are needed to determine the factors that affect quality of life and high frequency of psychopathology in patients with CD. The present study indicates that children and adolescents diagnosed with CD should be followed up by a child psychiatrist for a successful adherence to diet, and hence an optimal quality of life and mental health.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Sevinç E, Çetin FH, Coşkun BD. Psychopathology, quality of life, and related factors in children with celiac disease. J Pediatr (Rio J). 2017;93:267–73.