congenital adrenal hyperplasia (CAH) newborn screening can prevent neonatal mortality in children with the salt-wasting form of the disease and prevent incorrect gender assignments, which can occur in females. However, the occurrence of false-positive results in preterm or low-birth-weight newborns creates some diagnostic difficulties, with consequent therapeutic implications. This study aimed to report the results of a pilot project for neonatal CAH screening conducted in the state of Minas Gerais, Brazil from 09/2007 to 05/2008 with a three-year follow-up.
Methodsdried blood specimens were collected on filter paper cards three to seven days after birth of all newborns in the period. Samples were analyzed for 17-hydroxyprogesterone using an enzyme-linked immunosorbent assay (ELISA).
Resultsa total of 159,415 children were screened. The apparent incidence of the classic variant of the disease was 1:9,963, based on initial diagnoses following newborn screening. During the follow-up period, eight of 16 children initially diagnosed with CAH were reclassified as unaffected, resulting in a revised incidence of 1:19,927. The false-positive rate was 0.31%, and the positive predictive value was 2.1%. Sensitivity and specificity were 100% and 99.7%, respectively.
Conclusionsnewborn screening is an important public health policy in developing countries such as Brazil, where CAH remains underdiagnosed. It has great potential to identify children with the disease who otherwise cannot be diagnosed earlier. Long-term follow-up and monitoring of all children with positive screening results are crucial to ensure a correct diagnosis and to calculate a reliable incidence ratio of the disease.
a triagem neonatal para hiperplasia adrenal congênita (HAC) pode evitar a morte de recém-nascidos com a forma perdedora de sal e o registro civil incorreto das meninas. Entretanto, a ocorrência de resultados falso-positivos em recém-nascidos pré-termos ou com baixo peso ao nascer gera dificuldades diagnósticas, com consequentes implicações terapêuticas. O objetivo do estudo foi avaliar os resultados do projeto piloto de triagem neonatal para HAC realizado no estado de Minas Gerais, Brasil, de setembro de 2007 a maio de 2008 com acompanhamento de três anos.
MétodosA dosagem da 17-hidroxiprogesterona foi realizada por ensaio imunoenzimático (ELISA), em amostras de sangue seco coletadas em papel-filtro, três a sete dias após o nascimento de todos os recém-nascidos no período.
ResultadosForam triadas 159.415 crianças. Observou-se incidência de 1:9.963 para a forma clássica da doença, baseando-se nos diagnósticos iniciais. Durante o período de acompanhamento, 8 de 16 crianças inicialmente diagnosticadas com HAC foram reclassificadas como não afetadas, resultando em uma incidência corrigida de 1:19.927. A taxa de falsos positivos foi de 0,31%, e o valor preditivo positivo foi de 2,1%. A sensibilidade e a especificidade foram 100% e 99,7%, respectivamente.
Conclusõesa triagem neonatal é uma importante política de saúde pública para países em desenvolvimento como o Brasil, onde a HAC continua subdiagnosticada. Ela possui grande potencial para identificar crianças que poderiam não ter a doença reconhecida precocemente. O acompanhamento em longo prazo e o monitoramento de todas as crianças com resultados positivos na triagem são cruciais para confirmação diagnóstica e para o correto cálculo da incidência da doença.
Congenital adrenal hyperplasia (CAH) consists of a group of inborn autosomal recessive disorders that are characterized by the deficiency of one of the enzymes involved in cortisol synthesis in the adrenal cortex. Over 90% of CAH cases are due to 21-hydroxylase deficiency (21-OHD), which is one of the most common inborn errors of metabolism, with a variable incidence according to ethnicity and geography.1–3
The global incidence of the classic form of CAH is 1:15,000 live births, as determined by screening programs. Frequencies varying from 1:10,000 to 1:14,000 have been observed in Europe. In North America, the incidence varies from 1:15,000 to 1:16,000. The reported rates of CAH have been as high as 1:280 among the Yupik people of Alaska and 1:2,100 on the French island of Réunion in the Indian Ocean; both of these populations are geographically isolated.4 The reported incidence of CAH in the two Brazilian states that have routinely included CAH in their public newborn screening programs is 1:11,655 in the South (Santa Catarina) and 1:10,325 in the Midwest (Goiás).5,6
Newborn screening for CAH, which is now performed in many countries, has reduced the number of deaths by enabling earlier diagnosis.1 In females, the classic form of the disease can be diagnosed through the detection of ambiguous genitalia (AG) at birth. In males, however, the absence of overt physical signs at birth can lead to avoidable deaths caused by salt-losing crises.
The main goals of screening are to detect the severe, salt-wasting (SW) form of the disease; to prevent shock, brain damage or death, by implementing pre-symptomatic treatment; and to prevent or shorten the period of incorrect gender assignment that can occur in females.7,8 However, the occurrence of false-positive results in sick children, preterm or low birth weight newborns creates some diagnostic difficulties, with consequent therapeutic implications.9 Newborn screening for CAH also provides knowledge of the real incidence of the disease in the population.
The objective of this study was to provide the results of a pilot project for neonatal CAH screening developed in the state of Minas Gerais (MG), Brazil, aimed at establishing a routine program.
MethodsThe pilot project for neonatal CAH screening was included in the newborn screening program of the State of Minas Gerais (PTN-MG) from September of 2007 to May of 2008.
This study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (UFMG), Brazil (ETIC 392/07) and by the Minas Gerais State Health Department. The study includes only data of children whose legal guardian provided written, informed consent. The PTN-MG has been implemented by the state health administration in partnership with the Center for Newborn Screening and Genetic Diagnostics (Núcleo de Ações e Pesquisa em Apoio Diagnóstico - NUPAD), UFMG, since September of 1993. The PTN-MG covers all municipalities in the state, and routinely screens for four diseases: congenital hypothyroidism, phenylketonuria, cystic fibrosis, and sickle cell disease. The newborn screening program in Minas Gerais follows the Brazilian public health recommendations, which aim to achieve 100% population coverage and define the newborn screening process in five steps: laboratory testing, active surveillance of suspected cases, diagnostic confirmation, treatment, and follow-up by a multidisciplinary team. Although recommended, universal newborn testing has not yet been fully implemented in many Brazilian states, due in part to financial inequality and Brazil's large territory. Minas Gerais is one of the largest Brazilian states, with 853 municipalities and approximately 19,600,000 inhabitants (according to the 2010 census by the Instituto Brasileiro de Geografia e Estatística [IBGE]).
A strict protocol is routinely followed to ensure reliable results. Whole blood is drawn via a heel prick and dried on filter paper cards (S&S 903®) three to seven days after birth. Dried blood spot specimens are obtained in health care centers (or in hospitals for preterm or sick newborns) and then mailed to NUPAD, where they are processed. A positive result triggers an immediate outreach process. The NUPAD team makes telephone contact with the health care center (or hospital), in order to either get a new sample collected or to refer the child to medical consultation, according to the specific protocol for each disease and the degree of alteration of the result.
Blood samples are processed and analyzed at NUPAD's laboratory, and the results are delivered to the health care centers (or hospitals) as quickly as possible. There are approximately 20,000 births monthly in Minas Gerais, and nearly the same number of newborns are screened through the aforementioned public program. The current population coverage of the program is 98%.
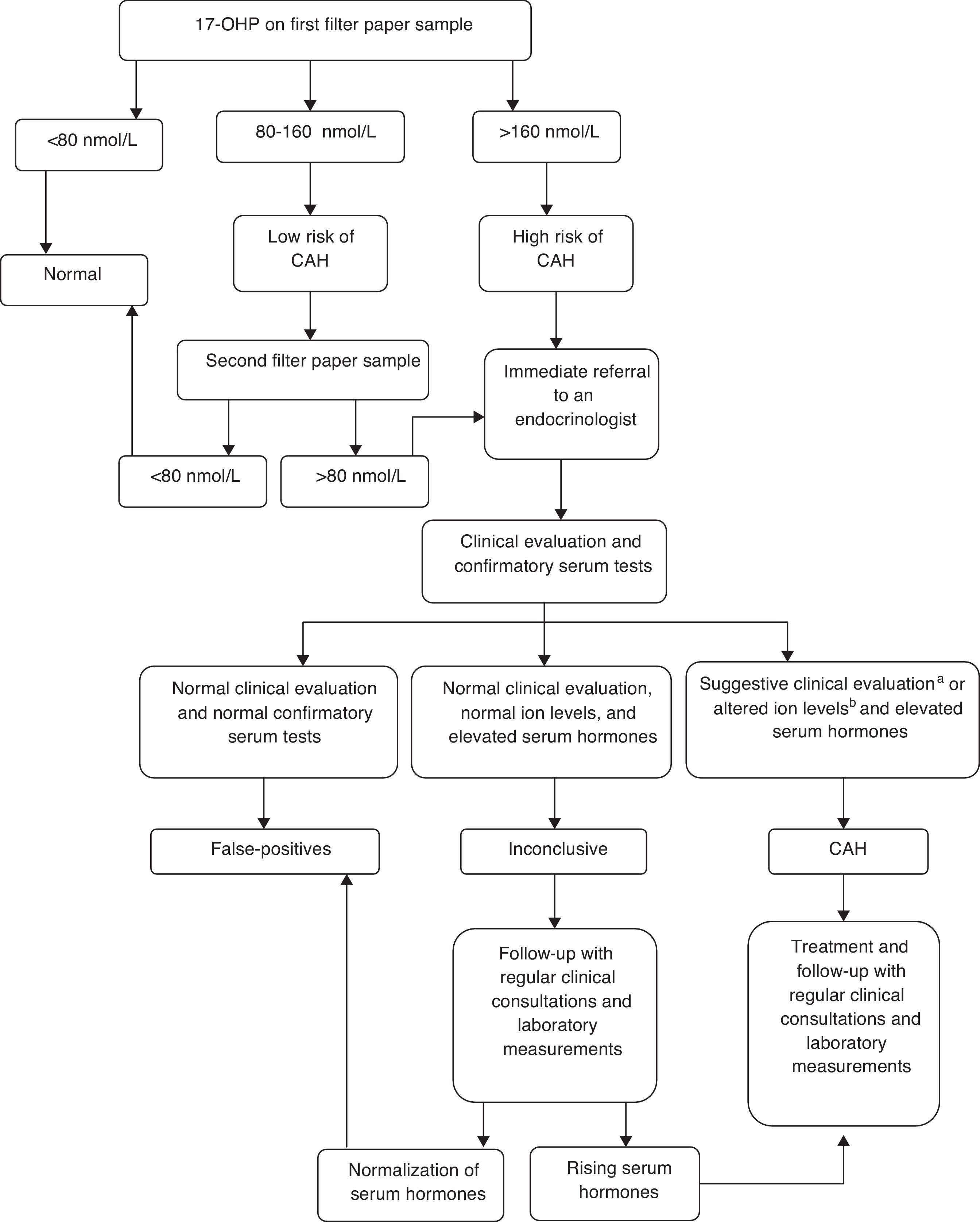
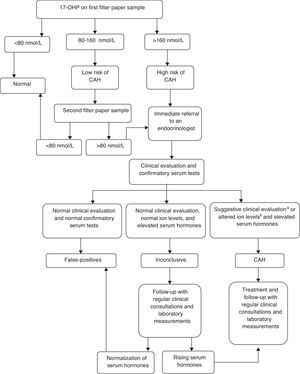
Newborn screening for CAH was implemented as part of a research study to prepare the state for an expansion in its program, which is expected to include this disease in the routine panel. It was performed by measuring 17-hydroxyprogesterone (17-OHP) levels using the sample isolated from dried blood spot specimens for the routine screening by PTN-MG. The 17-OHP levels were measured using the Umelisa 17-OH Progesterona Neonatal (Havana, Cuba) assay with a reference value (RV) of 80 nmol/L (conversion factor: 1 nmol/L of blood=0.73 ng/mL of serum) for normal weight (≥ 2,500g) and full term infants (≥ 37 weeks of gestation). A flow diagram based on established protocols in literature was proposed, and the cut-offs 80 nmol/L and 160 nmol/L were adopted for defining the procedures. (Fig. 1).
Following the preliminary data analysis, a specific 17-OHP cut-off value was determined for preterm infants (< 37 weeks of gestation) and children with birth weights below 2,500g, based on the 99th percentiles of the results analyzed. All samples with 17-OHP values below 160 nmol/L were considered to be normal.
All children who met the criteria and needed a follow-up were referred to pediatric endocrinologists at the outpatient clinic of the University Hospital, where they were clinically evaluated. Confirmatory serum tests for 17-OHP, androstenedione, testosterone, sodium (Na+), and potassium (K+) were conducted for all children at their first visit to the endocrinologist. Serum 17-OHP was measured using radioimmunoassay (RV<204 ng/dL). Androstenedione (RV<1.6 ng/mL) and testosterone (RV=male term: 0.75-4.0 ng/mL; female term: 0.20-0.64 ng/mL; male preterm: 0.37-1.98 ng/mL; and female preterm: 0.05-0.22 ng/mL) were measured using chemiluminescence.
Children who presented clinical evidence of salt loss (weight loss or insufficient weight gain and/or dehydration signs), and females with signs of virilization (Prader stages I-V) plus increased serum hormones (17-OHP, androstenedione, and testosterone), low Na+ levels (< 135 mmol/L), and high K+ levels (> 5.5 mmol/L) were assigned a CAH diagnosis. They were treated with steroids: 10 to 15mg/m2 of hydrocortisone acetate and 0.1 to 0.2mg of fludrocortisone, daily.
Diagnoses were deemed as inconclusive when the results of the confirmatory serum hormones were altered, but the ion levels were normal and clinical signs were absent. Children who were diagnosed with CAH or who had inconclusive diagnoses were followed-up in regular clinical consultations, and had laboratory measurements of their 17-OHP and androstenedione levels. Inconclusive cases were monitored until the children's 17-OHP levels normalized. At that point, these cases were classified as false positives, and the children were discharged from the program.
Data were obtained from the screening records and analyzed using Microsoft® SQL Server™ 2000 (Washington, EUA) software. To calculate the incidence of CAH, the number of CAH cases confirmed by three years of follow-up visits was divided by the number of screened children. Positive predictive value (PPV), sensitivity, and specificity were calculated.
ResultsFrom September of 2007 to May of 2008, 159,415 children were screened for CAH in Minas Gerais, which corresponds to almost all the children born in the state during this period. Children had a median age of 6 days at screening (mean=8±14 days).
During this period, 16 children were assigned a diagnosis and began treatment for CAH, and the incidence of the classic form of the disease was determined to be 1:9,963 based on the initial diagnoses. However, after three years of follow-up visits, only eight of these children (three SW males) were confirmed to have CAH, and maintained under continued treatment. The other eight children received glucocorticoid treatment during the first year of life (subsequently discontinued), and a diagnosis of CAH was discarded following clinical evaluations and normal 17-OHP serum levels after withdrawal of medication. These children exhibited high 17-OHP levels and at least one clinical sign that suggested CAH (insufficient weight gain, mild hyponatremia, or both) at their first visit. During the follow-up, a complete normalization of 17-OHP was achieved without periodic adjustments of the hydrocortisone dosage. They remained asymptomatic after withdrawing medication, and were considered false positives.
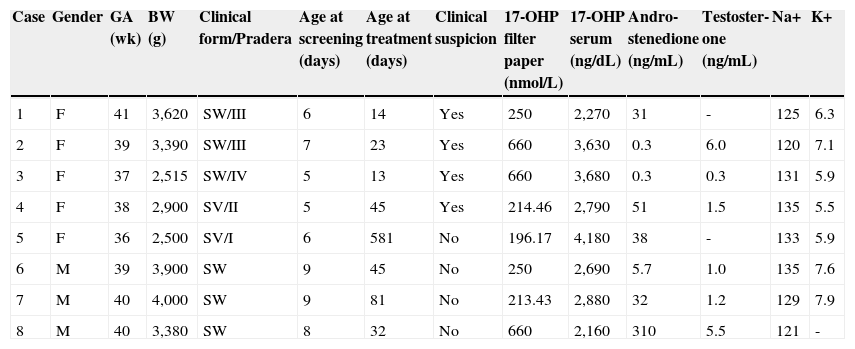
Therefore, based on the final results of this pilot project, the incidence of the classic form of CAH in Minas Gerais, Brazil was determined to be 1:19,927, with a male-to-female ratio of 1:1.6. Table 1 summarizes the characteristics of the eight affected children.
Clinical and laboratory findings of children with congenital adrenal hyperplasia diagnosed by the neonatal screening project.
| Case | Gender | GA (wk) | BW (g) | Clinical form/Pradera | Age at screening (days) | Age at treatment (days) | Clinical suspicion | 17-OHP filter paper (nmol/L) | 17-OHP serum (ng/dL) | Andro-stenedione (ng/mL) | Testoster-one (ng/mL) | Na+ | K+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 41 | 3,620 | SW/III | 6 | 14 | Yes | 250 | 2,270 | 31 | - | 125 | 6.3 |
| 2 | F | 39 | 3,390 | SW/III | 7 | 23 | Yes | 660 | 3,630 | 0.3 | 6.0 | 120 | 7.1 |
| 3 | F | 37 | 2,515 | SW/IV | 5 | 13 | Yes | 660 | 3,680 | 0.3 | 0.3 | 131 | 5.9 |
| 4 | F | 38 | 2,900 | SV/II | 5 | 45 | Yes | 214.46 | 2,790 | 51 | 1.5 | 135 | 5.5 |
| 5 | F | 36 | 2,500 | SV/I | 6 | 581 | No | 196.17 | 4,180 | 38 | - | 133 | 5.9 |
| 6 | M | 39 | 3,900 | SW | 9 | 45 | No | 250 | 2,690 | 5.7 | 1.0 | 135 | 7.6 |
| 7 | M | 40 | 4,000 | SW | 9 | 81 | No | 213.43 | 2,880 | 32 | 1.2 | 129 | 7.9 |
| 8 | M | 40 | 3,380 | SW | 8 | 32 | No | 660 | 2,160 | 310 | 5.5 | 121 | - |
BW, birth weight; F, female; GA (wk), gestational age in weeks; K+, potassium; M, male; Na+, sodium; SV, simple virilizing; SW, salt-wasting; 17-OHP, 17-hydroxyprogesterone
* stages of genital virilization
All the children with CAH were considered at high risk upon screening, and were immediately referred for medical evaluation. The median 17-OHP value of the filter paper blood samples from the children who were ultimately confirmed to have CAH was 250 nmol/L (196-660 nmol/L).
From the eight children diagnosed with CAH, six (75%) had the salt-wasting form, and two had the simple virilizing form. All five female newborn exhibited clinical signs of AG at birth (Prader stages I-IV) that suggested a diagnosis before the release of the screening results. One female (Prader I genitalia) and all three male children were diagnosed primarily via neonatal screening. None of the affected females had incorrect gender assignment. Consanguinity was identified among the parents of Cases 3 and 7; however, none of the children had a positive family history of CAH.
The average age at screening of the children diagnosed with CAH was 7±2 days. The median age at diagnosis (and at the beginning of treatment) was 39 days (13-581 days). The diagnosis of the simple virilizing form of CAH was delayed for one female child, who was born prematurely (Case 5) with a Prader I genitalia. She was evaluated at 49 days of age; however, no treatment was initiated until the age of 19 months, when the diagnosis was confirmed.
The median serum 17-OHP level (RV<204 ng/dL) was high: 2,835 ng/dL (2,160-4,180 ng/dL). The 17-OHP levels of the children with the salt-wasting form did not differ from those of the children with the simple virilizing form (p=0.43). The median serum androstenedione levels (RV<1.6 ng/mL) were very high: 32 ng/mL (0.3-310 ng/mL). The serum testosterone levels were high, and did not differ between male and female children (p=0.83). The Na+ and K+ levels in the children with the salt-wasting form of CAH were in the abnormal range: 127±6 mmol/L (RV=135-145 mmol/L) and 7.0±0.8 mmol/L (RV=3.5-5.5 mmol/L), respectively, at diagnosis.
The false-positive rate and the PPV were calculated for the first six months of the project. During this period, 106,476 children were screened for CAH, and 328 cases were determined to be false positives after screening and follow-up visits. No false-negative cases were identified during the first three years of follow-up, but active search was not performed. Therefore, the false-positive rate was 0.31%, and the PPV was 2.1%. Sensitivity and specificity were 100% and 99.7%, respectively. Of the 315 children with false-positive diagnoses who were monitored by the PTN-MG, 63% were either born prematurely, had a low birth weight, or experienced both conditions. The false-positive cases were followed-up for a median of 17 months after birth (2.6-34 months) until their 17-OHP levels normalized.
DiscussionAn incidence ratio of 1:19,927 was calculated for the classic form of CAH in Minas Gerais, following the screening pilot project. This incidence was lower than that of the other Brazilian states where CAH screening has been performed: 1:7,500 and 1:13,809 in the South and 1:10,325 in the Midwest.5
Goiás (in Midwestern Brazil) was the first Brazilian state to include CAH in its public program of newborn screening, which was implemented in 1997 and is now widely established. An incidence ratio of 1:10,325 was reported for the classic form of CAH in the state, after follow-up of an average of 16 months.5
Another Brazilian state that has routinely included CAH in its public program of screening since 2000 is Santa Catarina (in Southern Brazil), with a reported incidence ratio of 1:11.655 among 378.337 newborns.6
The incidence of CAH in another Southern state (1:7,500) was reported as one of the highest in the world.10 However, this number was disputed by a recent study where the authors emphasized that those figures were obtained through voluntary, self-paid tests with unconfirmed clinical outcomes.5
This large variation in reported prevalence might be due to Brazil's large territory and diversity, but it might also be due to methodological differences across the studies.
The screening in Minas Gerais was performed over eight months, but the clinical and laboratory follow-ups with the children who had positive screening tests lasted up to 34 months, until the diagnosis was either confirmed or proven to be a false-positive result.
One explanation for the lower incidence of CAH in Minas Gerais observed in the present study compared with those of other Brazilian states may be the lengthy follow-up period, which enabled the exclusion of false positives. A high incidence of 1:9,963 was initially incorrectly assessed; after the clinical and laboratory follow-up, approximately half of the initial diagnoses of CAH were determined to be false-positives. The children with false-positive diagnoses exhibited high confirmatory serum levels of 17-OHP, and at least one clinical sign that suggested CAH at the beginning of the follow-up. CAH was discarded and the children remained asymptomatic after withdrawing medication. Therefore, only the confirmed cases of CAH were considered in the calculation of disease incidence. This points to follow-up monitoring as an essential step for a reliable CAH diagnosis.
Difficulties with CAH diagnoses are common9 and may have accounted for the initially high apparent incidence of the disease in the present pilot study. Decisions regarding treatment should always be postponed for asymptomatic children and for those with only slightly elevated 17-OHP levels.11 Tests with higher specificities for diagnostic confirmation, such as a molecular genetic analysis of the CYP21 gene, have been suggested as a complementary analysis in such cases.9,12
The present study was the first to examine the incidence of CAH in Minas Gerais, a large state in the Southeastern region of Brazil, with a territory of 586,528km2. The disease incidence herein reported is close to those reported in Japan (1:18,000), New Zealand (1:21,270), and in Northeastern Italy (1:21,380).7,13,14
The duration of this study might be considered a hindrance for drawing conclusions. However, the incidence calculated in this screening pilot project is highly credible, due to the state-wide coverage of the PTN-MG (nearly 100%) and to the large number of screened children despite the short period of time devoted to the pilot.
The evaluation of this pilot project points to the potential for reducing CAH morbidity and mortality rates among the affected children.
Clinical suspicion for CAH was low at birth: half of the cases in the present study were diagnosed by screening alone. One female with the simple virilizing form of the disease and Prader I genitalia, as well as three male children with the salt-wasting form of the disease, could not have been diagnosed earlier without the use of screening, and the males could have died from dehydration and shock. It is known that more female than male children are diagnosed in the absence of screening (4:1), which indicates that there are unrecognized deaths due to CAH in male children.15,16
CAH treatment onset was late in this pilot study, mainly due to the initial difficulties to implement a protocol for CAH in the PTN-MG without previous experience with the management of this disease within the state program of newborn screening. According to the Working Group on Neonatal Screening of the European Society for Pediatric Endocrinology, the results of newborn screening for CAH should ideally be available within ten days after birth.17 This timeline is important, because delays in beginning treatment raise concerns about the potential benefits of implementing screening programs for CAH. Delayed diagnoses may invalidate the primary goal of CAH screening, i.e. to avoid salt-wasting crises and thereby reduce the morbidity and mortality of the disease. Data from the Netherlands published in 2001 showed that specimen collection is usually performed within two days of life, and all the children with CAH diagnosed via screening were able to begin treatment before the tenth day of life, thus preventing severe salt loss.18 However, during the pilot project at Minas Gerais, it was possible to identify children with the disease who otherwise could not be diagnosed earlier, even with an average age of seven days at screening.
Reduced false-positive rates and improved PPVs might be obtained through both optimizing the 17-OHP cut-off points according to a higher number of birth weight categories or gestational age, and implementing a second-tier test. These strategies are used in many countries19–23 to offset the fact that serum 17-OHP levels may take several months to return to normal in false-positive cases. This time can represent significant psychological stress for the family.
Because of the poor management of CAH in developing countries, the authors believe that implementing a program of newborn screening for CAH is an important public health policy with confirmed benefits, as herein reported. Newborn screening programs represent one of the great advances for the early care of treatable diseases in childhood.24 Based on the development of this pilot program, it can concluded that the implementation of a routine program of newborn screening for CAH would be beneficial. In addition, long term follow-up and monitoring of all children with positive screening results are crucial to ensure a correct diagnosis and to calculate a reliable incidence ratio. Furthermore, to avoid the occurrence of overtreatment, improved diagnostic methods and follow-up procedures should be introduced before newborn screening for CAH is implemented. Multicenter and long-term analyses are needed to evaluate these data appropriately. The results herein reported may help overcome difficulties in the implementation of future programs.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Scott Grosse from the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, for his helpful comments on the manuscript.
Please cite this article as: Pezzuti IL, Barra CB, Mantovani RM, Januário JN, Silva IN. A three-year follow-up of congenital adrenal hyperplasia newborn screening. J Pediatr (Rio J). 2014;90:300–7.
Study conducted at the Núcleo de Ações e Pesquisa em Apoio Diagnóstico (NUPAD), Faculdade de Medicina/Hospital das Clínicas, Universidade Federal de Minas Gerais (UFMG), Minas Gerais, MG, Brazil.