This review aimed to provide a critical overview on the pathogenesis, clinical findings, diagnosis, imaging investigation, treatment, chemoprophylaxis, and complications of urinary tract infection in pediatric patients.
Source of dataData were obtained independently by two authors, who carried out a comprehensive and non-systematic search in public databases.
Summary of findingsUrinary tract infection is the most common bacterial infection in children. Urinary tract infection in pediatric patients can be the early clinical manifestation of congenital anomalies of the kidney and urinary tract (CAKUT) or be related to bladder dysfunctions. E. coli is responsible for 80–90% of community-acquired acute pyelonephritis episodes, especially in children. Bacterial virulence factors and the innate host immune systems may contribute to the occurrence and severity of urinary tract infection. The clinical presentation of urinary tract infections in children is highly heterogeneous, with symptoms that can be quite obscure. Urine culture is still the gold standard for diagnosing urinary tract infection and methods of urine collection in individual centers should be determined based on the accuracy of voided specimens. The debate on the ideal imaging protocol is still ongoing and there is tendency of less use of prophylaxis. Alternative measures and management of risk factors for recurrent urinary tract infection should be emphasized. However, in selected patients, prophylaxis can protect from recurrent urinary tract infection and long-term consequences. According to population-based studies, hypertension and chronic kidney disease are rarely associated with urinary tract infection.
ConclusionMany aspects regarding urinary tract infection in children are still matters of debate, especially imaging investigation and indication of antibiotic prophylaxis. Further longitudinal studies are needed to establish tailored approach of urinary tract infection in childhood.
Esta revisão teve como objetivo fornecer uma visão crítica da patogênese, achados clínicos, diagnóstico, investigação por imagem, tratamento, quimioprofilaxia e complicações da infecção do trato urinário em pacientes pediátricos.
Fonte de dadosOs dados foram obtidos de forma independente por dois autores que fizeram uma pesquisa abrangente e não sistemática em bancos de dados públicos.
Síntese dos achadosA infecção do trato urinário é a infecção bacteriana mais comum em crianças. Em pacientes pediátricos, pode ser a manifestação clínica precoce de anomalias congênitas do rim e trato urinário (CAKUT) ou estar relacionada a disfunções da bexiga. A E. coli é responsável por 80–90% dos episódios agudos de pielonefrite adquirida na comunidade, principalmente em crianças. Os fatores de virulência bacteriana e o sistema imunológico inato do hospedeiro podem contribuir para a ocorrência e gravidade da infecção do trato urinário. A apresentação clínica de infecções do trato urinário em crianças é altamente heterogênea, com sintomas que podem ser bastante obscuros. A cultura de urina ainda é o padrão-ouro para o diagnóstico de infecção do trato urinário e os métodos de coleta de urina em centros individuais devem ser determinados com base na precisão das amostras coletadas. O debate sobre o protocolo de imagem ideal ainda está em andamento e há uma tendência a um menor uso da profilaxia. Medidas opcionais e o manejo dos fatores de risco para infecção do trato urinário recorrente devem ser enfatizados. Entretanto, em pacientes selecionados, a profilaxia pode proteger contra infecção do trato urinário recorrente e consequências em longo prazo. Segundo estudos populacionais, hipertensão e doença renal crônica raramente são associadas à infecção do trato urinário.
ConclusãoMuitos aspectos relacionados à infecção do trato urinário em crianças ainda são motivo de debate, principalmente a investigação por imagem e a indicação de profilaxia com antibióticos. Estudos longitudinais adicionais são necessários para estabelecer uma abordagem personalizada da infecção do trato urinário na população pediátrica.
Urinary tract infections (UTIs) are among the most common bacterial infections in children. Up to 8% of children will experience at least one UTI between the ages of 1 month and 11years,1,2 and up to 30% of infants and children experience recurrent infections during the first six to 12 months after initial UTI.3,4 In the United States, there are about 1.5 million pediatric ambulatory visits annually for UTIs.5 The overall US health care costs for management and treatment of UTI in 2013 was $630 million.6 UTIs cause short-term morbidity such as fever, dysuria, and flank pain, and may also result in long-term renal injury, such as permanent kidney scarring.7
A fundamental issue in the topic of the management of UTI in children is that a single episode may be the sentinel event for an underlying renal abnormality and in 30% of children with congenital anomalies of the kidney and urinary tract (CAKUT), UTI can be the first sign.8,9 Therefore, since the 1960s, the management of UTI in children has been based on the conception that recurrent episodes, particularly with vesicoureteral reflux (VUR), increase the risk of chronic kidney disease (CKD), hypertension, and ultimately end-stage renal disease (ESRD).10 Consequently, the guidelines on the management of UTIs in children are elaborated on the assumptions that prompt diagnosis and treatment and comprehensive imaging investigation might prevent an unfortunate chain of deleterious events and long-term renal injury.
Over the last two decades, the scenario of the management of children with a febrile UTI has changed. The old model proposed that all children with UTI were to be investigated using ultrasound (US), a micturition cystourethrogram (MCUG), and some form of nuclear imaging, such as dimercaptosuccinic acid (DMSA). The aim of these investigations was to identify all children with CAKUT, especially those with VUR and renal scarring. In addition, children with a febrile UTI were hospitalized for intravenous antibiotics and children with VUR of any grade were treated with prophylactic antibiotics.11 Several randomized clinical trials and prospective cohort studies have called into question all these old paradigms. The results of this new body of knowledge led to a review of the existing guidelines once they failed to show any evidence of a change in clinical evolution driven by antibiotic prophylaxis or imaging tools. Moreover, improved prenatal US has revealed that major kidney damage in children is frequently related to the presence of congenital hypodysplasia, associated with urologic abnormalities.12–15 Consequently, recent guidelines on the management of UTIs in children have shown a shift from aggressive imaging investigation and the indiscriminate use of prophylactic antibiotics to a more restrictive and targeted approach.16,17
Despite these advances, the management of UTIs in the pediatric population remains challenging and controversial. Diagnosis, treatment, and follow-up of children with UTI are important issues for general pediatricians and involve multiple decisions.18 It is consensual that a correct diagnosis, appropriate treatment, and a subsequent selected imaging investigation in children with UTI is still pivotal because of the association between UTI, underlying urological abnormalities, and its consequences. Therefore, a prompt diagnosis and immediate initiation of treatment remain important in preventing long-term renal damage. However, it must be pointed out that establishing a suitable approach and identifying children with risk of renal parenchymal damage is not a simple task.
This review article discusses recent recommendations for the diagnosis, treatment, prophylaxis, and imaging of UTI in children based on evidence, and when this is lacking, based on expert consensus.
Source of dataData were obtained independently by two authors who carried out a comprehensive and non-systematic search in the PubMed, Embase, LILACS, Cochrane, Scopus and SciELO databases. Search strategies included Medical Subject Heading terms for “urinary tract infection,” “CAKUT,” “renal scarring,” “vesicoureteral reflux,” “renal ultrasonography,” “renal scintigraphy,” “antibiotic prophylaxis,” and “chronic kidney disease.” No time or language restrictions were established. The search emphasized recent consensus statements, guidelines, meta-analyses, systematic reviews, randomized clinical trials, and prospective cohort studies. The publications were critically selected by the authors.
Summary of findingsPathogenesis of UTIThe role of bacteriaThe urinary tract is normally sterile, except for the distal part of the urethra. Physiologically, the periurethral area has bowel bacteria. In healthy young girls the predominant bacteria is Eschericha coli (E. coli), whereas, in boys, after the first 6 months of life, Proteus mirabilis predominates. On the other hand, bowel bacteria do not usually form the periurethral flora of older children. It should be pointed out, however, that colonization with Gram-negative bacteria generally precedes the occurrence of UTI.19 In some occasions, the prescription of broad-spectrum antibiotics for other infections may produce changes in the normal flora.20
E. coli is responsible for 80–90% of community-acquired acute pyelonephritis episodes, especially in children. Less common uropathogenic bacteria include Proteus mirabilis, Klebsiella spp., and Staphylococcus saprophyticus.21,22 Infectious agents of UTI acquired during hospitalization depend on the hospital environment and underlying host factors.21,22 Bacterial virulence factors and the innate host immune systems may contribute to the occurrence and severity of UTI.23–27
UTI may occur via two routes: hematogenic and ascendant. The hematogenic route is typical in newborns, while the ascendant route characteristically develops after the neonatal period. In newborns, UTI may manifest as sepsis, largely with nonspecific clinical features, including anorexia, vomiting, poor sucking, irritability, lethargy, convulsions, pallor, hypothermia and, sometimes, jaundice.28 As with most infections, in this age group, there is high probability of bacteremia and high rate of mortality (around 10%) due to the spread of infection to other sites, leading to meningitis, for instance.28,29 The ascendant route comprises the migration, fixation, and proliferation of uropathogenic bacteria in the urinary tract. Uropathogenic bacteria may reside for long periods in the gastrointestinal tract before spreading to the periurethral area. After spreading via the perineum to the periurethral area, bacteria ascend the urinary tract against urine flow, and establish infection by means of several mechanisms. The main mechanisms include fimbriae that promote adhesion to urothelial cells, flagella-mediated motility, resistance to antibacterial defenses, and other adaptation strategies.23,26,27
In this regard, the subtype of E. coli strain that causes acute pyelonephritis in healthy children has genes that confer virulence, forming the so-called “pathogenicity islands”.30–32 The sequential activation of these genes increases host tissue attack and bacterial survival. The presence of fimbriae promotes bacterial adhesion to the mucosa that facilitates tissue attack30 by increasing the exposure to other virulence factors, such as hemolysin and lipopolysaccharide (LPS). These toxins secreted by E. coli may affect cellular functions or induce cell death. Uropathogenic strains of E. coli can be identified by the presence of surface antigen expression (OKH serotypes) or of surface expression of P-fimbriae.30,33,34 Different types of fimbriae recognize different oligosaccharide receptor epitopes. Type 1 fimbriae bind to mannosylated epitopes present in the Tamm–Horsfall glycoprotein, in secretory immunoglobulin A (IgA), in bladder cell uroplakins, or in integrin molecules.35–37 S-fimbriae bind to receptors on sialylated glycoproteins and glycolipids, while P-fimbriae recognize Galα1-4Galβ epitopes in the glycolipids, which are antigens in the P blood group system.38
Other virulence factors are LPS, capsular polysaccharide, and hemolysin. LPS is an endotoxin of Gram-negative bacteria that contains lipid A anchored in the outer membrane, as the component responsible for the toxic effects including fever and acute phase response. Other components of LPS are the oligosaccharide core and the repeating oligosaccharide that determines the O-antigen. LPS activates toll-like receptor 4 (TLR4) signaling, after binding to soluble or cell surface-associated CD14.39,40 Capsular polysaccharides are formed from oligosaccharide polymers surrounding bacteria. Capsules confer to bacteria resistance against host defenses by counteracting lytic effects of complement and phagocytosis.41 Hemolysins are cytotoxic, pore-forming proteins that permeate the cell membrane. Hemolysin production was first observed in the 1940s in E. coli causing acute pyelonephritis.
Besides mechanisms of virulence, uropathogenic bacteria may also compete with host cells for nutrients, such as iron. All uropathogenic strains express some molecules responsible for iron uptake. For example, enterobactin is expressed by nearly all E. coli strains, but most E. coli strains causing acute pyelonephritis produce aerobactin, which is a high-affinity iron-binding protein, as well as other iron-sequestering proteins, including yersiniabactin, ChuA, and Iro.42–44
The role of host immune responseHost resistance to UTI depends for the most part on innate immune defenses, mainly during the acute phase of the disease. The response to uropathogenic E. coli is activated by P-fimbriae mediated adhesion to glycolipid receptors, leading to activation of TLRs, of which TLR4 has been considered the most important.45,46 Activation of TLR4 signaling results in the release of transcription factors such as IRF3, which trigger neutrophil recruitment and cytokine production in order to kill bacteria. These mechanisms determine the symptoms and signs of UTI. Urothelial cells produce interleukin-8 (IL-8), which attracts neutrophils to urinary tract leading to pyuria.24,25,47 Infection itself enhances the expression of IL-8 receptors, further stimulating neutrophil attraction and activation. Interleukin-6 (IL-6) is also secreted by urothelial cells. IL-6 activates C-reactive protein (CRP) production and stimulates the production of mucosal IgA.25
Another source of innate immune defense are the antimicrobial peptides (AMPs), which are natural antibiotics produced by nearly all organisms.48,49 AMPs are small cationic proteins expressed by phagocytic and epithelial cells, either constitutively or through induction by invading agents.48
Further supporting the role of innate immunity in UTI is the fact that genetic variation affecting innate immunity influences host susceptibility. For example, mutations in the TLR4 gene promoter lead to low expression of TLR4, which was detected in children with asymptomatic bacteriuria when compared to age-matched controls or children with acute pyelonephritis. In addition, single nucleotide polymorphisms (SNPs) in the gene promoter of the transcription factor IRF3 have been identified in about 80% of patients with recurrent episodes of acute pyelonephritis. Reduced expression of CXCR1, the IL-8 receptor, due to SNPs in the CXCR1 gene, was also found in children with frequent episodes of acute pyelonephritis.50–52 Individuals of blood group P lack functional receptors for P-fimbriae, while children with blood group P1 have an increased risk of acute pyelonephritis. Very few AMPs have been described in the human kidney and urinary tract, which include defensins, cathelicidin, hepcidin, and ribonuclease 7. Other proteins with antimicrobial activity present in the kidney and urinary tract are Tamm–Horsfall protein, lactoferrin, lipocalin, and secretory leukocyte proteinase inhibitor.48,49,53
It should also be mentioned that a specific immune response develops after three to seven days in patients with acute pyelonephritis. As an attempt to stimulate specific immune mechanisms, experimental vaccines against antigens of uropathogenic E. coli have been tested.54 Besides vaccines, other alternative methods and therapeutic strategies to prevent and/ or control UTIs include receptor analogues, pilicides and curlicides, bacterial interference, or phagotherapy.54
The role of host urinary tract malformationsUTIs may be the sentinel event for underlying congenital anomalies of the kidney and urinary tract (CAKUT), although normal anatomy is more common.8 In 30% of children with CAKUT, UTI can be the first sign.9 If pediatricians fail to detect patients at risk of CAKUT, the upper urinary tract may be damaged.
Hypothetically, anatomical or functional alterations of normal urinary flux may certainly predispose to episodes of UTI, and these episodes probably occur in neonates or young infants. In this regard, the VUR has been associated with approximately 20% of neonatal cases of UTI, although the incidence of VUR is not significantly different between genders, birth weight, gestational age, or mode of delivery.55 In a study with infants less than 2 months of age from a neonatal intensive care unit, a rate of anatomic abnormalities in patients with UTI of less than 5% was detected. However, VUR was associated with a younger age at UTI presentation.56 In another study including 45 male infants with first UTI episode occurring early in life, renal ultrasound scan (RUS) and voiding cystourethrogram (VCUG) found CAKUT in half of the cases.57 The most common anomalies were VUR, duplicated collecting system, posterior urethral valves, ureteropelvic junction obstruction, and renal hypodysplasia.57 The DMSA scan revealed renal scars in those with VUR grade 3 or higher.22 Similarly, renal anomalies were found in 47% of febrile infants less than 30 days of age at the first UTI episode.56 However, even in the absence of any abnormalities detected on the RUS or VCUG, infants with UTI can have an abnormal DMSA scan, indicating renal cortical damage. The question is if the renal cortical damage would be an effect rather than a cause of a UTI.9
Clinical findingsEarly and prompt diagnosis of UTIs is paramount to initiating therapy and thereby limiting morbidity and renal damage. In children, however, the diagnosis is rarely straightforward. The clinical presentation of UTIs in children is highly heterogeneous, sometimes misleading, with symptoms that can be quite obscure. As a consequence, unfortunately many UTIs are likely either not diagnosed or diagnosed late.58 Therefore, it is important that the pediatrician or the primary care providers have a high index of suspicion for UTIs in children. The evaluation must include a thorough history and the importance of the physical exam in pediatric patients cannot be overstated.
The clinical manifestations of the UTIs are clearly related with the age of the children and the site of the infection. Smellie et al.,59 in a classic study of 200 children (3 days to 12 years of age) with UTI, demonstrated that the most common symptoms in the first 2 years of life were failure to thrive, feeding problems, vomiting, and fever. In the 2- to 5-year-old child, fever and abdominal pain were the most common symptoms, and after 5 years of age, the classic symptoms and signs of UTI (fever, dysuria, urgency, and costovertebral angle tenderness) predominated. While the history and physical examination represent the cornerstone of an accurate diagnosis, in nonverbal children the clinical suspicion of UTI can be troublesome due to the nonspecific nature of symptoms. The classic presentations of dysuria, frequency, and flank pain in adults are unreliable when applied to pediatric UTI, particularly in infants. The presenting complaints in children tend to be vague, including fever, irritability, lethargy, poor feeding, failure to thrive, and gastrointestinal complaints. In addition, evidence of infection outside the urinary tract does not exclude the possibility of UTI.59,60 To make matters more complicated, symptoms and signs of respiratory or gastrointestinal infections are often present in febrile infants and in children with documented UTI.59,60
Newborns and infants younger than 3 months may have, at onset, vague and nonspecific symptoms of illness that are difficult to interpret, including failure to thrive, diarrhea, irritability, lethargy, malodorous urine, hypothermia, fever, asymptomatic jaundice, and oliguria or polyuria.22 As with most bacterial infections in this age group, there is an elevated probability of bacteremia, sepsis, and high rate of mortality (around 10%) due to the spread of infection to other sites.61,62 In this age group, UTI may also present with less acute, insidious symptoms, such as food refusal, occasional vomiting, pallor, and jaundice.63 In fact, it has been recommended that testing for UTI be part of the evaluation of asymptomatic jaundice in infants younger than 8 weeks, especially those with elevated conjugated bilirubin levels. The American Academy of Pediatrics (AAP) recommends that infants with elevated direct bilirubin levels be screened for UTIs. However, those with elevated unconjugated bilirubin levels should not be excluded, especially if other concerning clinical features are present.63,64
In infants between 3 months and 2 years old, fever is the main symptom, and often the only sign of infection. High temperatures associated with nonspecific manifestations like appetite loss, vomiting, abdominal pain, dehydration, and poor weight gain are commonly found in this age group. It can be rarely associated with specific signs or symptoms related to the urinary tract, such as urinary dysuria and foul-smelling urine.61,65,66 The pediatrician should consider investigating UTI in infants with unexplained fever. The prevalence of UTI in infants and young children with fever that is not localizable by history or physical examination is high. In a meta-analysis, Shaikh et al.67 have shown a pooled prevalence of 7.0% (CI: 5.5–8.4) of UTI among the 14 studies of febrile infants less than 24 months of age. Among males, prevalence rates were highest during the first 3 months of life and declined thereafter. In females, prevalence rates were highest during the first 12 months. According to the AAP guideline, the presence of UTI should be considered in neonates and children between 2 months to 2 years of age with unexplained fever (with strong evidence).68 In the same guideline, the experts pointed out that the two sexes are not affected equally. The prevalence of UTI in febrile girls with 2 months to 2 years is more than twice that in boys (relative risk, 2.27). The prevalence of UTI in girls younger than 1year of age is 6.5%, while, in boys, it is 3.3%. The prevalence of UTI in girls between 1 and 2 years of age is 8.1%, whereas, in boys, it is 1.9%. In an updated guideline, the AAP recommended that if a clinician decides that a febrile infant with no apparent cause for the fever requires antimicrobial therapy to be administered because of ill appearance or another pressing reason, the clinician should ensure the collection of urine specimens for both culture and urinalysis before antibiotic administration. If a clinician assesses a febrile infant with no apparent source for the fever as not being so ill as to require immediate antimicrobial therapy, then the clinician should assess the likelihood of UTI.69
In this regard, Shaikh et al.70 recently developed and validated a calculator for estimating the probability of UTI in young febrile children. Electronic medical records of febrile children aged 2–23 months who were brought to the emergency department of Children’s Hospital of Pittsburgh were reviewed. The authors created an independent training database with 1686 patients and a validation database with 384 patients. They tested five multivariable logistic regression models for predicting risk of UTI; one clinical model had only clinical variables and the remaining had laboratory results. The clinical model had lower accuracy than the laboratory models, indicating the nonspecific signs and symptoms of UTI in young children (clinical model area under the curve [AUC] of 0.80 [95% CI 0.77–0.82] vs. 0.97 [95% CI, 0.96–0.98] to 0.98 [95% CI, 0.98–0.99] for the laboratory models). Models including a Gram-stained smear had better accuracy than those that did not use this exam. The authors concluded that accurate diagnosis of UTI may decrease the delay in starting the treatment and may avoid unnecessary use of antibiotics.70
Older children are better able to verbalize symptoms and, for this reason, specific symptoms of UTI are more commonly identified. Abdominal pain and fever are the most common presenting symptoms in children between 2 and 5 years of age. After 5 years, the classic urinary tract symptoms, including dysuria, frequency, suprapubic or flank discomfort, incontinence, and costovertebral angle tenderness are usually present. Less common signs such as secondary enuresis in a previously toilet-trained child or frank hematuria can also occur. The pediatrician must be aware, however, that sometimes even older children may be less able to describe localized symptoms.61,71,72
Adolescent girls are more likely to present with typical cystitis symptoms including frequency, urgency, dysuria, cloudy urine, hematuria, and lower abdominal discomfort. The prevalence of UTI among adolescent boys is very low. Adolescents are better able to provide history and participate in physical exams. Sexual activity is a special issue for this population that requires additional attention. Sexually transmitted infections (STI) are an important differential diagnosis in adolescents with urinary symptoms. Adolescent girls with vaginitis or a sexually transmitted infection (STI) may present with symptoms similar to UTI. In addition, adolescent girls who are diagnosed with cystitis may have a concurrent vaginitis or STI.5,73
Concerning the differentiation between pyelonephritis and cystitis, host and bacterial biomarkers were recently investigated in blood and urine samples from 61 children with febrile UTI.74 To detect children with pyelonephritis, a DMSA scan was performed within two weeks of UTI diagnosis. Inflammatory proteins were measured in blood and urine samples, and in children with UTI caused by E. coli, polymerase chain reaction for four previously identified virulence genes was also performed. The best urinary markers that differentiated pyelonephritis from cystitis were the chemokines CXCL1, CXCL9, and CXCL12, C-C motif chemokine ligand 2, INF-γ, and IL-15. The best blood marker for pyelonephritis was procalcitonin. However, E. coli virulence genes did not associate with pyelonephritis.
The physical examination of children with UTI can be nonspecific. Occasionally, an abdominal mass may be palpated secondary to an enlarged kidney. In older children, palpation of the flank or abdomen may provoke discomfort. Suprapubic palpation can reveal a palpable bladder. Examination of the external genitalia and perineum is important and may reveal the possible origin of symptoms like balanitis, meatal ulcer, or vulvovaginitis. Regardless of age, all children should have their sacral region examined for dimples, pits, or a sacral fat pad, because the presence of these signs is associated with neurogenic bladder. The magnitude of the temperature elevation might assist with the clinical evaluation. Low-grade fevers are more likely to implicate a lower tract infection, whereas temperatures greater than 39.0°C are indicative of upper tract infection.66 Hypertension can be transitory in acute pyelonephritis. However, if elevated blood pressure persists, the suspicion of CAKUT or renal parenchyma lesions should be considered.
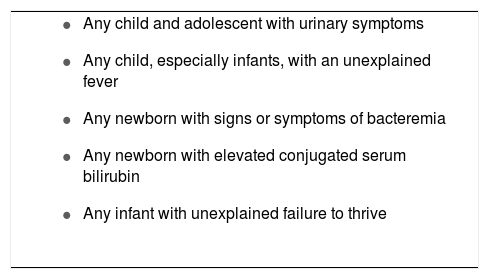
In summary, diagnosis of UTI in children, especially in younger infants, can be difficult and requires a high index of suspicion. Some key points must be considered by the pediatrician to prevent missing the diagnosis of UTI in children. Many guidelines and opinions of experts have recommended an investigation for possible UTI in some selected conditions (Box 1). In addition, it is a pivotal issue that the pediatrician recognizes children at risk for UTI (Box 2). For instance, possible urinary tract anomaly, voiding dysfunction, and constipation must be investigated during the history taking.75 In this regard, the pediatrician should inquire about fetal ultrasonography, as the majority of urinary tract anomalies, a well-known strong risk factor for UTI, are currently suspected in utero.75,76 Therefore, the pediatrician with a high index of suspicion, a thorough and accurate history, and a complete physical exam might be able to prevent the delay of UTI diagnosis and thus avoid kidney damage.
Conditions to consider for the investigation of urinary tract infection in children.
|
The main methods of urine collection include clean-catch, plastic bag, bladder catheterization, and suprapubic aspiration (SPA). These four methods have variable contamination rates and invasiveness.77
Most commonly urine is obtained from clean-catch urine samples, especially for toilet-trained children.69,78 In newborns, infants, and when voided specimens cannot be obtained, the best way to collect urine is still controversial. Clean-catch urine is also possible to obtain in non-toilet trained children. In these cases, the patient is placed in the lap of a parent or nurse holding a sterile foil bowl underneath the genitalia. A systematic review of five studies comparing clean voided urine specimens with bladder tap specimens reported a wide variation among studies, with sensitivity ranging between 75% and 100% and specificity varying between 57% and 100%.79 Conversely, Ramage et al.80 previously detected a good correlation between results of urine culture obtained by this method and by SPA. In regard to urine contamination, a study with 120 infants and children showed a 25% contamination rate with samples from clear-voided urine when compared to samples from SPA.81
Collection in a sterile plastic bag attached to the cleaned genitalia is a technique often used in several centers.82 Although a culture-negative urine bag sample is reliable, this technique has a high rate of false-positive cultures because of contamination by periurethral flora.82 In a cross-sectional study of 303 non toilet-trained children under age 3 years at risk for UTI, sensibility and specificity of the urinalysis collected by clean-voided bag were compared with catheter urine specimens using the catheter culture as the gold standard.83 The bag dipstick was more sensitive than the catheter dipstick for the entire study sample: 0.85 vs. 0.71, respectively. However, specificity was consistently lower for the bag specimens than for the catheter specimens: 0.62 vs. 0.97, respectively.83
International guidelines generally recommend that urine should be collected by bladder catheterization17,69,78 or SPA under ultrasound control.17,78 SPA is the most sensitive method for obtaining an uncontaminated urine sample. When urine is collected by SPA, any colony count is considered to represent significant bacteriuria. All other methods of urine collection (clean catch, bladder catheterization, and plastic bag collections) require passage of urine through the urethra. SPA has been considered the standard method for obtaining urine that is uncontaminated by perineal flora. Variable success rates for obtaining urine have been reported, ranging from 23% to 90%.69 When RUS guidance is used, success rates improve.84 Despite invasiveness, the technique has limited risks when employed by expert physicians. SPA is recommended for boys with severe phimosis, girls with tight labial adhesions, and in case of external genital infection or presence of complex genital abnormalities. Bladder catheterization is considered an alternative to SPA, although with higher rates of contamination. Urine obtained through catheterization for culture has a sensitivity of 95% and a specificity of 99% in comparison to that obtained via SPA.85
In conclusion, methods of urine collection in individual centers should be determined based on the accuracy of voided specimens.
Urine cultureUrine culture is still the gold standard for diagnosing UTI. In freshly voided urine, a growth of more than 108 colony-forming units (CFU) per liter (105 per mL) of a unique bacterium is regarded most frequently as the cutoff between contamination and UTI. However, what is not broadly understood is that CFU quantification is a semiquantitative test. The method is based on the microbiology technician unfailingly differentiating 10 and 100 separate CFUs on an agar plate, which has been streak plated with 1mL of urine. Considering the intrinsic imprecision of the method, physicians should take into account signs and symptoms of UTI to treat a child. It must be mentioned that some children with UTI do not reach the traditional diagnostic threshold of 108 CFU/L.
As an example, Upadhyay et al.86 found that 20% of children with UTI based on SPA had CFU below 108/L (105/mL) on voided specimens. The CFU of these children with UTI was 106/L (103/mL) to 107/L (104/mL). Indeed, different cutoffs for significant bacteriuria are adopted for SPA or catheter specimens based on the lower risk of contamination in such specimens. The cutoff used for bladder catheter specimens is > 107 CFU/L (> 104 CFU/mL), while any bacterial growth in urine obtained by SPA indicates a UTI.87
Imaging studies for urinary tract infectionsMost children undergo one or more imaging studies following their first UTI aiming to identify abnormalities, which increases the risk of recurrent UTI or kidney damage. In the last decade, however, newer guidelines assume that imaging is only of value if subsequent management reduces the risk of UTI, kidney damage, and its long-term sequelae. These guidelines were released by the National Institute of Health and Care Excellence (NICE) in the UK, from the AAP, and from the Italian Society of Pediatric Nephrology (ISPN).17,69,78 All of them suggest limited investigations for children with UTI. The NICE guidelines provide imaging recommendations for children of all ages, whereas the AAP guidelines apply to children aged 2–24 months, and the ISPN guidelines refer to children aged 2–36 months.17,69,78 The NICE guidelines recommend that children aged over 6 months with their first uncomplicated UTI require no investigations following the episode and that children under 6 months should have only RUS.17 The AAP and ISPN guidelines recommend that all infants aged 2–24 months with febrile UTIs should undergo RUS, although they recognize that prenatal US is likely to identify most serious urinary tract abnormalities.69,78 None of these recent guidelines recommend routine VCUG or DMSA scans, but they recommend further evaluation if the ultrasound is abnormal, if the child is critically ill and fails to respond promptly to antibiotics, and in case of recurrent infections. Some studies have evaluated the impact of fewer investigations and concluded that the recent guidelines are safe to follow.88–90 On the other hand, other authors consider that potentially important abnormalities will be missed if the newer guidelines are followed.91 It should also be mentioned that these guidelines assume that most serious urinary tract abnormalities would be identified in antenatal US and that high-quality RUSs interpreted by expert pediatric radiologists are always available. However, in many situations, those assumptions are not true.
In a spite of the imaging protocol adopted, RUS is generally considered the first-line investigation for urinary tract malformations, since the method is noninvasive and can identify structural anomalies including obstructive uropathies, kidney hypodysplasia, and urinary tract dilatations.17,69,78 However, the main limitations of RUS are the dependence on the equipment and the operator, and the impossibility to obtain data on renal function. Compared with VCUG and DMSA scans, RUSs are poor predictors of the presence of VUR or of kidney damage, respectively.17
The termed ‘bottom-up’ approach is traditionally adopted for imaging evaluation of UTI. RUS and VCUG are recommended after the first episode of UTI in all pediatric patients despite sex and age group. VCUG is regarded as the reference standard for identifying VUR and for providing information on the bladder and urethra. More recently, it has been debated whether the presence and severity of VUR on VCUG might influence decisions on the management of VUR. In this regard, subgroup analyses in randomized controlled trials have found no difference in the efficacy of antibiotics in preventing UTI between children with and without VUR92 or between mild (grades I or II) and severe (grades III or IV) VUR.93 Some authors consider that since the presence or severity of VUR does not influence the efficacy of treatment, routine VCUG following the first UTI is no longer justified. The remaining clear indication for a VCUG is to evaluate the bladder and urethra in children suspected of having obstructive uropathy, such as posterior urethral valves.
To avoid all children from having unnecessary VCUGs, some authors have suggested a “top-down” approach to imaging studies, with RUSs and DMSA scans performed first and VCUG only performed if the DMSA scan shows acute kidney parenchymal injury.94 The DMSA scan is a sensitive test for detecting acute parenchymal injury following UTI, with a sensitivity of 86% and specificity of 91%.95 However, DMSA scans cannot differentiate between damage due to UTI and congenital kidney damage. In addition, most acute changes resolve over time regardless of whether antibiotic prophylaxis is used.92,96 The present authors conducted a retrospective cohort study with the aim to evaluate the diagnostic accuracy of DMSA scan and RUS in identifying high-grade VUR in 533 children after a first episode of UTI.97 The findings showed that if a negative diagnosis was established only when both test results were normal, sensitivity was 97% and the diagnostic odds ratio was 25. Only nine children (6.3%) with severe reflux would be missed by an absence of alterations in both tests. Nevertheless, a systematic review of 13 studies evaluating DMSA scans for the identification of dilating VUR (grades III–V) concluded that DMSA scans are poor predictors of dilating VUR,98 with considerable heterogeneity between studies. It should be also taken into account that, in many countries, there is limited availability of DMSA scans and that these are expensive for families.
Both VCUG and DMSA scans are associated with significant radiation (both equivalent to 40–50 chest X-rays or four months of natural background radiation), and are unpleasant and time-consuming tests for the child and their families. In addition, a nationwide population-based retrospective cohort study in Taiwan found that the overall risk of cancer was 1.92-fold greater in children who had undergone VCUG compared with matched controls, with highest risk for genital and urinary system cancers.99
A recent line of investigation on the ideal imaging protocol is the use of machine learning algorithms to develop predictive models for the probability of recurrent UTI associated with VUR in children after first infection. In this regard, the Advanced Analytics Group of Pediatric Urology and the ORC Personalized Medicine Group evaluated 500 subjects, including 305 from the Randomized Intervention for Children with Vesico-Ureteral Reflux (RIVUR) and 195 from the Careful Urinary Tract Infection Evaluation (CUTIE) trials. Most subjects were females (90%) and they had a mean age of 21±19 months. Recurrence of UTI occurred in 72 subjects, of whom 53 also exhibited VUR. The final predictive model included age, sex, race, weight, systolic blood pressure percentile, dysuria, urine albumin-to-creatinine ratio, prior antibiotic exposure, and current medication. The model predicted recurrent UTI related to VUR, with an AUC of 0.761 (95% CI: 0.714–0.808).100
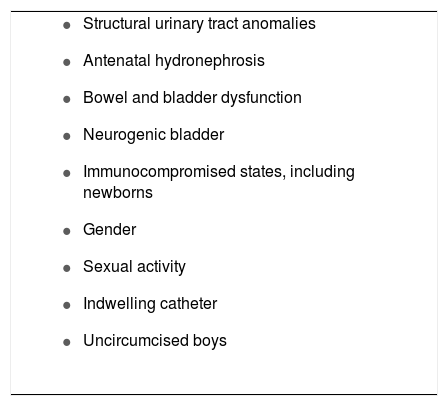
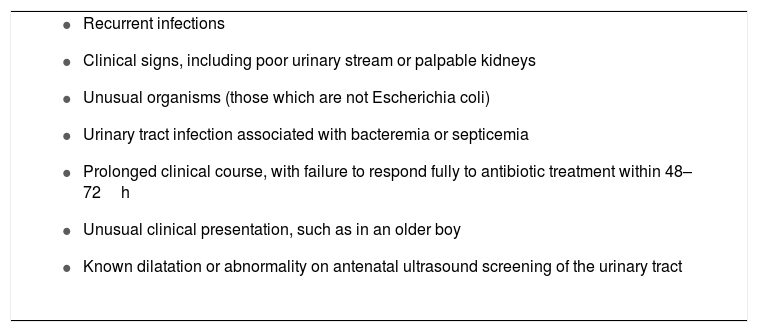
The debate on the ideal imaging protocol is still ongoing, but experts do agree that longitudinal prospective studies are still needed to establish tailored imaging protocols for the approach of UTI in childhood. An interesting approach was proposed by Marks et al.16 They suggested that targeting investigations for a selected group of children (as opposed to protocol-based investigations of all children with UTI) would be clinically safe and effective, and would avoid the distress and cost of unnecessary invasive investigations. Certain clinical risk factors have been defined in the literature and can help identify which infants and children with febrile UTI have a high risk of having an abnormal urinary tract, and consequently warrant investigation (Box 3).
Features of high-risk children that warrant investigation for an abnormal urinary tract.
|
The aims of the management of children with UTI are (1) resolution of the acute symptoms of the infection; (2) prompt recognition of concomitant bacteremia, particularly in infants less than 2 months of age and (3) prevention of renal damage by eradication of the bacterial pathogen, identification of abnormalities of the urinary tract, and avoidance of recurrent infections.101 Prompt treatment of UTI in preschool children might prevent renal scarring. For instance, Coulthard et al.102 reported that treating children’s UTI in less than three days after the onset of the symptoms more than halves the risk of them acquiring kidney scars. Accordingly, Shaikh et al.103 showed that there was a significant association between delay in treatment of febrile UTI and permanent renal scarring. The authors analyzed data of 482 children, 90% females and 78% with VUR, and found, after adjusting other covariates, that a delay of 48h or more would increase the odds of new renal scarring by about 47%.
The clinical management of UTIs in children should be tailored according to the age of the patient, severity of presentation, and infection location (cystitis vs. pyelonephritis). Antibiotic treatment is the cornerstone of treatment for acute UTI. The decision to initiate empiric treatment should be based on clinical suspicion of UTI that includes careful history and physical exam, and positive urinalysis in an appropriately collected urine specimen.73 The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.69 Most patients can be treated in an outpatient basis with oral therapy, if the child has a nontoxic appearance, can tolerate oral medications, and the family complies with recommendations.73 On the other hand, inpatient parenteral therapy should be considered for acutely ill children, children who cannot tolerate oral therapy, or when adherence with the prescribed regimen is in question.6 The AAP currently recommends that parenteral antibiotic therapy and hospitalization be considered for children who appear to be severely ill or dehydrated and those who are unable to retain oral intake.69 Children with a renal or perinephric abscess should also be treated initially with parenteral therapy and surgical drainage should be considered. Parenteral therapy should also be considered in children who are immunocompromised and in those with indwelling devices.6
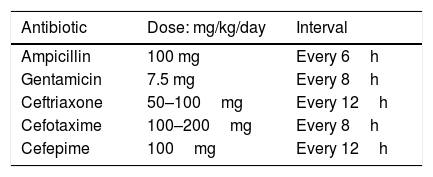
Infants aged 3 months or less with UTI should be treated initially with intravenous antibiotics due to the risk of urosepsis and the higher possibility of structural urinary tract anomaly, although data on prevalence of uropathies are imprecise.18,104–106 In addition, infants younger than 60–90 days are more likely to have their course of disease change abruptly because of their incompletely developed immune system.107,108 Broad coverage for group B streptococci and Enterobacteriaceae using the intravenous route is required during the first 12 weeks of life, pending results of blood and cerebrospinal fluid cultures. Once blood and cerebrospinal fluid cultures are confirmed as negative, the systemic signs have resolved, and the infant is afebrile, antimicrobial therapy may be completed using the oral route for a total duration of therapy of seven to 14 days.18,101,109 Antibiotic parenteral treatment regimens are detailed in Table 1.
Current treatment recommendations for children over the age of 3 months with clinical suspected pyelonephritis are based on eight randomized controlled trials and summarized in a Cochrane review.110 This review, based on three randomized trials (960 children), provides good evidence that oral antibiotics are an effective treatment for acute febrile pyelonephritis. For instance, Hoberman et al.111 compared three days of intravenous cefotaxime followed by 11 days of oral cefixime vs. 14 days of oral cefixime alone in 306 children 1–24 months of age; there was no difference in outcome. A more recent study involving 502 children (>1 month and <7 years of age) had similar results.96 Five trials (including 534 children) using intravenous antibiotics for 48–72h followed by oral antibiotics demonstrated no difference in DMSA abnormality or resolution of symptoms compared with seven to 14 days of intravenous antibiotics.110 Thus, it appears that oral antibiotics may be appropriate for the first febrile UTI in children older than 3 months of age. This review corroborates recent guidelines, which recommend that oral antibiotics should be given for seven to ten days unless the child is seriously unwell or unable to take oral antibiotics, in which case intravenous antibiotics are indicated.18
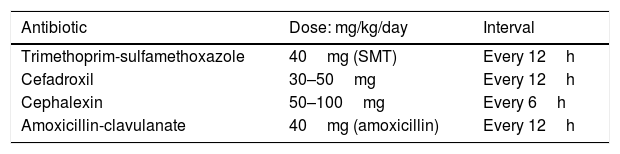
Empiric oral antibiotics usually recommended are detailed in Table 2. The initial choice of antibacterial therapy is preferably based on the knowledge of the predominant uropathogens in the patient’s age group, antibacterial sensitivity patterns in the practice area, the clinical status of the patient, and the opportunity for close follow-up.101
A common choice for treatment of UTI orally in the well-appearing child includes a sulfonamide-containing antimicrobial (trimethoprim-sulfamethoxazole [TMP-SMX]) or a cephalosporin. Antimicrobials that are excreted in the urine, but fail to achieve therapeutic concentrations in the bloodstream like nitrofurantoin, are not recommended for treatment of febrile infants or children in whom renal involvement is suspected.68 Of particular interest, Edlin et al.112 described resistance patterns in pediatric urinary isolates from 192 hospitals throughout the United States and found that up to 24% of E. coli cultured were resistant to TMP-SMX and 45% were resistant to ampicillin. On the other hand, resistance was found in less than 10% of E. coli for cephalosporins, amoxicillin-clavulanate, ciprofloxacin, and nitrofurantoin. Likewise, a surveillance study of E. coli isolates of 967 children with UTI in a tertiary hospital from 1992 to 1994 revealed that 30% of 1636 isolates were resistant to TMP-SMX.113 Risk factors associated with TMP-SMX resistance were young age, multiple inpatient hospital admissions, and previous antimicrobial therapy for greater than four weeks in the past six months. In Brazil, Reis et al.114 recently described a similar pattern. The authors conducted a retrospective study in 1641 patients with community-acquired UTI for five years (2010–2014). Resistance to ampicillin was observed in 55.9% of the isolated species, TMP-SMX showed 33.6% bacterial resistance, ciprofloxacin 18.4%, levofloxacin 18.0%, gentamicin 6.3%, cefepime 3.7%, and amikacin showed the lowest frequency, at 1.3%. Nevertheless, an important recent issue has emerged regarding the use of fluoroquinolones for the treatment of uncomplicated UTI in all age groups. Pharmacovigilance risk assessment committees from two leading international agencies, the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have released warnings that fluoroquinolones should not be prescribed for patients who have other treatment options for infectious diseases, including uncomplicated UTIs, because the risks outweigh the benefits in these patients and other antibiotics to treat these conditions are available.115,116
There is one systematic review with meta-analysis of six randomized controlled trials (RCTs) including 523 children (aged 2 weeks to 16 years) with microbiologically proven UTI and acute clinical pyelonephritis. These RCTs made comparisons of different classes of antibiotics. Reported outcomes were persistence of bacteriuria at 48–72h, resolution of clinical symptoms, symptomatic recurrence, and adverse effects. Three RCTs compared third generation cephalosporins with other antibiotics, including amoxicillin-clavulanate and TMP-SMX. There was no difference in the reduction of persistent bacteriuria at 48h (two RCTs, RR 5.5, 95% CI, 0.30–1.28), recurrent or persistent UTI five to ten days after the end of therapy (three RCTs, RR 0.42, 95% CI, 0.03–6.23), or the incidence of gastrointestinal adverse effects (three RCTs, n 5 108, RR 0.55, 95% CI, 0.10–3.16).110,117
Alternative options for outpatient management include outpatient parenteral therapy for patients with acute pyelonephritis. Several studies have reported that once-daily parenteral administration of gentamicin or ceftriaxone in a day treatment center is safe, functional, and cost effective in children with UTI.118,119
Treatment options for afebrile children and adolescents with cystitis symptoms are based on many well-conducted trials and systematic reviews.120–122 These studies have shown that short duration therapy (three to four days) is as effective as standard therapy (seven to 14 days) in eradicating urinary bacteria. The NICE guidelines recommend three days of treatment, with the choice of antibiotic directed by local guidelines.123
Prevention of the recurrent UTI and antibiotic prophylaxisPrevention of recurrent UTI is a debatable issue in the pediatric setting. Patients with significant urinary tract abnormalities or frequent symptomatic UTIs may benefit from prophylactic antibiotics.101,124 The basis for this practice was established by Smellie et al.,125 who examined the effect of antibiotic prophylaxis in children with recurrent UTI and structurally normal urinary tracts. In this study, 45 children with radiologically normal urinary tracts were given prophylactic doses of cotrimoxazole or nitrofurantoin, or no prophylaxis, after treatment of a symptomatic UTI. During the initial 10 months of the study, the 25 children on prophylaxis had significantly fewer episodes of UTI. Further studies have confirmed that nitrofurantoin, sulfonamides, and cotrimoxazole are effective in reducing the recurrence rate of infection in patients with normal urinary tracts as long as the drug is given.126,127 More recently, in a multicenter clinical trial, Craig et al.92 demonstrated the efficacy of prophylaxis in predisposed children. The authors randomized 576 children to receive either daily TMP-SMX or placebo for 12 months. During the study, UTI developed in 36 of 288 patients (13%) in the group receiving TMP-SMX (antibiotic group) and in 55 of 288 patients (19%) in the placebo group (hazard ratio in the antibiotic group, 0.61; 95% CI, 0.40–0.93; p=0.02).
Nevertheless, the use of long-term antimicrobial prophylaxis to prevent UTI in children with and without VUR remains controversial.63 One meta-analysis investigated antibiotic prophylaxis in children and included six RCTs with a total of 388 participants, predominantly girls younger than 14 years of age, who were identified as being at risk of recurrent UTI, but without any predisposing anatomic or neurologic abnormalities.128 Compared with placebo, four studies reported that the incidence of recurrence was reduced in the antibiotic-treated group, although with a wide range (21%–69%) in the recurrence of repeat positive cultures (RR 0.44, 95% CI, 0.19–1.00). However, when analysis was limited to high-quality studies, the results were not statistically significant. The authors concluded that more evidence in the form of properly randomized double-blinded trials is needed to support the routine use of antibiotic prophylaxis in preventing recurrent UTI in children. Another issue is the effect of prophylaxis on developing a multidrug-resistant recurrent UTI. Selekman et al.129 recently demonstrated in a meta-analysis that children with VUR treated with prophylaxis were more likely to have a multidrug-resistant infection (33% vs. 6%, p<.001) and were more likely to receive broad-spectrum antibiotics (68% vs. 49%, p=.004). Those receiving prophylaxis had 6.4 times the odds (95% confidence interval: 2.7–15.6) of developing a multidrug-resistant infection. In 2007, NICE published its recommendations that healthcare professionals in the United Kingdom should not use antibiotic prophylaxis routinely in infants and children following first time UTI, and only selectively in recurrent UTI.130
Although the effectiveness of antimicrobial prophylaxis for the prevention of UTI has not been fully demonstrated, in the present authors’ experience, some selected patients with recurrent episodes may benefit from this approach by reducing the morbidity and possibly preventing kidney damage. First, following treatment of UTI, the present authors have used prophylactic antibiotic coverage for the young child until urinary tract abnormalities have been excluded by imaging studies. After, the decision regarding continuous antibiotic prophylaxis is based on the results of these imaging studies and/or clinical features, such as age and gender of the children. Concomitantly, it is important to establish fundamental interventions that might reduce recurrent UTI. In this regard, the prompt identification and adequate treatment of urinary tract abnormalities – such as vesicoureteral reflux, posterior urethral valves, or ureteropelvic obstruction – are relevant. It is beyond the scope of this review to explore the myriad facets of the treatment of specific uropathies. Additional interventions that have been associated with a decrease in symptomatic UTI in children with recurrent UTI include treatment of constipation and management of voiding dysfunction.101,131
Obstacles to the effectiveness of antimicrobial prophylaxis are adherence to a daily regimen, side effects associated with the various agents, and the potential for emergence of antimicrobial resistance. According to AAP, to overcome these issues, evidence of effectiveness with a well-tolerated, safe product would be required, and parents would need adequate education to understand the value and importance of adherence.69
Agents of choice for prophylaxis of recurrent UTI are nitrofurantoin and cotrimoxazole.101 In this regard, the earlier mentioned review also identified two RCTs that compared antibiotic classes in prophylaxis of UTI (nitrofurantoin versus trimethoprim, and nitrofurantoin versus cefixime).69 Nitrofurantoin was found to be superior to trimethoprim, but no different from cefixime in reducing the incidence of recurrent repeat-positive urine cultures. In turn, nitrofurantoin was three times more likely to be discontinued because of the adverse effects such as of nausea, vomiting, or stomachache.
In summary, there has been a tendency of less use of prophylaxis due to dispute about its efficacy, increasing bacterial resistance, and a propensity to low adherence. Alternative measures and management of risk factors for recurrent UTI should be emphasized. However, in selected patients carefully followed, prophylaxis can protect from recurrent UTI and long-term sequelae.132
Complications and prognosisThe involvement of renal parenchyma in UTI may lead to an inflammatory reaction with risk of permanent damage. The long-term consequences of such damage include hypertension and impaired renal function,133–138 but the frequency of these complications is still poorly known. The main issue is the difficulty in following patients over several decades, which is needed to obtain reliable findings.
Earlier studies from specialized centers reported high rates of hypertension and chronic kidney disease (CKD) in children and young adults with kidney damage following UTI.138,139 However, a few years later, population-based studies of individual with previous UTI did not find similar results. In this regard, Wennerstrom et al. evaluated glomerular filtration rate (GFR) and 24h ambulatory blood pressure measured 16–26 years after first UTI in 57 (77%) of 74 people with renal parenchyma damage and in a matched group of 51 adults without kidney damage (control group) from the same cohort.140,141 The mean GFR of 99mL/min/1.73 m2 in the group with kidney damage did not differ significantly from that in the control group (102mL/min/1.73 m2).141 Only eight patients (six in the kidney damage group and two in the matched group) exhibited GFR below 80mL/min/1.73 m2. Similarly, ambulatory blood pressure measurements did not differ between individuals with and without renal parenchyma damage. Blood pressure exceeded the reference values in 9% of those with kidney damage vs. 6% in those without.140
However, more recently, a systematic review made an estimative for the risk of renal damage after childhood UTI of approximately 15%.142 Subsequently, 193 young people randomly sampled from a cohort of 1161 children were evaluated following their first childhood UTI and followed up six to 17 years later.143 Patients with congenital kidney dysplasia or obstruction (24 in total) were excluded. Twenty-two among 150 patients (15%), who were submitted to RUS, presented renal damage and/or decreased kidney growth. Recurrence of UTI and VUR grades III-V were more commonly detected in this subgroup. However, GFR and blood pressure remained within normal range in all participants of the study.143 In 2015, Gebäck et al. evaluated a population-based cohort of women followed for a median period of 35 years from their first UTI in childhood. GFR was estimated by51 Cr-edetic acid clearance, while renal parenchyma damage was diagnosed by DMSA scan.144 Eighty-six among 111 women initially recruited completed the investigation; 58 with renal damage and 28 without. Of those with renal damage, one had stage 3 CKD, 14 had stage 2, and 43 had stage 1. Bilateral damage was positively associated with lower GFR. However, most women with UTI-associated renal damage had impressively well-preserved renal function.144
ConclusionsDespite its high prevalence, UTI in pediatric patients still has many unsolved issues. First, in neonates and infants, signs and symptoms of UTI are often unspecific, delaying the diagnosis. Should a urine sample be collected in all cases of fever without a known cause? In the present authors’ opinion, this is the one the most important recommendations for a timely diagnosis in this age group. Second, which is the best way to collect urine in non toilet-trained children? On one hand, some guidelines recommend urine catheterization; on the other hand, some authors argue in favor of urine bag collection, despite the prevalence of false positive findings. Third, must the bacteria colony count for infants be equal or higher than 108 CFU per liter (105 per mL)? Fourth, should all children after a single episode of UTI have their urinary tract investigated by imaging? If yes, which is the best protocol? Fifth, in regard to antibiotic prophylaxis, which subgroup of children will benefit from its long-term use? Which criteria should be taken into account to prescribe and to stop antibiotic prophylaxis? Sixth, is there a real risk for CKD and hypertension for children with UTI?
In conclusion, these and other questions are still not solved. However, some general advice must be taken into account by the pediatrician. Early diagnosis of UTI is very important, especially for neonates and infants. Risk factors for recurrence of UTI and for CAKUT should always be considered. The protocol of urine collection must consider the limitations and risks of each method, and also the local practice and feasibility. Imaging evaluation is critical to detect CAKUT, but always depends on the quality of equipment and the experience of the radiologist. In the present authors’ point of the view, a comprehensive RUS by a trained radiologist is advisable for all children who have had a confirmed episode of febrile UTI. Antibiotic prophylaxis is useful to prevent recurrence of UTIs in patients with obstructive uropathies and high-grade reflux. All decisions in regard to a child with UTI must be based on detailed clinical history and physical examination and careful clinical judgment to avoid, on one hand, unnecessary invasive exams and, on the other hand, future adverse outcome for renal function.
FundingThis study was partially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil). Dr. AC Simões e Silva and Dr. EA Oliveira received a research grant from CNPq.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Simões e Silva AC, Oliveira EA, Mak RH. Urinary tract infection in pediatrics: an overview. J Pediatr (Rio J). 2020;96(S1):65–79.