To describe the epidemiological situation of tuberculosis in children under 19 years of age in Brazil and to review the latest publications on disease risk, diagnosis, treatment, and prevention.
Source of dataNotifiable Diseases Information System (2018), World Health Organization estimates, and PubMed articles selected using the descriptor “Tuberculosis,” delimited by type of study, period, age, and language.
Synthesis of dataIn 2018, in Brazil, 9.4% of notifications were in children under 19 years. The pulmonary form predominated in 80.1% of the cases. The cure rate was 76.8%, lethality was 0.8%, and abandonment was 10.4%. The prevalence of drug-resistant tuberculosis (2011–2016) was 0.5%. It has been found that the risk of disease can reach up to 56% in children under 5 years, influenced by helminth co-infections, malaria, chronic viral infections, live attenuated virus vaccines, and hypovitaminosis D. Exposure to a bacilliferous patient for periods shorter than 30minutes is sufficient for the development of infection and/or disease. In Brazil, microbiological screening is recommended, but the use of the scoring system, modified in 2019, has been maintained. Studies on infection detection have supported the use of the tuberculin skin test. In the treatment, the great advance was the introduction of dispersible formulations, adjustment of the recommended doses, and shortened regimens for latent infection. Several vaccine studies (stages 1–3) are ongoing, but no BCG-licensed substitute has been implemented yet.
ConclusionsThere has been progress in treatment, but major challenges need to be overcome to improve diagnosis, monitoring, and outcome of cases, aiming to eliminate tuberculosis.
Descrever a situação epidemiológica da tuberculose nos menores de 19 anos no Brasil e revisar as últimas publicações sobre risco de adoecimento, diagnóstico, tratamento e prevenção.
Fonte dos dadosBanco de notificação Brasil (2018), estimativas da Organização Mundial da Saúde e artigos do PubMed selecionados pelo descritor Tuberculosis, delimitaram-se tipo de estudo, período, idade e língua.
Síntese dos dadosEm 2018, no Brasil, 9,4% das notificações foram nos menores de 19 anos. Predominou a forma pulmonar em 80,1% dos casos. A taxa de cura foi de 76,8%, letalidade 0,8% e abandono 10,4%. A prevalência de tuberculose drogarresistente (2011 a 2016) foi 0,5%. Encontrou-se que o risco de adoecimento pode chegar até 56%, nos menores de cinco anos, influenciado por coinfecções com helmintos, malária, infecções virais crônicas, vacinas de vírus vivos atenuados e hipovitaminose D. A exposição ao doente bacilífero por períodos menores de 30 minutos é suficiente para o desenvolvimento de infecção e/ou doença. No Brasil, recomenda-se a pesquisa microbiológica, porém mantem-se o uso do Sistema de Pontuação, modificado em 2019. Estudos sobre detecção da infecção respaldaram o uso da prova tuberculínica. No tratamento, o grande avanço foi a introdução das formulações dispersíveis, adequação das doses preconizadas e esquemas encurtados para infecção latente. Vários estudos de vacinas (fases de 1 a 3) estão em andamento, mas ainda sem substituto licenciado para a BCG.
ConclusõesObservaram-se progressos no tratamento, porém ainda há grandes desafios para melhorar o diagnóstico, monitoramento e desfecho dos casos em busca da eliminação da tuberculose.
For the first time in history, in September 2018, tuberculosis (TB) was a central topic at a high-level United Nations meeting, aimed at outlining strategies for the elimination of TB by 2030. This meeting highlighted the need to improve measures of prevention, diagnosis, and treatment of this disease, which continues to have high mortality rates worldwide. At the age range of children and adolescents, the importance of improving diagnostic tools to reduce the number of undiagnosed cases, the so-called ‘missing cases,’ was also reiterated, as well as the need for more appropriate medications, especially for children.1
The World Health Organization (WHO) estimated 1000,000 new cases of tuberculosis in children under 14 in 2017. Of these, 55 % went undiagnosed and/or unreported. In children under 5 years, this percentage was 69 %, while in the others it was 40 %. When assessing the number of deaths, 80 % occurred in children under 5 years; in 96 % of these, children did not have access to anti-TB treatment.2
Aiming to improve these indicators, the WHO has published the “Roadmap towards ending TB in children and adolescents.” It guides the narrowing of TB control program actions within the child healthcare network, including primary health care, nutrition, HIV, and immunization, among others. The objective is to emphasize new prevention, diagnosis, and treatment tools, especially dispersible drugs for children.2 It also emphasizes the empowerment of and partnership with civil society, communities, and families affected by TB to give relevance to the disease and reduce its stigma, which is as old as the history of mankind.
Considering the abovementioned facts, this review described the epidemiological situation of TB in children under 19 years, as well as the latest publications on the risk of the disease, diagnosis, and treatment of active and latent disease. Thus, this study aimed to analyze TB in childhood and adolescence from different perspectives.
MethodTo describe the epidemiological situation of TB in children under 18 years old, the Brazilian Notifiable Diseases Information System database (2017–2018), unpublished data, and WHO2 estimates were used.
A search was carried out in the PubMed database using the descriptor “Tuberculosis” and a total of 253,156 articles were found. After refining the search to original articles, clinical studies, or meta-analyses, published from January 01, 2018 to June 30, 2019, in children under 18 years old, human beings, in English, Spanish, or Portuguese, a total of 104 articles remained. After the evaluation of all abstracts, 26 articles related to tuberculosis in children and adolescents were selected. While reading these articles, other articles mentioned in these references and recommendations for TB control by the Brazilian Ministry of Health (MoH) and the WHO were included.
This study was approved by the Human Research Ethics Committee of Hospital de Clínicas of Universidade Federal do Paraná under CAE No. 17876919.8.0000.0096.
TB epidemiology in children and adolescents in BrazilThe WHO’s overall estimates show that Brazil failed to detect approximately 12,000 TB cases in 2017. Of these, 8500 (71 %) were in children under 14 years, namely 3500 in children under 4 and 5000 in children between 5 and 14 years.3 One possibility for this under-detection is the difficulty in TB diagnosis. These data show the importance of prioritizing actions for this age group, expanding decentralization, and training professionals from primary health care services in the diagnosis and treatment of these cases.4
In 2018, Brazil reported 75,709 new cases of TB, including 1552 (3.3 %) in children under 14 years. It is noteworthy that the WHO estimates the percentage of cases in this age group would be 10 % of the total reported cases for the 30 countries with high burden of the disease, including Brazil.3 However, these are global estimates and there are no national studies on these data, which deserve caution in their interpretation.
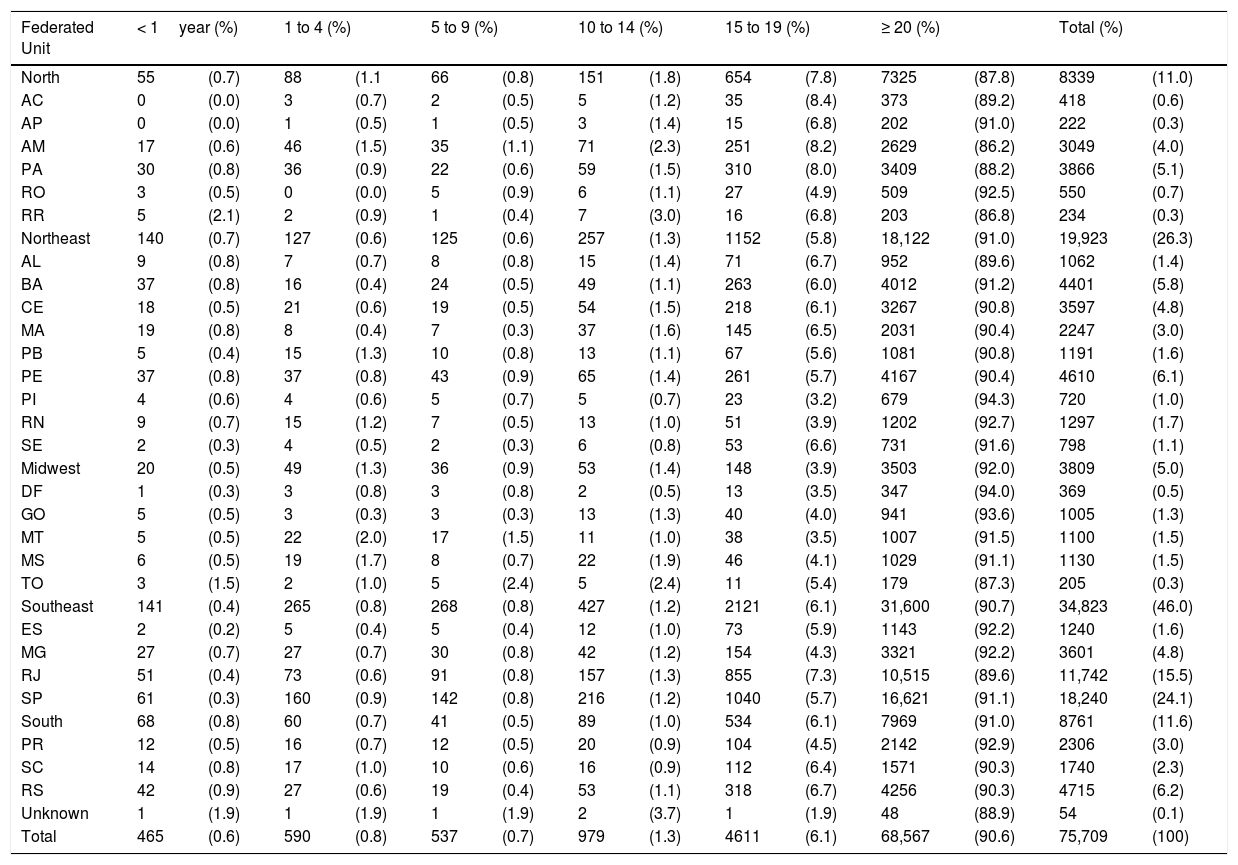
The distribution of new cases notified in Brazil (2018), according to the age group and federated units, is shown in Table 1.
Distribution of new cases of tuberculosis in Brazil, by age group and state, 2018.
| Federated Unit | < 1year (%) | 1 to 4 (%) | 5 to 9 (%) | 10 to 14 (%) | 15 to 19 (%) | ≥ 20 (%) | Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | 55 | (0.7) | 88 | (1.1 | 66 | (0.8) | 151 | (1.8) | 654 | (7.8) | 7325 | (87.8) | 8339 | (11.0) |
| AC | 0 | (0.0) | 3 | (0.7) | 2 | (0.5) | 5 | (1.2) | 35 | (8.4) | 373 | (89.2) | 418 | (0.6) |
| AP | 0 | (0.0) | 1 | (0.5) | 1 | (0.5) | 3 | (1.4) | 15 | (6.8) | 202 | (91.0) | 222 | (0.3) |
| AM | 17 | (0.6) | 46 | (1.5) | 35 | (1.1) | 71 | (2.3) | 251 | (8.2) | 2629 | (86.2) | 3049 | (4.0) |
| PA | 30 | (0.8) | 36 | (0.9) | 22 | (0.6) | 59 | (1.5) | 310 | (8.0) | 3409 | (88.2) | 3866 | (5.1) |
| RO | 3 | (0.5) | 0 | (0.0) | 5 | (0.9) | 6 | (1.1) | 27 | (4.9) | 509 | (92.5) | 550 | (0.7) |
| RR | 5 | (2.1) | 2 | (0.9) | 1 | (0.4) | 7 | (3.0) | 16 | (6.8) | 203 | (86.8) | 234 | (0.3) |
| Northeast | 140 | (0.7) | 127 | (0.6) | 125 | (0.6) | 257 | (1.3) | 1152 | (5.8) | 18,122 | (91.0) | 19,923 | (26.3) |
| AL | 9 | (0.8) | 7 | (0.7) | 8 | (0.8) | 15 | (1.4) | 71 | (6.7) | 952 | (89.6) | 1062 | (1.4) |
| BA | 37 | (0.8) | 16 | (0.4) | 24 | (0.5) | 49 | (1.1) | 263 | (6.0) | 4012 | (91.2) | 4401 | (5.8) |
| CE | 18 | (0.5) | 21 | (0.6) | 19 | (0.5) | 54 | (1.5) | 218 | (6.1) | 3267 | (90.8) | 3597 | (4.8) |
| MA | 19 | (0.8) | 8 | (0.4) | 7 | (0.3) | 37 | (1.6) | 145 | (6.5) | 2031 | (90.4) | 2247 | (3.0) |
| PB | 5 | (0.4) | 15 | (1.3) | 10 | (0.8) | 13 | (1.1) | 67 | (5.6) | 1081 | (90.8) | 1191 | (1.6) |
| PE | 37 | (0.8) | 37 | (0.8) | 43 | (0.9) | 65 | (1.4) | 261 | (5.7) | 4167 | (90.4) | 4610 | (6.1) |
| PI | 4 | (0.6) | 4 | (0.6) | 5 | (0.7) | 5 | (0.7) | 23 | (3.2) | 679 | (94.3) | 720 | (1.0) |
| RN | 9 | (0.7) | 15 | (1.2) | 7 | (0.5) | 13 | (1.0) | 51 | (3.9) | 1202 | (92.7) | 1297 | (1.7) |
| SE | 2 | (0.3) | 4 | (0.5) | 2 | (0.3) | 6 | (0.8) | 53 | (6.6) | 731 | (91.6) | 798 | (1.1) |
| Midwest | 20 | (0.5) | 49 | (1.3) | 36 | (0.9) | 53 | (1.4) | 148 | (3.9) | 3503 | (92.0) | 3809 | (5.0) |
| DF | 1 | (0.3) | 3 | (0.8) | 3 | (0.8) | 2 | (0.5) | 13 | (3.5) | 347 | (94.0) | 369 | (0.5) |
| GO | 5 | (0.5) | 3 | (0.3) | 3 | (0.3) | 13 | (1.3) | 40 | (4.0) | 941 | (93.6) | 1005 | (1.3) |
| MT | 5 | (0.5) | 22 | (2.0) | 17 | (1.5) | 11 | (1.0) | 38 | (3.5) | 1007 | (91.5) | 1100 | (1.5) |
| MS | 6 | (0.5) | 19 | (1.7) | 8 | (0.7) | 22 | (1.9) | 46 | (4.1) | 1029 | (91.1) | 1130 | (1.5) |
| TO | 3 | (1.5) | 2 | (1.0) | 5 | (2.4) | 5 | (2.4) | 11 | (5.4) | 179 | (87.3) | 205 | (0.3) |
| Southeast | 141 | (0.4) | 265 | (0.8) | 268 | (0.8) | 427 | (1.2) | 2121 | (6.1) | 31,600 | (90.7) | 34,823 | (46.0) |
| ES | 2 | (0.2) | 5 | (0.4) | 5 | (0.4) | 12 | (1.0) | 73 | (5.9) | 1143 | (92.2) | 1240 | (1.6) |
| MG | 27 | (0.7) | 27 | (0.7) | 30 | (0.8) | 42 | (1.2) | 154 | (4.3) | 3321 | (92.2) | 3601 | (4.8) |
| RJ | 51 | (0.4) | 73 | (0.6) | 91 | (0.8) | 157 | (1.3) | 855 | (7.3) | 10,515 | (89.6) | 11,742 | (15.5) |
| SP | 61 | (0.3) | 160 | (0.9) | 142 | (0.8) | 216 | (1.2) | 1040 | (5.7) | 16,621 | (91.1) | 18,240 | (24.1) |
| South | 68 | (0.8) | 60 | (0.7) | 41 | (0.5) | 89 | (1.0) | 534 | (6.1) | 7969 | (91.0) | 8761 | (11.6) |
| PR | 12 | (0.5) | 16 | (0.7) | 12 | (0.5) | 20 | (0.9) | 104 | (4.5) | 2142 | (92.9) | 2306 | (3.0) |
| SC | 14 | (0.8) | 17 | (1.0) | 10 | (0.6) | 16 | (0.9) | 112 | (6.4) | 1571 | (90.3) | 1740 | (2.3) |
| RS | 42 | (0.9) | 27 | (0.6) | 19 | (0.4) | 53 | (1.1) | 318 | (6.7) | 4256 | (90.3) | 4715 | (6.2) |
| Unknown | 1 | (1.9) | 1 | (1.9) | 1 | (1.9) | 2 | (3.7) | 1 | (1.9) | 48 | (88.9) | 54 | (0.1) |
| Total | 465 | (0.6) | 590 | (0.8) | 537 | (0.7) | 979 | (1.3) | 4611 | (6.1) | 68,567 | (90.6) | 75,709 | (100) |
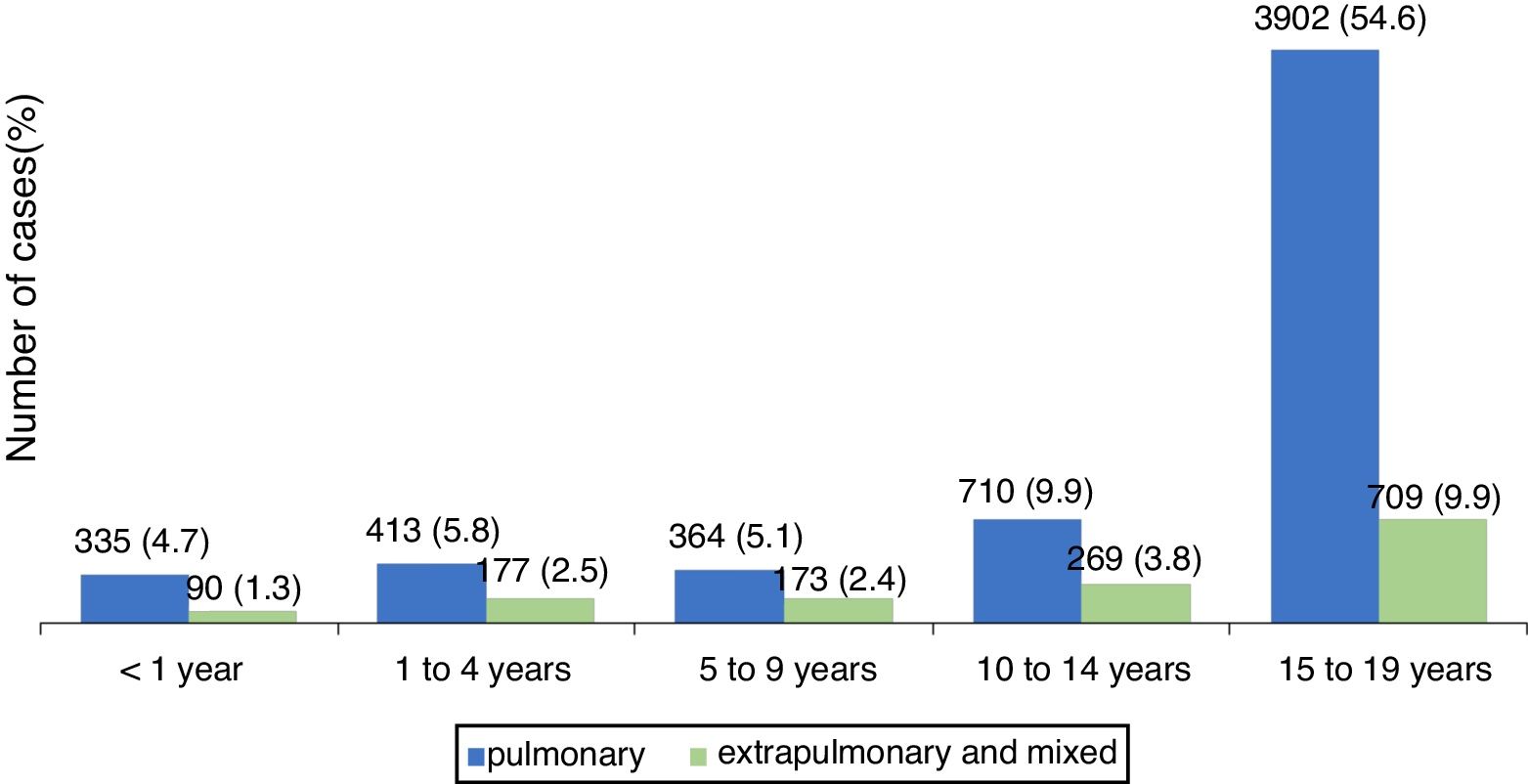
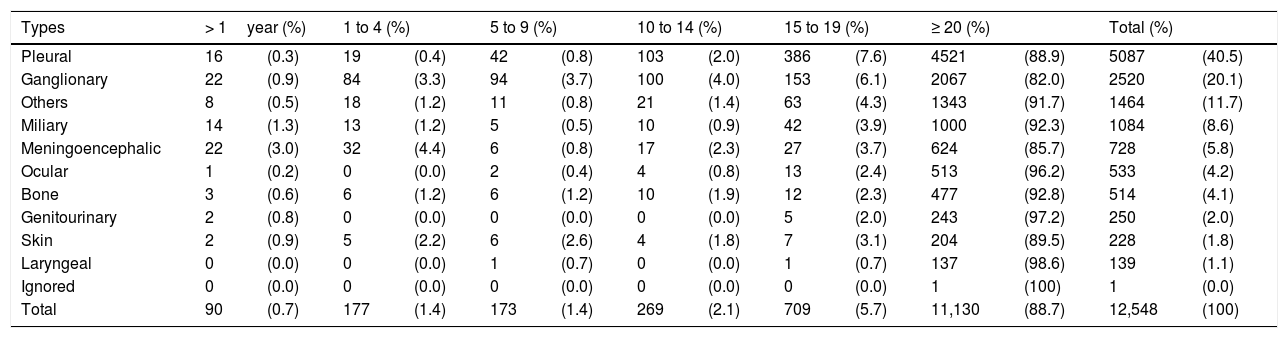
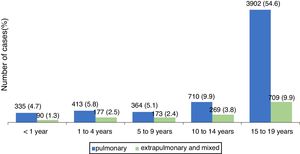
The pulmonary form predominated in those under 19 years, accounting for 80.1 % of cases. Fig. 1 shows the distribution, by type of TB, per age group. The distribution of the less frequent extra pulmonary forms varied with age. In children under 1year, the meningeal and ganglionic forms showed similar number of cases, while in the others, there was a predominance of the ganglionic form, excluding adolescents, in whom the pleural form was predominant. The distribution of extra pulmonary and/or mixed cases (pulmonary associated with extra pulmonary) is shown in Table 2.
Distribution of new cases of extrapulmonary and mixed tuberculosis by age group, Brazil, 2018.
| Types | > 1year (%) | 1 to 4 (%) | 5 to 9 (%) | 10 to 14 (%) | 15 to 19 (%) | ≥ 20 (%) | Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pleural | 16 | (0.3) | 19 | (0.4) | 42 | (0.8) | 103 | (2.0) | 386 | (7.6) | 4521 | (88.9) | 5087 | (40.5) |
| Ganglionary | 22 | (0.9) | 84 | (3.3) | 94 | (3.7) | 100 | (4.0) | 153 | (6.1) | 2067 | (82.0) | 2520 | (20.1) |
| Others | 8 | (0.5) | 18 | (1.2) | 11 | (0.8) | 21 | (1.4) | 63 | (4.3) | 1343 | (91.7) | 1464 | (11.7) |
| Miliary | 14 | (1.3) | 13 | (1.2) | 5 | (0.5) | 10 | (0.9) | 42 | (3.9) | 1000 | (92.3) | 1084 | (8.6) |
| Meningoencephalic | 22 | (3.0) | 32 | (4.4) | 6 | (0.8) | 17 | (2.3) | 27 | (3.7) | 624 | (85.7) | 728 | (5.8) |
| Ocular | 1 | (0.2) | 0 | (0.0) | 2 | (0.4) | 4 | (0.8) | 13 | (2.4) | 513 | (96.2) | 533 | (4.2) |
| Bone | 3 | (0.6) | 6 | (1.2) | 6 | (1.2) | 10 | (1.9) | 12 | (2.3) | 477 | (92.8) | 514 | (4.1) |
| Genitourinary | 2 | (0.8) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 5 | (2.0) | 243 | (97.2) | 250 | (2.0) |
| Skin | 2 | (0.9) | 5 | (2.2) | 6 | (2.6) | 4 | (1.8) | 7 | (3.1) | 204 | (89.5) | 228 | (1.8) |
| Laryngeal | 0 | (0.0) | 0 | (0.0) | 1 | (0.7) | 0 | (0.0) | 1 | (0.7) | 137 | (98.6) | 139 | (1.1) |
| Ignored | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (100) | 1 | (0.0) |
| Total | 90 | (0.7) | 177 | (1.4) | 173 | (1.4) | 269 | (2.1) | 709 | (5.7) | 11,130 | (88.7) | 12,548 | (100) |
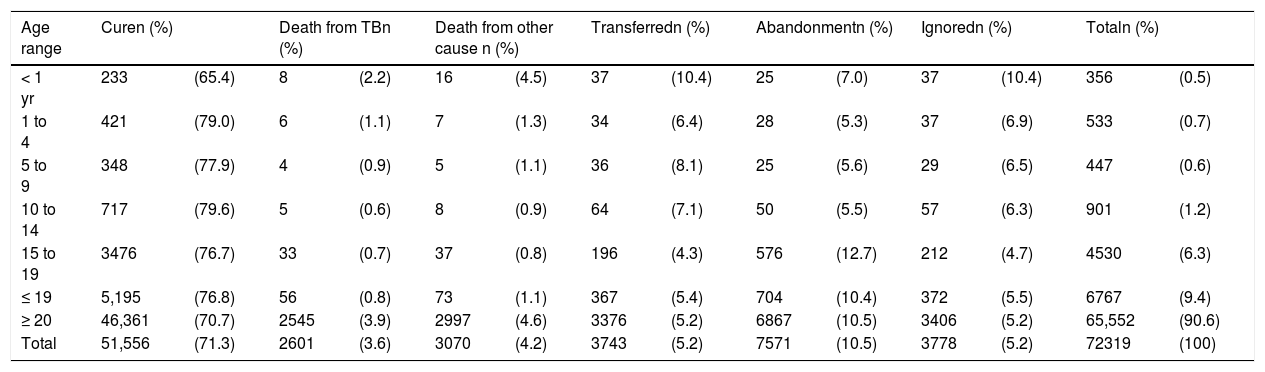
Regarding the closure of cases reported in 2017, children under 1year of age had a cure rate of 65 %, abandonment of 7 %, and lethality of 2 %. It is noteworthy that the lethality at this age is double when compared to the other age groups of childhood and adolescence, showing the severity of the disease in this group. It is important to observe that abandonment showed its highest rate in adolescents, which was 12.7 %, even above that at the age range of 20 years; this reflects the importance of the directly observed treatment (DOT) in this population. Other outcomes are shown in Table 3.
Treatment outcome of new cases of tuberculosis by age group, Brazil, 2017.
| Age range | Curen (%) | Death from TBn (%) | Death from other cause n (%) | Transferredn (%) | Abandonmentn (%) | Ignoredn (%) | Totaln (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1 yr | 233 | (65.4) | 8 | (2.2) | 16 | (4.5) | 37 | (10.4) | 25 | (7.0) | 37 | (10.4) | 356 | (0.5) |
| 1 to 4 | 421 | (79.0) | 6 | (1.1) | 7 | (1.3) | 34 | (6.4) | 28 | (5.3) | 37 | (6.9) | 533 | (0.7) |
| 5 to 9 | 348 | (77.9) | 4 | (0.9) | 5 | (1.1) | 36 | (8.1) | 25 | (5.6) | 29 | (6.5) | 447 | (0.6) |
| 10 to 14 | 717 | (79.6) | 5 | (0.6) | 8 | (0.9) | 64 | (7.1) | 50 | (5.5) | 57 | (6.3) | 901 | (1.2) |
| 15 to 19 | 3476 | (76.7) | 33 | (0.7) | 37 | (0.8) | 196 | (4.3) | 576 | (12.7) | 212 | (4.7) | 4530 | (6.3) |
| ≤ 19 | 5,195 | (76.8) | 56 | (0.8) | 73 | (1.1) | 367 | (5.4) | 704 | (10.4) | 372 | (5.5) | 6767 | (9.4) |
| ≥ 20 | 46,361 | (70.7) | 2545 | (3.9) | 2997 | (4.6) | 3376 | (5.2) | 6867 | (10.5) | 3406 | (5.2) | 65,552 | (90.6) |
| Total | 51,556 | (71.3) | 2601 | (3.6) | 3070 | (4.2) | 3743 | (5.2) | 7571 | (10.5) | 3778 | (5.2) | 72319 | (100) |
The prevention of active tuberculosis through the treatment of latent tuberculosis infection (LTBI) is one of the main strategies for reducing the incidence of tuberculosis and achieving the goals of the End TB Strategy.3
The global burden of LTBI is not known and is estimated at up to a quarter of the world's population; however, there is wide variation by country and age group.5,6 In Brazil, its treatment is not subject to compulsory notification in all federated units, but it is recommended to notify in a specific national form.7 Recently, the LTBI Treatment Notification System was implemented in the country to monitor, evaluate, and surveil these cases, which will provide knowledge of the magnitude of this problem.
The WHO estimates that in 2014, 25,000 children and adolescents up to 15 years of age became ill with drug-resistant TB (DRTB) and less than 10 % had access to treatment. Another matter of concern is that there are no data on DRTB stratified by age in the pediatric and adolescent age groups reported to the WHO, making it difficult to know the real extent of this disease.2
Brazil has had the Tuberculosis Special Treatment Information System (Sistema de Informação de Tratamentos Especiais da Tuberculose [SITETB]) since 2013. The assessment of notified cases in children under 19 years, from 2011 to 2016, reported 181 cases of DRTB, corresponding to a prevalence of 0.5 % of all reported TB cases in the same age group and period. It is noteworthy that the cases of DRTB accounted for 67 % of SITETB notifications in children under 19 years and, among the cases of resistance, most were multiresistant (88 %).8
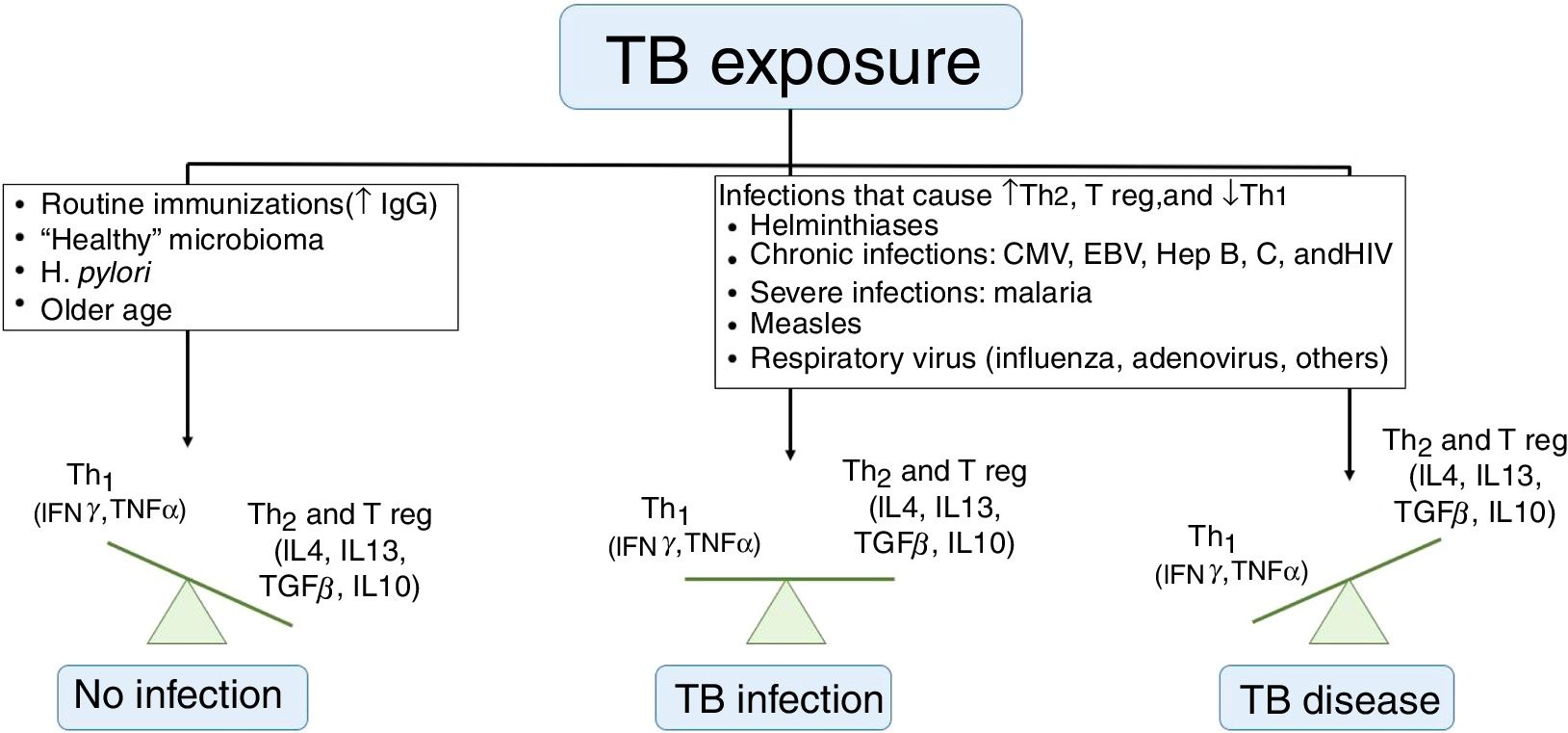
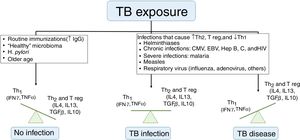
Risk of illnessIt is known that not every child with a latent infection will develop active TB. Children under 5 years or those with immunodeficiency have an increased risk of disease progression, but the understanding of risk factors for this occurrence is limited. Whittaker et al. described that an infection by another pathogen (viruses, bacteria, fungi, or parasites) is the most often involved process for this progression, by dysregulating the Th1, Th2, and T-regulatory immune responses (Fig. 2). Many of these diseases have geographic distributions overlapping TB, making it difficult to establish a causal correlation. Vaccines, especially those using live attenuated viruses, can collaborate in reducing the risk of disease by stimulating the production of immunoglobulins and interferon gamma.9
Factors that influence tuberculosis immune response.
TB, tuberculosis; IgG, immunoglobulin G; Th, T helper lymphocyte; Treg, T regulatory cells; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; CMV, cytomegalovirus; EBV, Epstein-Barr virus; Hep, hepatitis.
Adapted from Whittaker et al.9
The time of disease evolution is described as 5%–10% throughout life, with 50 % of cases concentrated in the first two to five years after the infection, with more incidents in children under 5 years.10 Trauer et al. proposed that the risk of active TB after infection is higher than the traditionally accepted estimates, especially among children under 5 years, and may be as high as 56 % in some projections.11
Studies that assessed the disease risk among contacts from countries with different incomes showed varying rates of active TB. In low/middle-income countries, the percentage was 3.1 %, while in high-income countries it was 1.4 %. However, in both situations, the percentages were higher (10.0 % and 4.7 %, respectively) in children under 5 years.12
Another issue questioned in relation to the disease is related to the risk of infection. How long would the time of contact have to be for an individual to become infected? Normally the guidelines do not have this answer. Each case should be analyzed by observing the source case’s bacillary load and the environment: ventilation, size of the space, proximity of the case, and duration of contact. This last item is the most difficult to establish, because it depends directly on the previous ones. The Australian guideline (2015) assigned eight hours as the minimum time for an individual to become infected, and a shorter time should be considered when more susceptible people are involved or when there are high-exposure procedures (cough induction or intubation).13 The WHO’s 2013recommendations consider the period of risk during flights in those lasting more than eight hours.14 However, in 2017, Luzzati et al. described the investigation of an outbreak in Trieste, Italy, with the identification of secondary cases in children, in which the contact with the source case was occasional, within a period of less than 30min.15 This leads us to question the need for long-lasting contacts for the individual to become infected.
Another topic of study is the correlation between vitamin D deficiency and TB.16,17 Such an association has already been established, but studies are required to determine whether the deficiency would be the cause or the consequence, as well as whether vitamin D supplementation would influence disease progression avoidance or improve patient response to treatment.
Diagnosis of pulmonary TBTB is classified as pulmonary, extrapulmonary, and/or mixed,7 so the diagnostic tools vary according to its location. Pulmonary TB is the most prevalent form and, therefore, will be the focus of this review.
The pathophysiology and clinical presentation of TB differ according to age. The WHO classifies as children individuals aged up to 14 years, while in Brazil, based on these differences, considers up to 10 years. This age cutoff defines the research flows and therapeutic conducts. Children have a nonspecific clinical picture similar to usual childhood infections and are paucibacillary. As for adolescents (older than 10 years), they develop disease similar to that in adults, and can have bacilliferous pictures. Therefore, according to the MoH guideline, the diagnosis of pulmonary TB in Brazil varies according to age.7
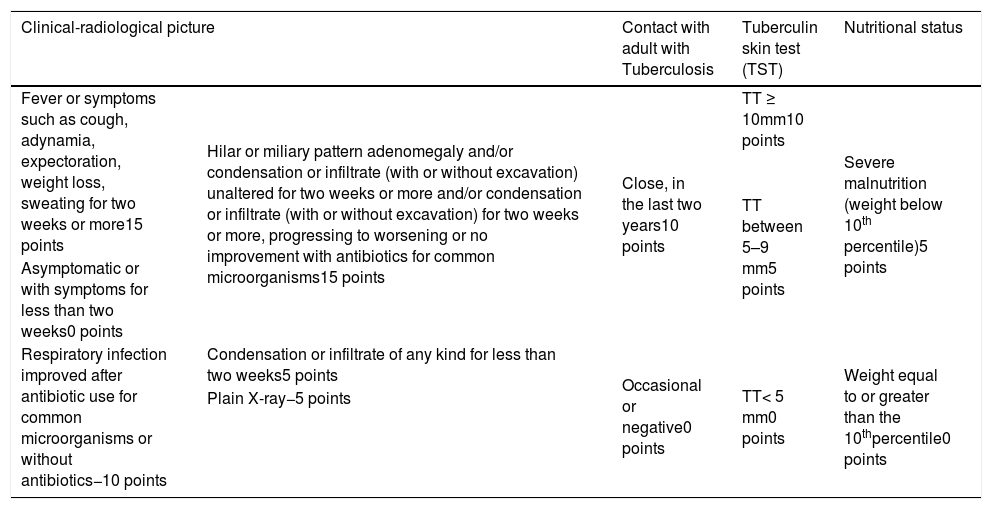
In children under 10 years of age, due to paucibacillary form and sputum collection difficulties, the diagnosis is based on a scoring system, which evaluates the clinical and epidemiological history, tuberculin skin test (TST) response, chest X-ray, and the patient's nutritional status (Table 4). This score is dynamic and evaluates the case evolution, with different scores according to time of evolution and response to therapies for usual infections; it has been adopted in Brazil since 2002. It is the best-studied, most-validated regimen, with consistent sensitivities and specificities among the various existing systems in the world.18 However, as the TST scores change, further studies should be developed to assess its current sensitivity and specificity.
Diagnosis of pulmonary tuberculosis in children and adolescents with negative smear or undetected rapid molecular test for tuberculosis (RMT-TB).
| Clinical-radiological picture | Contact with adult with Tuberculosis | Tuberculin skin test (TST) | Nutritional status | |
|---|---|---|---|---|
| Fever or symptoms such as cough, adynamia, expectoration, weight loss, sweating for two weeks or more15 points | Hilar or miliary pattern adenomegaly and/or condensation or infiltrate (with or without excavation) unaltered for two weeks or more and/or condensation or infiltrate (with or without excavation) for two weeks or more, progressing to worsening or no improvement with antibiotics for common microorganisms15 points | Close, in the last two years10 points | TT ≥ 10mm10 points | Severe malnutrition (weight below 10th percentile)5 points |
| TT between 5–9 mm5 points | ||||
| Asymptomatic or with symptoms for less than two weeks0 points | ||||
| Respiratory infection improved after antibiotic use for common microorganisms or without antibiotics−10 points | Condensation or infiltrate of any kind for less than two weeks5 points | Occasional or negative0 points | TT< 5 mm0 points | Weight equal to or greater than the 10thpercentile0 points |
| Plain X-ray−5 points | ||||
Interpretation:
≥40 points (very likely diagnosis): it is recommended to start treatment.
30 to 35 points (possible diagnosis): indicative of tuberculosis; it is advised to start treatment at the clinicians discretion.
<25 points (unlikely diagnosis): Investigation should continue. Differential diagnosis should be made with other lung diseases and complementary diagnostic methods can be employed, such as sputum smears and culture of induced sputum, or gastric lavage, bronchoscopy, puncture histopathology, and other rapid tests.7
Those over 10 years old, with similar radiological clinical pictures to adults, have their diagnosis established by microbiological tests. However, in the absence of bacteriological confirmation, the recommendation of the MoH is that the diagnosis should be based on the scoring system.
The WHO advises, whenever possible, to seek bacteriological confirmation through smear microscopy, culture, or rapid molecular test and does not recommend the “therapeutic test,” which consists of trying to establish the diagnosis of TB by performing the treatment and awaiting clinical improvement.19 For the MoH, the use of the score provides early diagnosis and therapeutic intervention, even in basic health units, without the need for more sophisticated complementary exams and/or specialized professionals.7 Bacteriological confirmation should always be attempted, although this should not delay the start of the treatment.
Despite the relevance of the topic, little has been published in the last year regarding this specific point. Other clinical collection options, such as induced sputum, string test, or oral swab, are being evaluated, but there are still many challenges and controversies, as recently discussed by Rauter et al.20
The technique of induced sputum collection consists of performing hypertonic inhalation of an airway irritant prior to the collection of the material. In individuals who can spontaneously expectorate, sputum is collected, but in children the material is collected by nasal aspiration. The accuracy of these samples is similar to that of gastric lavage,20 but it has the disadvantage of generating aerosols and, therefore, a biosecure site suitable for collection is needed.
The efficacy of the string test or Corda Dulce has yet to be established. In this methodology, the children swallow a capsule containing a “string” that remains for one hour in the stomach and it is then removed. This string adsorbs mycobacteria and is sent to the laboratory for routine examinations such as smear microscopy, culture, and rapid molecular testing. This technique has some advantages because it does not require hospitalization or biosecure conditions for collection. Its drawback is that not all children can swallow the capsules. However, further studies need to be performed to demonstrate the tolerability and accuracy of the method.21 The oral swab has been studied as a promising alternative, but the studies have only been carried out in adults.22–24
Microbiologically, the rapid molecular test has not yet been able to surpass the culture for the diagnosis of pulmonary tuberculosis in childhood, but due to the rapid culture result, when available, it should always be performed. This technique has now been modified with the production of the new GeneXpert ULTRA cartridge (Xpert®, CA, USA), which has been shown to be more sensitive, improving diagnosis, especially in populations with HIV, children under 10 years, and cases of extrapulmonary tuberculosis.25
The TST has several limitations, such as administration technique, reader-dependent result, need for two visits to perform the test, different reference values in the literature to consider the test positive, possibility of interference with other mycobacteria, especially the M. bovis vaccinal strain and, in recent years, the lack of supplies, PPD-RT 23 (Purified Protein Derivatite, RT: Reset Tuberculin, 23. Statens Serum, Copenhagen, Denmark). Therefore, more sensitive and specific tests are under study. Currently, interferon-gamma release assays (IGRA) are the most promising, but with limitations of use in children from 2 to 5 years old due to the low rate of agreement with TST and high rates of indeterminate cases. Moreover, they have a high cost, require better laboratory infrastructure, and have showed no superiority in any study when compared with TST. Velasco-Arnaiz et al. compared the tests in children under 5 years old and concluded that there is no need to perform both in unvaccinated populations, due to the high agreement between them. Among vaccinated children, the disagreement was higher (κ=0.190), and it was not possible to establish whether the cases were infected with other mycobacteria or by M. tuberculosis. Thus, in the presence of disagreement, the authors suggest considering the child as being infected. In that study, there was no difference in the results between infected patients and those with the disease, nor was there greater sensitivity of IGRA in relation to TST in patients.26
In 2018, Aggerbeck et al. published a phase-3 study with a new C-Tb tuberculin test (Statens Serum Institut–Copenhagen, Denmark). It is a skin test proposed for LTBI diagnosis, aimed at overcoming some difficulties of the usual TST with PPD-RT 23 and IGRAs. It is administered and read in the same way as the TST, but consists of the more specific antigens, ESAT-6 and CFP-10, the same as of IGRAs. The positivity cutoff is universal, with 5-mm skin induration. The results showed a safe test with positivity rates similar to that of TST and IGRA (83 % agreement), even in children under 5 years and in HIV-positive patients. However, in HIV-positive individuals with CD4<100 cells/μL, the positivity rates were decreased in all three tests.27
Evidence of inflammatory activity is classically investigated in association with TB. Velasco-Arnaiz et al. evaluated the association of procalcitonin and C-reactive protein with TB in children under 6 years of age and described the normality of this marker in cases of pulmonary TB, and an increase in both procalcitonin and C-reactive protein in pneumococcal pneumonia. Thus, it is suggested that children with TB contacts who have increased procalcitonin and C-reactive protein levels should be evaluated for the possibility of treatment for bacterial pneumonia and further re-evaluation for TB in two to three weeks.28
Treatment of active diseaseThe recommended TB treatment regimens in Brazil, as well as the diagnosis, follow the same age cutoffs. The latest guideline showed a change in the dose ranges for children, according to the WHO recommendations, so that the indicated serum concentrations of these drugs could be reached. Rifampicin should be prescribed at a dose of 15mg/kg (10–20mg/kg) and isoniazid 10mg/kg (7–15mg/kg), while pyrazinamide was maintained at a dose of 35mg/kg (30–40mg/kg).7
The recommended treatment time is two months for pyrazinamide and six months for the other drugs.7 Specifically, in relation to the meningeal form, it is extended to 12 months, following a standardized WHO regimen. The importance of isoniazid and pyrazinamide is emphasized, due to their excellent penetration and high concentration, with potent CSF bactericidal activity, whereas rifampicin has lower penetration and concentration in this site.29 As for the forms with bone involvement, the recommended treatment time is six to 12 months, at clinical discretion.7
A review by Donald et al. showed that the use of ethambutol at a dose of 20mg/kg (15–25mg/kg) is safe in children and can be used in the pediatric age range. In this review, with 3811 children, optic neuritis was attributed to ethambutol in only 0.05 % of children using this drug at a dose of 15–30mg/kg/day.30 In Brazil, its use is still recommended only in children over 10 years old, but when necessary, it can be safely used in younger children.7
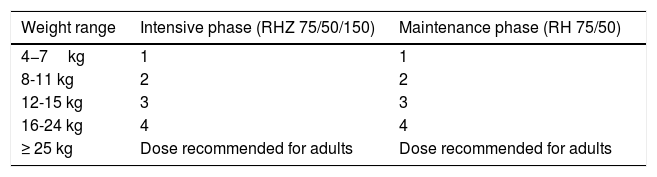
Another important advancement for children has been the development of the combined fixed dose since 2015, in the form of dispersible tablets: child-friendly, fruit-flavored formulations, which makes the treatment much more palatable and pleasant for children, in line with the new WHO recommendations. Each tablet contains 75mg of rifampicin, 50mg of isoniazid, and 150mg of pyrazinamide for the intensive phase; tablets with 75mg rifampicin and 50mg isoniazid are used for the treatment maintenance phase (Table 5). Dispersible tablets with isolated doses of pyrazinamide, ethambutol, isoniazid, and other drugs used for TB31 are under preparation.
Therapeutic regimen for tuberculosis, with friendly formulations, according to different weight ranges.
| Weight range | Intensive phase (RHZ 75/50/150) | Maintenance phase (RH 75/50) |
|---|---|---|
| 4−7kg | 1 | 1 |
| 8-11 kg | 2 | 2 |
| 12-15 kg | 3 | 3 |
| 16-24 kg | 4 | 4 |
| ≥ 25 kg | Dose recommended for adults | Dose recommended for adults |
R, rifampicin; H, isoniazid; Z, pyrazinamide.
Adapted from the World Health Organization.31
It is noteworthy that Brazil is in the phase of acquisition of pediatric dispersible formulations, showing its commitment to prioritizing TB control in this age group.
Children are still growing and developing, and because of this, the drugs have a wide pharmacokinetic variability and may have serum concentrations that are below the therapeutic level. A study carried out in Africa found that rifampicin and pyrazinamide, at the new doses recommended by the WHO, do not reach adequate serum concentrations in children under 12kg. Notably, serum rifampicin levels in children, regardless of the weight, were lower than those in adults. New studies are underway to evaluate the bioavailability of drugs in different geographic regions, taking into account possible genetic factors, weight, age, and formulation used.32
A study carried out in India evaluated the serum concentrations reached by isoniazid (10mg/kg) and pyrazinamide (35mg/kg) and compared them in children under and over 3 years of age, as well as with nutritional status. The concentration of pyrazinamide was lower in children under three years old and in children with low body mass index, while isoniazid showed no significant differences when these variables were evaluated.33
In Brazil, the regimens used and made available for the treatment of DRTB in children are the same as in adults, extending from 18 to 24 months.7 The WHO recommends the possibility of a shortened regimen, from nine to 12 months, but with little evidence in the pediatric population.34
Kumar et al. evaluated the pharmacokinetics of second-line medications used to treat MDR-TB (multidrug-resistant tuberculosis) in Indian children and adolescents, with a median age of 16 years, and showed that levofloxacin reached higher serum concentrations in females,35 similar to a study by Denti et al. with South African children.36 The latter study used macerated levofloxacin tablets in children and showed lower serum concentrations than in adults, despite equivalent doses, suggesting the need for further studies to evaluate the effects of different formulations and presentations.36
Kumar et al. also showed that ethionamide had lower serum concentrations in children under 12 years and that the serum concentrations of pyrazinamide were decreased in children and adolescents with low weight.35
These differences in drug pharmacokinetics according to gender, age, and weight should be considered when facing unfavorable outcomes, even when using appropriate doses and with good treatment adherence.
New drugs are being tested and released for use in pediatrics, such as bedaquiline and delamanid in long-term regimens. Bedaquiline can be used in patients aged 6–17 years and delamanid in patients from 3 years of age.34 Pretomanid, a new drug recently approved by the FDA, still requires studies before being used in children.37
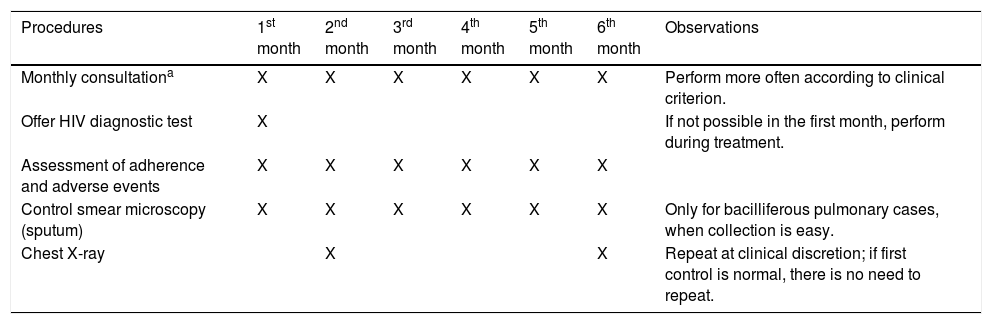
Follow-up of treatment in children and adolescentsChildren and adolescents should be monitored as described in Table 6.
Clinical consultations and follow-up exams for tuberculosis treatment in children and adolescents.
| Procedures | 1st month | 2nd month | 3rd month | 4th month | 5th month | 6th month | Observations |
|---|---|---|---|---|---|---|---|
| Monthly consultationa | X | X | X | X | X | X | Perform more often according to clinical criterion. |
| Offer HIV diagnostic test | X | If not possible in the first month, perform during treatment. | |||||
| Assessment of adherence and adverse events | X | X | X | X | X | X | |
| Control smear microscopy (sputum) | X | X | X | X | X | X | Only for bacilliferous pulmonary cases, when collection is easy. |
| Chest X-ray | X | X | Repeat at clinical discretion; if first control is normal, there is no need to repeat. |
Adapted from the Ministry of Health, Brazil.7
Children, usually paucibacillary and without bacteriological confirmation, will not undergo control smear and should be followed periodically (at least once a month) according to their clinical and radiological evolution. Follow-up at referral outpatient clinics with specialist pediatricians is recommended in cases of greater complexity, considering that better treatment outcomes, such as reduced mortality, were observed in the follow-up with professionals from these services.38
A study with Paraguayan children showed that the rifampicin and pyrazinamide dose by dry blood spot on filter paper could be a way of monitoring the plasma concentrations of these drugs,39 especially when good adherence to treatment is certain and progress is unfavorable.
Children rarely have adverse events; thus, biochemical tests are recommended based only on individual clinical criteria at the beginning or during therapy.
The importance of DOT at all ages is reiterated, guiding the family and the patient, according to their understanding, about the consequences of irregular adherence and treatment abandonment.7
Treatment of latent infectionLTBI is defined as a state of persistent immune response without active disease. The groups that would benefit from the identification of this status and the need for treatment should be defined. Whether the diagnosis will be made through TST or IGRA, the TST value considered positive, and the priority populations for treatment may vary in different countries, according to the prevalence of TB in the region and local guidelines.5
The pharmacological treatment of LTBI is the main intervention capable of preventing its progression to active tuberculosis. Its indication depends on the outcome of the TST or IGRA, age, likelihood of infection, and risk of illness.
In Brazil, the TST is considered positive with a skin induration ≥ 5mm, regardless of age, immunological and vaccination status, or time elapsed since BCG vaccination.7 Recent studies have shown that the influence of BCG on TST depends more on the age at which BCG was administered than the interval between these administrations. This effect decreases over time, especially if the vaccine was administered before the age of 2, the period when it is performed in the Brazilian population.40 Seddon et al. confirmed this finding, showing that in this scenario (children under 2 years, vaccinated), the TST was negative in most cases.41
Patients with a history of contact or tuberculin conversion in the past year, those immunosuppressed by medication, or those with an underlying disease would be the priority groups to investigate LTBI and assess the need for treatment.
At-risk groups such as children with HIV infection, those pre-organ transplantation, or those who will start immunosuppressive therapy should be evaluated with TST, regardless of contact with TB. If the TST is positive, the LTBI must be treated. However, if there is a history of contact with a bacilliferous case, the individual should be treated even if the TST is negative.7
If the treatment of LTBI is necessary, the diagnosis of active disease should be previously ruled out, with clinical evaluation and chest X-ray.7
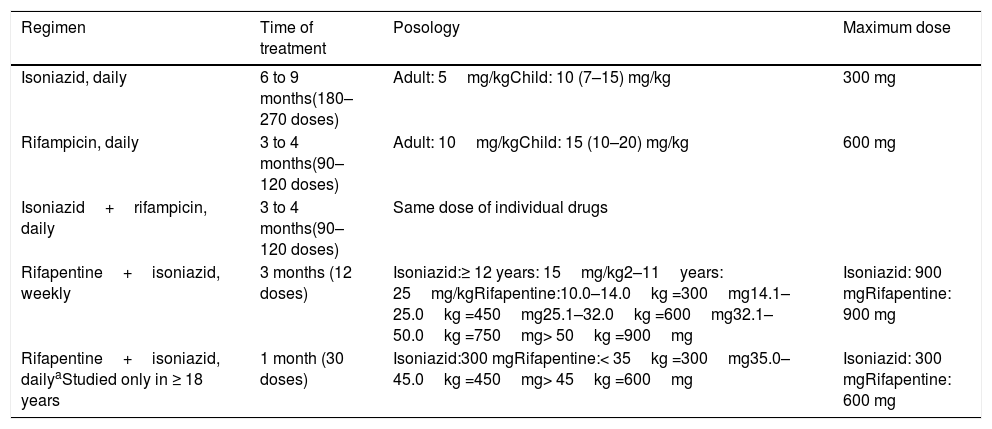
Currently, several therapeutic regimens are available (Table 7).42
Therapeutic regimen for treatment of latent tuberculosis infection.
| Regimen | Time of treatment | Posology | Maximum dose |
|---|---|---|---|
| Isoniazid, daily | 6 to 9 months(180–270 doses) | Adult: 5mg/kgChild: 10 (7–15) mg/kg | 300 mg |
| Rifampicin, daily | 3 to 4 months(90–120 doses) | Adult: 10mg/kgChild: 15 (10–20) mg/kg | 600 mg |
| Isoniazid+rifampicin, daily | 3 to 4 months(90–120 doses) | Same dose of individual drugs | |
| Rifapentine+isoniazid, weekly | 3 months (12 doses) | Isoniazid:≥ 12 years: 15mg/kg2–11years: 25mg/kgRifapentine:10.0–14.0kg =300mg14.1–25.0kg =450mg25.1–32.0kg =600mg32.1–50.0kg =750mg> 50kg =900mg | Isoniazid: 900 mgRifapentine: 900 mg |
| Rifapentine+isoniazid, dailyaStudied only in ≥ 18 years | 1 month (30 doses) | Isoniazid:300 mgRifapentine:< 35kg =300mg35.0–45.0kg =450mg> 45kg =600mg | Isoniazid: 300 mgRifapentine: 600 mg |
Adapted from the World Health Organization.34
Isoniazid reduces the risk of the disease by 60%–90%. This variation is due to the treatment duration and adherence. It is noteworthy that the number of doses, not the time of treatment, is the most important factor for therapeutic effectiveness. It is recommended that 270 doses be taken from nine to 12 months or 180 doses between six and nine months.7
Studies in children have shown the adherence, efficacy, and safety advantages of a four-month treatment with rifampicin, when compared to nine months of isoniazid.43,44
Considering this evidence, Brazil recommends rifampicin in children under 10 and adults over 50 years old, individuals with liver disease, and those who are isoniazid-intolerant, with 120 doses taken between four and six months.7
The most promising treatment regimen is rifapentine associated with isoniazid, for which studies have shown no inferiority to nine months of isoniazid alone.
The advantages of this regimen are greater adherence, lower or similar toxicity, and easy posology. There are two forms of prescription: weekly doses for three weeks (12 doses)45 or daily doses for four weeks (30 doses).46
In Brazil, the treatment of LTBI is not compulsorily reported, but notification using a specific national form is recommended.7
For contacts with DRTB, there is insufficient evidence for treatment. Their evaluation is recommended to identify possible cases of active or latent TB. When LTBI is identified, periodic follow-up for at least two years is recommended to identify early signs of disease progression. Levofloxacin has been studied as a prophylactic alternative for these cases.47
Primary chemoprophylaxisNewborns (NBs) exposed to cases of bacillary or laryngeal pulmonary TB may develop severe forms of the disease and should be monitored and investigated. In the prevention strategy for this population in Brazil, the use of rifampicin has recently been incorporated as an option to the use of isoniazid.7
The recommendation is maintained to not vaccinate with BCG at birth and to start chemoprophylaxis for three months; after this period, the TST is performed. If the TST result is positive, the chosen regimen is completed (isoniazid 180 doses or rifampicin 120 doses) and the newborn should not be vaccinated with BCG. If the TST is negative, discontinue treatment and vaccinate. If exposed newborns are inadvertently vaccinated, it is recommended to complete the treatment without the need for TST.7
When the source case is the mother, evaluate the possibility of congenital TB. There are no contraindications to breastfeeding as long as the mother does not have tuberculous mastitis. The use of a surgical mask is recommended when breastfeeding and caring for the child while sputum smear results remain positive.7
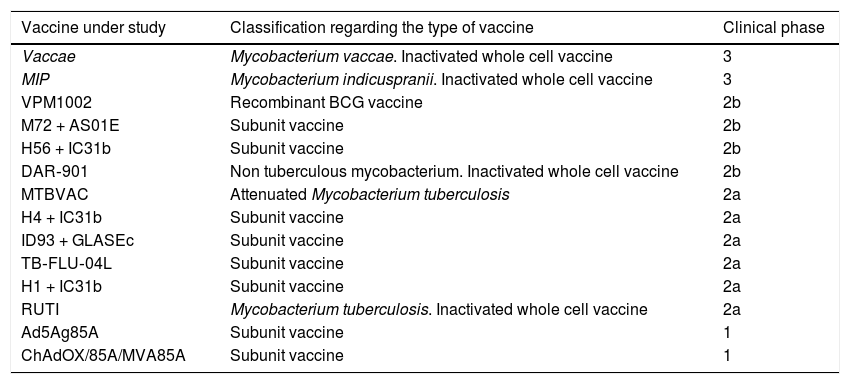
Active immunizationIn the last two decades, great progress has been made in search for a more effective tuberculosis vaccine, but there is still no substitute for the licensed BCG vaccine. There are approximately 14 vaccines for TB under study and they are in different phases of clinical trials, being tested pre- and post-exposure. They consist of vaccine subunits (associated with attenuated viral vectors or adjuvant proteins), whole-cell vaccines (genetically attenuated with M. tuberculosis, BCG recombinants, or dead M. vaccae or M. tuberculosis).48,49
Two vaccines are in phase 3, i.e., the phase that demonstrates the vaccine’s efficacy and reinforces its safety before it use is approved for the population. Most studies are in phase 2, which aims to assess immunogenicity. Two other vaccines are in phase 1, in which vaccine safety is studied. A summary of vaccines and their studies is described in Table 8.
Clinical phases of ongoing studies for tuberculosis vaccines.
| Vaccine under study | Classification regarding the type of vaccine | Clinical phase |
|---|---|---|
| Vaccae | Mycobacterium vaccae. Inactivated whole cell vaccine | 3 |
| MIP | Mycobacterium indicuspranii. Inactivated whole cell vaccine | 3 |
| VPM1002 | Recombinant BCG vaccine | 2b |
| M72 + AS01E | Subunit vaccine | 2b |
| H56 + IC31b | Subunit vaccine | 2b |
| DAR‐901 | Non tuberculous mycobacterium. Inactivated whole cell vaccine | 2b |
| MTBVAC | Attenuated Mycobacterium tuberculosis | 2a |
| H4 + IC31b | Subunit vaccine | 2a |
| ID93 + GLASEc | Subunit vaccine | 2a |
| TB‐FLU‐04L | Subunit vaccine | 2a |
| H1 + IC31b | Subunit vaccine | 2a |
| RUTI | Mycobacterium tuberculosis. Inactivated whole cell vaccine | 2a |
| Ad5Ag85A | Subunit vaccine | 1 |
| ChAdOX/85A/MVA85A | Subunit vaccine | 1 |
Adapted from Méndez-Samperio.48
Despite the widespread use of BCG, this vaccine is still the subject of many studies. In February 2018, the WHO questioned the benefits of repeating the BCG, due to lack of scientific evidence in vaccinated children who had not developed a vaccine scar, even with a negative TST or IGRA.50 Following the WHO recommendations, the Brazilian Ministry of Health has not recommended revaccination of children who did not develop the vaccine scar after six months of its administration and without the need for further testing (Informative Note No. 10/2019-CGPNI/DEVIT/SVS/MS).
Final considerationsThis review looked at TB from a different viewpoint in childhood and adolescence. It is important that greater relevance is given to this disease, thus causing children, adolescents, family, professionals, and civil society to feel that someone is listening to them, making this epidemic not so silent anymore. Advances in management have been observed. However, there is much to be done… more than just a look, a keener ear, giving an even greater voice to children and adolescents; taste and smell, so that new child-friendly formulas can be developed; and a caring touch that mobilizes and results in more effective actions. Only with the union of the five senses, children and adolescents will be able to replace the suffering, once imposed by TB, by a future of greater acceptance, of assertiveness in diagnosis and treatment, as well as prevention and perhaps TB elimination in the near future.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Tahan TT, Gabardo BM, Rossoni AM. Tuberculosis in childhood and adolescence: a view from different perspectives. J Pediatr (Rio J). 2020;96(S1):99–110.
Institution or service the study is associated with for indexing: Index Medicus/MEDLINE: Department of Pediatrics, Universidade Federal do Paraná, Brazilian Network of Tuberculosis Research (REDE TB –Rede Brasileira de Pesquisas em Tuberculose).