The aim of the present study was to create a translated version of the Pediatric Quality of Life Inventory™ 3.0 Diabetes Module (PedsQL™ 3.0 Diabetes Module) in Brazilian Portuguese that was conceptually equivalent to the original American English version and to linguistically validate it in a Brazilian pediatric population with type 1 diabetes mellitus and their parents or caregivers.
MethodsThe instrument was translated, back-translated, and then administered to 83 children/adolescents (5–18 years) with type 1 diabetes mellitus and their family members and to 25 parents/caregivers of patients aged between 2 and 4 years. The final translated version was tested for reliability by analyzing internal consistency, intraobserver (test–retest) reliability, and concurrent validity.
ResultsCronbach's alpha coefficient for the total score of the questionnaires of children/adolescents (α=0.85) and their parents (α=0.82) was above the recommended minimum of 0.70 for group comparisons. Intraobserver reliability and concurrent validity exhibited a significant positive correlation (p<0.001), indicating the reliability of the translated instrument. A moderate but significant positive correlation (r=0.40; p<0.001) was demonstrated between the total scores of patient self-report and parent proxy-report scales. There was no significant correlation between glycated hemoglobin (HbA1c) levels and the respective scores in the questionnaires answered by patients and their parents/caregivers.
ConclusionThe analysis of the translated version of the PedsQL™ 3.0 Diabetes Module revealed adequate psychometric characteristics with respect to reliability and validity following administration to a sample of Brazilian children/adolescents with type 1 diabetes mellitus and their caregivers.
Produzir uma versão do questionário Pediatric Quality of Life Inventory™ 3.0 Diabetes Module (PedsQL™ 3.0 Diabetes Module) para a língua portuguesa do Brasil, que fosse conceitualmente equivalente à versão original em inglês, e proceder à sua validação linguística numa população pediátrica brasileira portadora de diabetes melitus tipo 1 (DM1) e seus pais ou cuidadores.
MétodosA tradução do instrumento foi feita pela metodologia de tradução-tradução reversa, foi aplicado a 83 crianças/adolescentes (5-18 anos) portadores de diabetes mellitus tipo 1 com seus parentes e a 25 pais/cuidadores de pacientes entre 2 e 4 anos de idade. A confiabilidade da versão traduzida foi avaliada pelas seguintes análises: consistência interna, confiabilidade teste-reteste e validade concorrente.
ResultadosO coeficiente alfa de Cronbach para a pontuação total do questionário das crianças/adolescentes (α=0,85) e seus pais (α=0,82) excedeu o mínimo recomendado 0,70 para comparação entre grupos. Na confiabilidade intraobservador e validade concorrente observou-se correlação positiva e estatisticamente significativa (p<0,001), indicou indicando a fidedignidade do instrumento traduzido. Na comparação entre os escores totais obtidos por pais/cuidadores e crianças/adolescentes, houve uma correlação positiva, pequena, mas significativa (r=0,40; p<0,001). Não houve correlações estatisticamente significativas entre os níveis de hemoglobina glicada e os escores obtidos nos questionários respondidos pelos pacientes e seus pais/cuidadores.
ConclusãoAs análises do instrumento PedsQL™ 3.0 Módulo Diabetes demonstraram propriedades psicométricas adequadas em termos de confiabilidade e validade quando aplicado nessa amostra de crianças/adolescentes brasileiros portadores de DM tipo 1 e seus cuidadores.
Diabetes mellitus (DM) is one of the most common metabolic diseases worldwide, and its prevalence has increased in the past decades. Approximately 90% of cases in children aged under 15 years are classified as type 1 DM (DM1), with an autoimmune cause, representing one of the main chronic pediatric diseases. Unfortunately, DM diagnosed during childhood presents an increased risk of complications in an early and productive stage, leading to a reduction in mean life expectancy of 10 to 20 years, a result that is especially prevalent in developing countries.1
Prospective clinical studies have clearly shown that a strict glycemic control from disease onset can delay or even prevent the onset of DM-related chronic vascular complications.2,3 However, strategies to prevent severe recurrent nocturnal hypoglycemia must also be developed.4,5 In this sense, daily management of DM1 poses countless challenges to the achievement of satisfactory metabolic control, because it requires complex treatment through multiple insulin injections, frequent self-monitoring of blood glucose, strict mealtime schedule, regular physical exercise, and frequent contact with healthcare professionals,6,7 all of which could impact the quality of life of patients, especially children and adolescents.8,9
In the past decade, interest in health-related quality of life (HRQOL) has increased significantly and become an essential outcome measure in clinical trials and evaluations of healthcare services.10
The various aspects of health addressed by HRQOL are specific to each stage of cognitive development in children, and can reveal problems that even parents fail to notice. Thus, a standardized HRQOL assessment instrument would be highly useful for detecting physical and emotional concerns from the perspectives of both children and caregivers.10 To be useful in clinical practice, HRQOL questionnaires must meet the following criteria: (a) they must be brief, with reliability and validity to provide adequate non-biased information; (b) they must be designed for parents and children, being easy to interpret and score; and (c) they must be sensitive to sudden changes in patients’ attitudes.10,11 The lack of an effective instrument to assess HRQOL in children aged under 11 years motivated Varni et al. to develop the Pediatric Quality of Life Inventory (PedsQL™), which includes both a generic scale for chronic diseases (PedsQL™ 4.0 Generic Core Scales) and a scale specific to certain diseases,12 including a HRQOL scale for children and adolescents with DM1 between 2 and 18 years of age and their parents or caregivers, called Pediatric Quality of Life Inventory™ 3.0 Diabetes Module (PedsQL™ 3.0 Diabetes Module).13–16
The aims of the present study were to create a translated version of the PedsQL™ 3.0 Diabetes Module in Brazilian Portuguese that was conceptually equivalent to the original American English version13 and easy to understand, as well as to linguistically validate the instrument in a Brazilian pediatric population with DM1, making it possible to assess quality of life specifically related to this disease.
MethodsThe PedsQL™ 3.0 Diabetes Module is composed of self-report questionnaires on the quality of life of children/adolescents in the age ranges of 5–7, 8–12, and 13–18 years, paired with questionnaires assessing their quality of life from their parents’/caregivers’ perspectives (parent proxy-report); the latter also answer questionnaires about children in the age range of 2–4 years. Each questionnaire contains 28 items, classified into five domains or subscales as follows: (1) diabetes symptoms (11 items); (2) treatment barriers (4 items); (3) treatment adherence (7 items); (4) worry (3 items); and (5) communication (3 items). The initial instructions ask respondents to indicate to what extent each item has been problematic during the past month on a five-point scale (0=never a problem; 1=almost never a problem; 2=sometimes a problem; 3=often a problem; and 4=almost always a problem). Items are then reverse-scored and linearly transformed into a Likert scale with a 0–100 range (0=100, 1=75, 2=50, 3=25, 4=0), so that higher scores indicate better HRQOL. The questionnaire for children aged 5–7 years contains three choices for each item, specifically, “not at all a problem for you” (0=100), “sometimes a problem for you” (2=50), and “a problem for you a lot” (4=0) answers that are anchored to a face scale composed of a smiling, a middle, and a frowning face, respectively.12,13
The transcultural translation of the instrument followed the forward translation and back-translation method,17,18 in close collaboration with the Mapi Research Institute in Lyon, France and with the consent of the author, Dr. J.W. Varni.19 The translation included the following phases.
Phase 1Independent translations of the original version of the PedsQL™ 3.0 Diabetes Module from US English (source language) to Brazilian Portuguese (target language) were performed by two local professional translators (MMP and BFL), whose native language is Brazilian Portuguese. Both versions were discussed by the translators and researchers to obtain agreement on a single version of the questionnaire, which should be conceptually equivalent to the original questionnaire, using colloquial and simple language.
Phase 2The Portuguese version was then back-translated into American English by a translator (EA), whose native language is English. The original instrument was compared with the back-translated version at a meeting between the translator and the researchers, to detect misunderstandings, translation errors, and inaccuracies in the version produced in Phase 1. A report was sent to the group headed by Dr. J.W. Varni, author of the original PedsQL™; they suggested no changes.
Phase 3According to the standard cognitive interview of the PedsQL™ 3.0 Diabetes Module,13,19 the translated version was administered to groups of patients of each age range and their parents/caregivers. The aim of this phase was to determine whether the translated questionnaire was acceptable, used simple language, was easy to understand, and maintained the meanings of the American English terms. Additionally, the time needed for completion of the questionnaire was determined. Interviews with children were conducted separately (in different rooms) from those of their parents. The number of interviewees per age range was 2–4 years (n=4), 5–7 years (n=4), 8–12 years (n=5), and 13–18 years (n=5). Questionnaires were completed within approximately 15min per patient/parent, except for the age range of 5–7 years, which required 15–20min for completion. This is because for each question, the child had to choose a face that indicated the degree of difficulty of the respective item. Throughout the age ranges, the interviewees did not know the meaning of “identification card,” which is a small card containing information on the treatment of hypoglycemia, drugs used, emergency numbers, and of “fast-acting carbohydrate” (items 5 and 6 of the Treatment adherence domain). A report was produced in English and, after further review, Dr. J.W. Varni suggested including examples of fast-acting carbohydrates and showing an identification card.
Therefore, the final version in Brazilian Portuguese was created and designated PedsQL™ 3.0 Módulo Diabetes. To validate it (Phase 4 – Field test), patients regularly attended to at the Diabetes Outpatient Clinic of Instituto da Criança do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), at the Endocrinology and Metabolism Outpatient Clinic – Diabetes Unit – HCFMUSP, and at private practices, as well as those who voluntarily participated in activities coordinated by the Juvenile Diabetes Association – São Paulo were invited to answer the questionnaires from August 2014 to February 2017. Patients within the age range of 2–18 years, diagnosed with DM1 at least one year prior to the study, and accompanied by at least one parent or caregiver at the clinic appointment, were included. Exclusion criteria were the presence of physical and/or mental comorbidities and a need for daily insulin dose below 0.5U/kg/day. Other data collected included the age at diagnosis, duration of DM1, and glycated hemoglobin (HbA1c) levels, which were quantified using the Bio-Rad HPLC Variant II (Bio-Rad®, SP, Brazil) method at the time that the instrument was administered.
Questionnaires were answered by children/adolescents and their parents/caregivers separately on the day of a medical appointment (test phase) and re-administered after a 15-day to 3-month interval (retest phase). The researcher personally administered the questionnaires to children in the 5–7-year age range and to illiterate individuals, whereas children older than 8 years, adolescents, and adults answered the questionnaire in the waiting room, with no interference by the researcher.
The reliability of the Brazilian version (PedsQL™ 3.0 Módulo Diabetes) was assessed using the analyses of internal consistency, intraobserver reliability (test–retest), and concurrent validity, assessed through: (a) associations between scores on this instrument and those of a generic pediatric quality of life questionnaire previously translated and validated in Brazil (PedsQL™ Questionário pediátrico sobre qualidade de vida Versão 4.0 – Português [Brasil])16; (b) correlation between PedsQL™ 3.0 Módulo Diabetes total score and patient HbA1c levels; and (c) correlations between the scores obtained on patient self-report (5–18 years) and parent proxy-report scales.
The study was approved by the Ethics Committee for the Analysis of Research Projects (No. 555659). Patients and their relatives/caregivers were informed about the objectives of the study and asked to read and sign the informed assent/consent forms.
Statistical analysisDescriptive statistical analysis was used for clinical and laboratory patient characterization and for analysis of total score on the PedsQL™ 3.0 Módulo Diabetes and its subscales. Internal consistency (inter-item correlation) was assessed using Cronbach's alpha coefficient20: an alpha value>0.7 was considered fair, an alpha value>0.81, good, and an alpha value>0.91, excellent.21 Reliability scales≥0.70 are recommended for comparisons of patient groups, whereas those with a reliability criterion of 0.90 are recommended for the analysis of individual patient scores.12,13,22 Intraobserver reliability and concurrent validity were assessed using Pearson's correlation test. The degree of correlation (r) between variables was designated as small (0.3–0.49), medium (0.5–0.7), or large (>0.7).23 All analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 20.0. NY, USA),24 and the statistical significance level adopted was p<0.05.
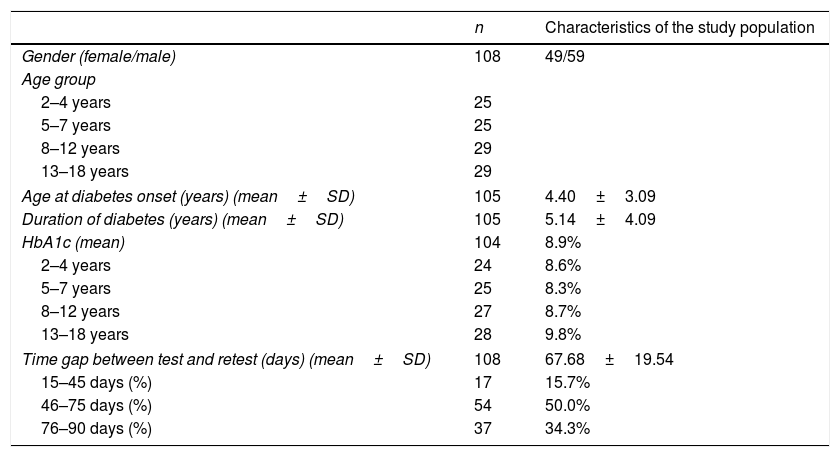
ResultsOf the 127 eligible participants, 83 patients (5–18 years) and 108 parents/caregivers (2–18 years) completed all questions of the instrument in the test and retest phases. Table 1 shows the distribution of the studied population according to age range, clinical characteristics, and mean HbA1c level.
Clinical and laboratory features from study sample population and time gap between test and retest phases.
| n | Characteristics of the study population | |
|---|---|---|
| Gender (female/male) | 108 | 49/59 |
| Age group | ||
| 2–4 years | 25 | |
| 5–7 years | 25 | |
| 8–12 years | 29 | |
| 13–18 years | 29 | |
| Age at diabetes onset (years) (mean±SD) | 105 | 4.40±3.09 |
| Duration of diabetes (years) (mean±SD) | 105 | 5.14±4.09 |
| HbA1c (mean) | 104 | 8.9% |
| 2–4 years | 24 | 8.6% |
| 5–7 years | 25 | 8.3% |
| 8–12 years | 27 | 8.7% |
| 13–18 years | 28 | 9.8% |
| Time gap between test and retest (days) (mean±SD) | 108 | 67.68±19.54 |
| 15–45 days (%) | 17 | 15.7% |
| 46–75 days (%) | 54 | 50.0% |
| 76–90 days (%) | 37 | 34.3% |
Among the parents/caregivers who completed the instrument (n=108), 34.3% were within the age range of 31–40 years, and 31.5% were within the age range of 41–50 years. Mothers comprised most of this sample (88.9%).
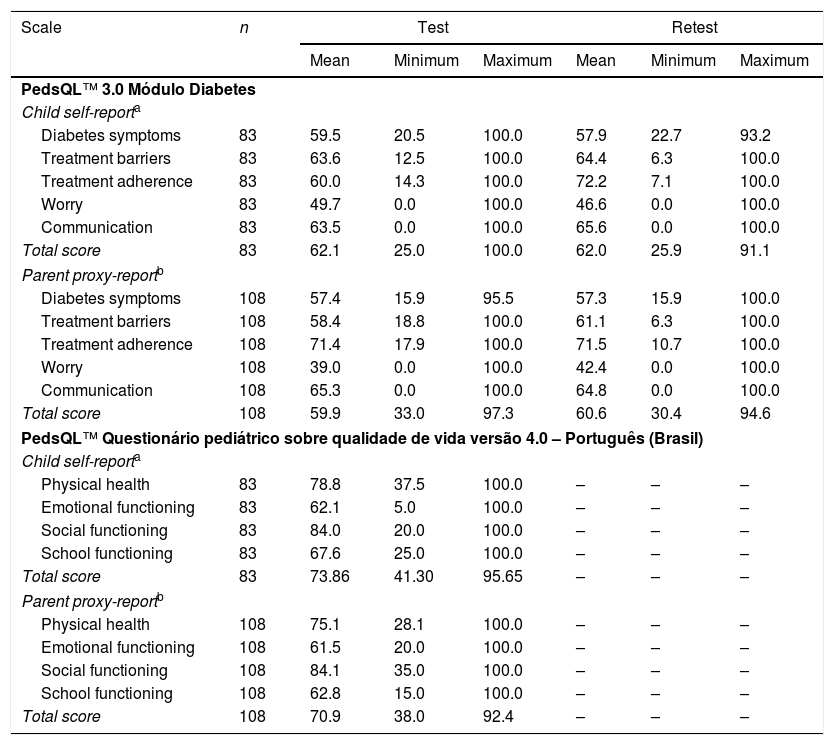
Descriptive analyses of the total score and subscale scores of the PedsQL™ 3.0 Módulo Diabetes (test–retest phases) and of the PedsQL™ Questionário pediátrico sobre qualidade de vida Versão 4.0 – Português (Brasil) of the studied population are presented in Table 2.
Scale descriptives for PedsQL™ 3.0 Módulo Diabetes (test–retest phases) and PedsQL™ Questionário pediátrico sobre qualidade de vida versão 4.0 – Português (Brasil) in the study sample.
| Scale | n | Test | Retest | ||||
|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean | Minimum | Maximum | ||
| PedsQL™ 3.0 Módulo Diabetes | |||||||
| Child self-reporta | |||||||
| Diabetes symptoms | 83 | 59.5 | 20.5 | 100.0 | 57.9 | 22.7 | 93.2 |
| Treatment barriers | 83 | 63.6 | 12.5 | 100.0 | 64.4 | 6.3 | 100.0 |
| Treatment adherence | 83 | 60.0 | 14.3 | 100.0 | 72.2 | 7.1 | 100.0 |
| Worry | 83 | 49.7 | 0.0 | 100.0 | 46.6 | 0.0 | 100.0 |
| Communication | 83 | 63.5 | 0.0 | 100.0 | 65.6 | 0.0 | 100.0 |
| Total score | 83 | 62.1 | 25.0 | 100.0 | 62.0 | 25.9 | 91.1 |
| Parent proxy-reportb | |||||||
| Diabetes symptoms | 108 | 57.4 | 15.9 | 95.5 | 57.3 | 15.9 | 100.0 |
| Treatment barriers | 108 | 58.4 | 18.8 | 100.0 | 61.1 | 6.3 | 100.0 |
| Treatment adherence | 108 | 71.4 | 17.9 | 100.0 | 71.5 | 10.7 | 100.0 |
| Worry | 108 | 39.0 | 0.0 | 100.0 | 42.4 | 0.0 | 100.0 |
| Communication | 108 | 65.3 | 0.0 | 100.0 | 64.8 | 0.0 | 100.0 |
| Total score | 108 | 59.9 | 33.0 | 97.3 | 60.6 | 30.4 | 94.6 |
| PedsQL™ Questionário pediátrico sobre qualidade de vida versão 4.0 – Português (Brasil) | |||||||
| Child self-reporta | |||||||
| Physical health | 83 | 78.8 | 37.5 | 100.0 | – | – | – |
| Emotional functioning | 83 | 62.1 | 5.0 | 100.0 | – | – | – |
| Social functioning | 83 | 84.0 | 20.0 | 100.0 | – | – | – |
| School functioning | 83 | 67.6 | 25.0 | 100.0 | – | – | – |
| Total score | 83 | 73.86 | 41.30 | 95.65 | – | – | – |
| Parent proxy-reportb | |||||||
| Physical health | 108 | 75.1 | 28.1 | 100.0 | – | – | – |
| Emotional functioning | 108 | 61.5 | 20.0 | 100.0 | – | – | – |
| Social functioning | 108 | 84.1 | 35.0 | 100.0 | – | – | – |
| School functioning | 108 | 62.8 | 15.0 | 100.0 | – | – | – |
| Total score | 108 | 70.9 | 38.0 | 92.4 | – | – | – |
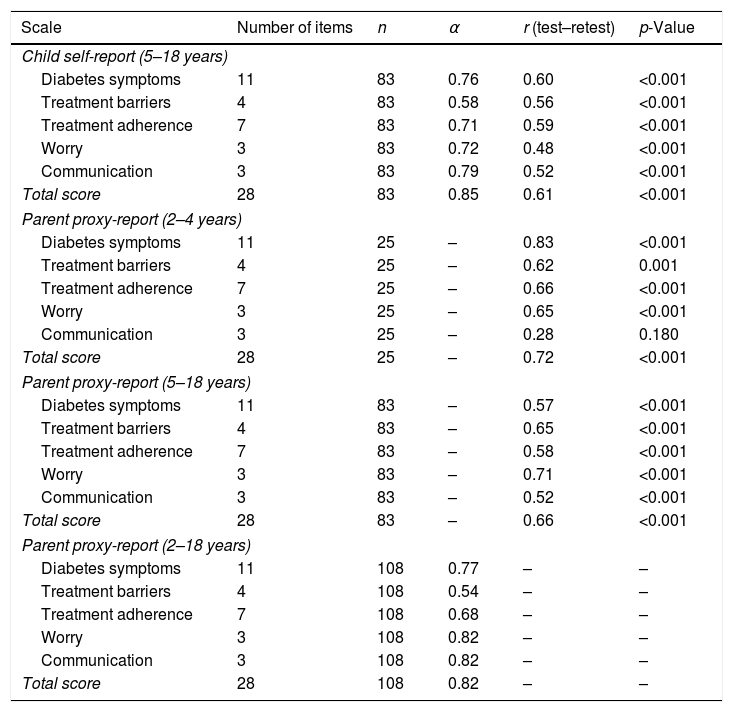
Cronbach's alpha coefficient for the fully translated PedsQL™ 3.0 Módulo Diabetes was 0.85 for children/adolescents and 0.82 for their parents/caregivers (Table 3). In a separate analysis of subscales, the following coefficients were obtained: diabetes symptoms (αchildren=0.76; αparents=0.77), worry (αchildren=0.72; αparents 0.82), communication (αchildren=0.79; αparents=0.82), treatment adherence (αchildren=0.71; αparents=0.68), and treatment barriers (αchildren=0.58; αparents=0.54).
Internal consistency (Cronbach's α-coefficient) and correlation between test–retest phases for PedsQL™ 3.0 Módulo Diabetes among parents/caregivers (2–18 years) and children/adolescents (5–18 years).
| Scale | Number of items | n | α | r (test–retest) | p-Value |
|---|---|---|---|---|---|
| Child self-report (5–18 years) | |||||
| Diabetes symptoms | 11 | 83 | 0.76 | 0.60 | <0.001 |
| Treatment barriers | 4 | 83 | 0.58 | 0.56 | <0.001 |
| Treatment adherence | 7 | 83 | 0.71 | 0.59 | <0.001 |
| Worry | 3 | 83 | 0.72 | 0.48 | <0.001 |
| Communication | 3 | 83 | 0.79 | 0.52 | <0.001 |
| Total score | 28 | 83 | 0.85 | 0.61 | <0.001 |
| Parent proxy-report (2–4 years) | |||||
| Diabetes symptoms | 11 | 25 | – | 0.83 | <0.001 |
| Treatment barriers | 4 | 25 | – | 0.62 | 0.001 |
| Treatment adherence | 7 | 25 | – | 0.66 | <0.001 |
| Worry | 3 | 25 | – | 0.65 | <0.001 |
| Communication | 3 | 25 | – | 0.28 | 0.180 |
| Total score | 28 | 25 | – | 0.72 | <0.001 |
| Parent proxy-report (5–18 years) | |||||
| Diabetes symptoms | 11 | 83 | – | 0.57 | <0.001 |
| Treatment barriers | 4 | 83 | – | 0.65 | <0.001 |
| Treatment adherence | 7 | 83 | – | 0.58 | <0.001 |
| Worry | 3 | 83 | – | 0.71 | <0.001 |
| Communication | 3 | 83 | – | 0.52 | <0.001 |
| Total score | 28 | 83 | – | 0.66 | <0.001 |
| Parent proxy-report (2–18 years) | |||||
| Diabetes symptoms | 11 | 108 | 0.77 | – | – |
| Treatment barriers | 4 | 108 | 0.54 | – | – |
| Treatment adherence | 7 | 108 | 0.68 | – | – |
| Worry | 3 | 108 | 0.82 | – | – |
| Communication | 3 | 108 | 0.82 | – | – |
| Total score | 28 | 108 | 0.82 | – | – |
During the test and retest phases (Table 3), for the parent/caregiver group (2–4 years), a significant correlation was observed between total scores (r=0.72; p<0.001) and all subscale scores (r=0.62–0.83; p<0.001), except in the domain communication (r=0.28; p=0.180), whereas those in age range 5–18 years presented a positive correlation (0.52–0.71; p<0.001) between all scores. A positive correlation was also observed in patient self-report scales (0.52–0.61; p<0.001) in both phases.
Concurrent validityIn comparing the total scores on the PedsQL™ 3.0 Módulo Diabetes and the PedsQL™ Questionário pediátrico sobre qualidade de vida Versão 4.0 – Português (Brasil), a significant positive correlation was observed between parent proxy-report scales of children in the age range of 2–4 and 5–18 years (r=0.48 and 0.55, respectively; p=0.016 and p<0.001), as well as between patient self-report scales (r=0.56; p<0.001).
A significant positive correlation was observed between the total scores of the parent proxy-report and patient (5–18 years) self-report scales (r=0.40; p<0.001), as well as in the subscales diabetes symptoms (r=0.43; p<0.001), treatment barriers (r=0.38; p<0.001), and communication (r=0.42; p<0.001).
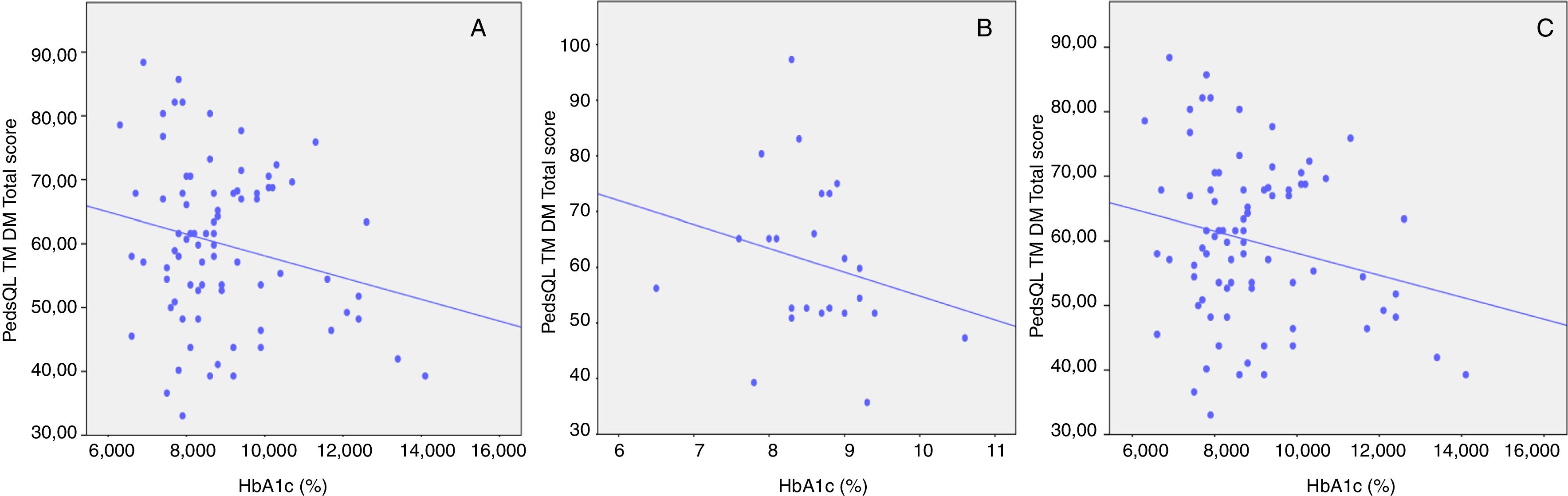
In contrast, in this population sample, no significant correlation was observed between glycemic control, based on HbA1c level, and total scores obtained in parent-child reports (r=−0.08 to −0.23; p>0.05; Fig. 1A–C).
DiscussionAfter the changes made to the second translated version of the PedsQL™ 3.0 Diabetes Module, the new instrument, now designated PedsQL™ 3.0 Módulo Diabetes, presented simple language and was easily understood by the studied population.
The scores obtained by the present sample of Brazilian children and adolescents with DM1 were lower than those obtained in studies in Kuwait,14 Italy,15 and Greece,25 and similar to those obtained in a study in Iran.26 Furthermore, the total score on the questionnaires completed by parents/caregivers was lower than that on the questionnaires completed by children/adolescents, as also observed in other studies.14,25
The internal reliability of the total scale of the translated questionnaire (αchildren=0.85; αparents=0.82) was above the recommended minimum (α=0.70) for comparison between groups,13,22 and exceeded the values found in the Greek,25 Italian,15 and Arabic versions.14 In the analysis of each subscale individually, the domains treatment adherence (α=0.71) and worry (α=0.72) in the questionnaires for children/adolescents (5–18 years) presented higher coefficients than those domains in the original American English13 and Italian15 versions. However, the domain treatment barriers in both questionnaires and treatment adherence in the parents’ questionnaire presented coefficients below 0.70, which suggests that the internal validity of the PedsQL™ 3.0 Diabetes Module lies in its entirety, thus not allowing for a fragmented application of its subscales. This trend was also observed in the original version13 and in the Greek,25 Italian,15 and Persian26 translations.
The intraobserver reliability was moderate for all age ranges. The results suggest an estimate of adequate reliability for the translated instrument; however, it should be noted that this finding might have been influenced by external factors, such as variables related to the disease itself and its treatment, as well as the socioeconomic and cultural conditions of the population.13,20
In the concurrent validation process, a small to medium correlation was observed between the PedsQL™ 3.0 Módulo Diabetes and the generic questionnaire, as was also observed in other versions,14,25 except in the original American English version.13 Although both questionnaires assess the construct “quality of life,” their items address different contexts of chronic diseases.27 In particular, the generic instrument focuses on physical ability and school functioning, which usually are not problematic for children and adolescents with DM1.
In the present study, a small and positive association was observed between patient self-report and parent proxy-report scales for both the total score (r=0.40) and most of the subscale scores (diabetes symptoms, treatment barriers, and communication; r=0.38–0.43). The original version of the instrument13 (r=0.28–0.47) and the Italian15 (r=0.28–0.54) and Greek25 (r=0.47–0.62) versions presented medium correlations in most domains. Such variations support the importance of not only assessing the perspectives of children/adolescents, but also those of their respective parents/caregivers, as these, even if different, represent additional aspects of the assessment of pediatric HRQOL.13,16
Although negative correlations of small to medium intensity have been observed between HbA1c and the original American English version of the questionnaire,13 other studies, such as the present, have not found such an association.25 Generally, quality of life scores tend to correlate modestly with clinical results, suggesting that clinical and human outcomes are relatively independent, i.e., they measure different and probably complementary domains.28 In turn, the results of recently published studies29,30 suggest that better metabolic control is associated with improved HRQOL in children and adolescents with DM1.
The main strengths of the present study are the inclusion of children and adolescents within the age range of 2–18 years, the confirmation of internal reliability of the translated version, the positive correlation with another questionnaire already validated for Brazil, and, finally, the fact that this is the first instrument that can be used to evaluate HRQOL specifically in a pediatric population (2–18 years) with DM1 in the Brazilian Portuguese language.
However, some limitations of the study should be mentioned: the relatively small sample size, the predominant use of a single tertiary center, and the lack of a factorial analysis that would enable an assessment of whether all items of the instrument measure a single dimension. In turn, both the original validation study13 and those of versions in other languages14,25,26 included a similar number of patients as the present study.
In conclusion, the PedsQL™ 3.0 Módulo Diabetes was found to be easily understood and quickly completed by children and adolescents within a broad age range. The calculation of scores by the researcher was also simple, which facilitates its use in clinical research. The group of parents and caregivers viewed the instrument as an important way to express their concerns and needs regarding the chronic disease of their children. The analyses performed confirmed that the instrument has the psychometric properties needed for reliability and validity and, hence, that it is applicable to Brazilian children/adolescents with DM1 and their caregivers.
Conflicts of interestsThe authors declare no conflicts of interest.
The authors would like to thank Dr. J.W. Varni, Mapi Research Institute, Endocrinology and Metabology ambulatory – Diabetes Division at HCFMUSP, Juvenile Diabetes Association – SP and all patients from the specialty ambulatory of Institute of Child at HCFMUSP and their parents’ participation in this study.
Please cite this article as: Garcia LF, Manna TD, Passone CG, Oliveira LS. Translation and validation of Pediatric Quality of Life Inventory™ 3.0 Diabetes Module (PedsQL™ 3.0 Diabetes Module) in Brazil-Portuguese language. J Pediatr (Rio J). 2018;94:680–8.
Study presented at Universidade de São Paulo (USP), Faculdade de Medicina, São Paulo, SP, Brazil, in order to obtain a Master's Degree in Pediatrics.