To describe the association between sleep duration and weight–height development in children and adolescents.
Source of dataA non-systematic search in the MEDLINE database was performed using the terms anthropometry, body composition, overweight, obesity, body mass index, growth, length, short stature, sleep, children, and infants and adolescents, limited to the last 5 years. The references cited in the revised articles were also reviewed, when relevant.
Synthesis of dataSleep disorders are prevalent in the pediatric population. Among them, insomnia, which leads to a reduction in total sleep time, is the most prevalent disorder. Evidence found in the current literature allows the conclusion that sleep time reduction has a role in the current pandemic of overweight and obesity. Studies associating sleep deprivation and deficit in height growth are still insufficient.
ConclusionsThe association between shorter sleep duration and risk of overweight and obesity is well established for all pediatric age groups. However, more evidence is needed to establish an association between insufficient sleep duration and height growth deficit. Pediatricians should include the encouragement of healthy sleep habits in their routine guidelines as an adjuvant in the prevention and management of excess weight.
O objetivo deste artigo é descrever a associação entre a duração de sono e o desenvolvimento pondero-estatural entre crianças e adolescentes.
Fontes de dadosFoi realizada uma busca não-sistemática na base de dados MEDLINE utilizando os termos antropometria, composição corporal, sobrepeso, obesidade, índice de massa corporal, crescimento, comprimento, baixa estatura, sono, crianças, lactentes e adolescentes, limitadas aos últimos cinco anos. As referências citadas nos artigos revisados também foram, conforme relevância, revisados.
Síntese dos dadosDistúrbios do sono são prevalentes na população pediátrica. Dentre eles, a insônia, que cursa com redução do tempo total de sono, é a mais prevalente. Evidências presentes na literatura atual permitem apontar que a redução do tempo de sono tem um papel na pandemia atual de sobrepeso e obesidade. Os estudos que associam a privação de sono com déficit no crescimento estatural ainda são insuficientes.
ConclusõesA associação entre menor duração de sono e risco para sobrepeso e obesidade está bem estabelecida para todas as faixas etárias da pediatria. Entretanto, maiores evidências são necessárias para que se possa estabelecer uma associação entre duração insuficiente de sono e déficit no crescimento estatural. O médico pediatra deve incluir nas suas orientações de rotina o estímulo a hábitos de sono saudáveis como coadjuvante na prevenção e manejo do excesso de peso.
Sleep disorders are prevalent in the population and can affect up to 30% of individuals between childhood and adolescence; according to their duration and severity, they are associated with changes in behavior, mood, attention, and school performance.1
Sleep is a physiological state that exerts an important homeostatic function. Although clinically present as a reduction of interaction with the environment and inactivity, it physiologically represents a period of intense activity, acting on tissue repair, memory consolidation, and somatic growth.2
Several endocrinological factors have their release associated with the circadian rhythm, with their activity and concentration oscillating according to the time and state of wakefulness or sleep of the individual.3 Sleep disturbances, especially insomnia, which occurs simultaneously with insufficient sleep duration, may influence the homeostatic balance maintenance, culminating in deleterious effects on body functions.
This review aimed to describe the association between reduction in sleep duration in childhood and adolescence and its association with weight changes and growth in stature.
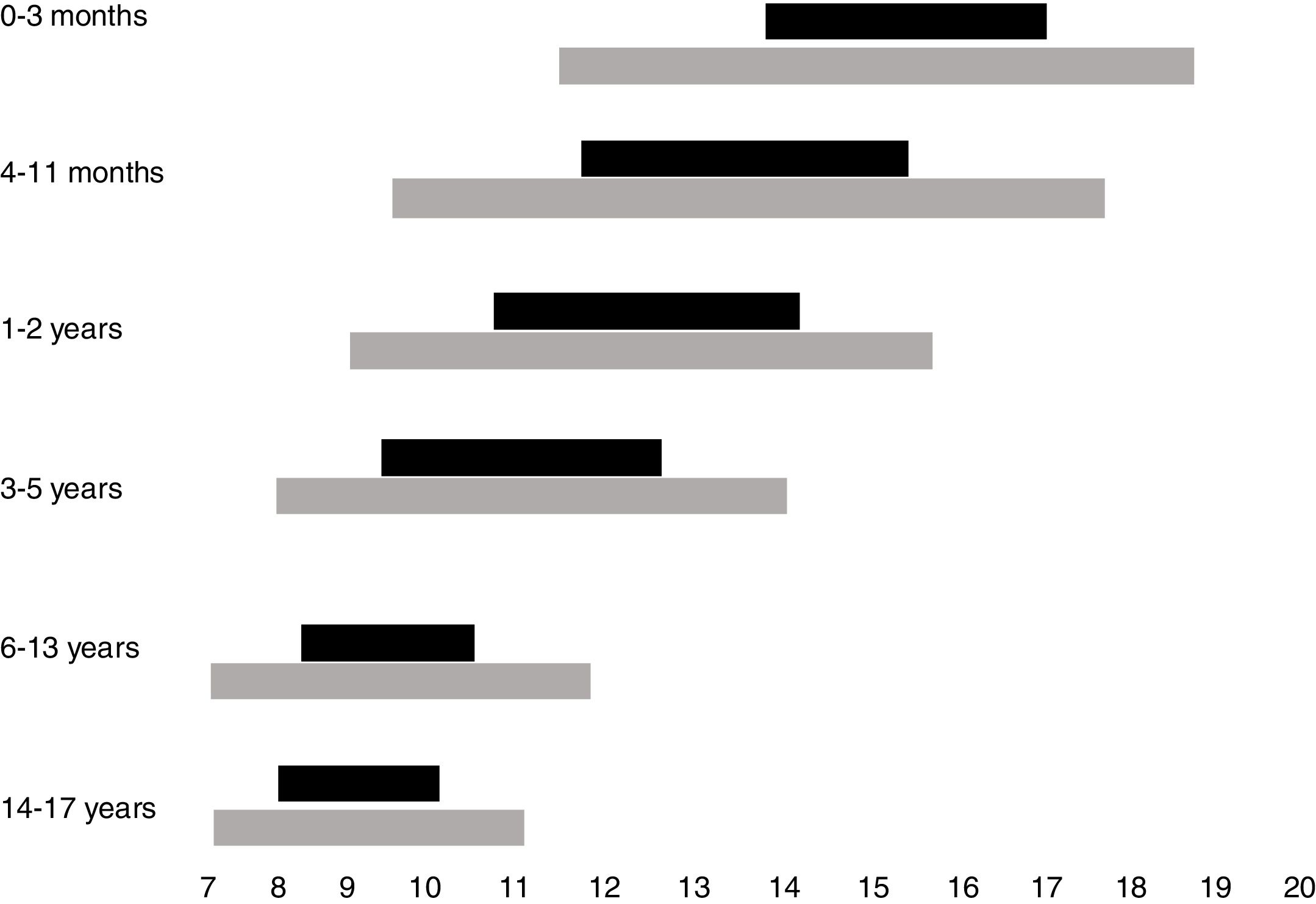
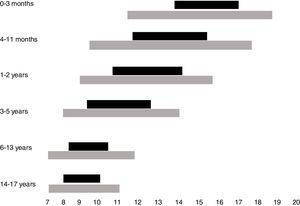
Normal sleep duration and architecture in childhood and adolescenceSleep architecture undergoes significant modifications throughout childhood. A newborn sleeps around two-thirds of the 24h, being interrupted by dietary needs and discomfort. Additionally, sleep begins with the active phase (precursor of rapid-eye movements [REM] sleep), which is the predominant phase. During the first year of life, there is a gradual consolidation of the circadian rhythm, with the concentration of the sleep period during nighttime from 6 months of age onwards, maintenance of one to two periods of daytime sleep and consequent reduction in the total sleep time in the 24h. Thus, whereas a newborn sleeps on average between 14 and 17h a day, a 12-month-old infant sleeps an average of 11–14h, starting with the non-REM phase (NREM), which predominates in the first half of the night, and takes up most of the total sleep period. Throughout childhood until adolescence, the recommended duration of sleep continues to decline, with the suppression of the need for daytime sleep at around 5 years of age.2
While the best parameter to define the ideal sleep requirement, in individual terms, is sleep duration, which allows the individual to fully exercise his/her diurnal activities, a consensus based on the current literature indicates the overall recommendations, on average, and the intervals that may be considered normal for each age group.4 Recommendations for sleep duration per age group, as well as variations accepted as possibly normal, are shown in Fig. 1.
The American Academy of Sleep Medicine classifies sleep disorders into seven categories: insomnia, sleep-related breathing disorders, hypersomnia of central origin, circadian rhythm sleep disorders, sleep-related movement disorders, and other sleep disorders.5 In childhood and adolescence, insomnia is the most prevalent disorder, affecting up to 30% of individuals in this age group.6 Insomnia is defined as difficulty to fall or stay asleep, waking up earlier than desired, resistance to initiate sleep, or difficulty to initiate sleep without external intervention (parents or caregivers), while being in an adequate environment for sleeping, i.e., without exposure to televisions, Smartphone, or tablets. The light frequency emitted by these devices affects the release of the hormone melatonin, responsible for the circadian cycle regulation, consequently reducing sleepiness and delaying the onset of sleep.7
When the above symptoms are present for at least three days a week and for at least three months, insomnia is classified as chronic. For the diagnosis of insomnia, the child/adolescent or his/her caregiver must demonstrate daytime impairment related to the reduction of sleep time, such as drowsiness, fatigue, decrease in academic, occupational and/or intellectual performance, or even mood swings or behavior alterations.5
In childhood, behavioral insomnia predominates, represented by two subtypes.8 The first characterizes insomnia by inadequate associations, in which the child depends on a stimulus, object, or environment to initiate or resume sleep. Thus, a child who needs, for instance, the maternal presence, lullabies, or feeding to initiate sleep, will need that same context to resume sleep the moment he/she awakens during the night. The second is represented by insomnia due to the lack of limits. In this context, which predominates among preschoolers and schoolchildren, there is a refusal by the child to go to bed, or recurrent demands for fluid intake or going to the bathroom during bedtime, avoiding going to bed. In this subtype, the delay in initiating sleep predominates, but not necessarily with a greater number of nocturnal awakenings. Mixed cases, with the presence of behavioral symptoms and secondary to the lack of limits, may occur.
In adolescence, circadian rhythm disorders, especially phase delays, are important factors contributing to the development of insomnia.6,8 Individuals with phase delays tend to delay sleep onset, usually for more than 2h beyond what is desired and expected to meet their social commitments.9 As a consequence, there is difficulty in waking up in the following morning, associated with behavioral symptoms with daytime hypersomnolence, as well as attention and behavior disorders. Phase delay can affect up to 16% of the adolescent population, being associated with hormonal changes inherent to puberty, social pressure, and inadequate sleep hygiene (irregularity of sleep onset between weekdays and weekends, access to electronic medias close to sleep time, use of licit or illicit stimulating substances, or consumption of caffeinated beverages).10
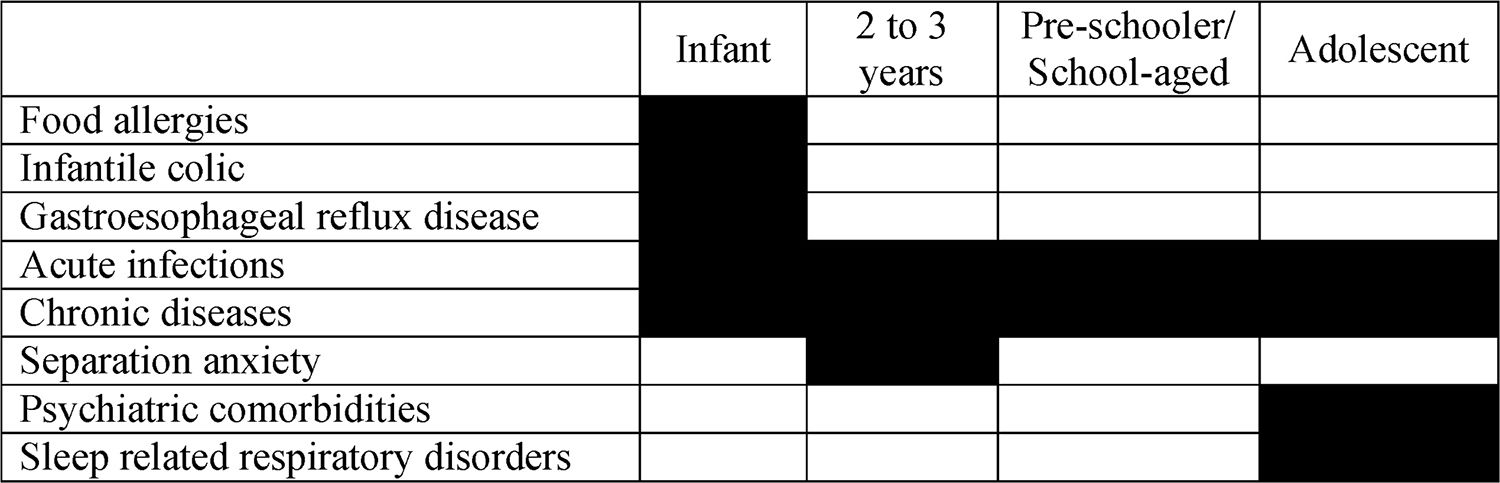
In the presence of insomnia, a differential diagnosis with organic diseases should be carried out.11 This suspicion is particularly important in infants, since gastroesophageal reflux disease and food allergies are often associated with insomnia and sleep-related breathing disorders in this age group, which may represent the key symptoms of the diseases. In these cases, an organic picture may be associated with behavioral insomnia, since the symptoms of the underlying disease may favor the adherence of negative associations at the time of sleep by the caregivers.11
Among acute infections in infants, acute otitis media should be considered as a diagnosis. Although sleep-related breathing disorders, such as sleep Obstructive apnea/hypopnea syndrome (OSAHS), are more common among schoolchildren and adolescents, this diagnosis should not be ignored as a possibility in infants and toddlers.
Table 1 shows the main diagnoses that need to be ruled out from a patient in the age group from childhood to adolescence with insomnia-related complaints.
Metabolic cycle and sleepThe circadian clock in humans, as well as other mammals, follows a period of approximately 24h of duration.12 Its main pacemaker is found in neurons of the suprachiasmatic nucleus, located in the hypothalamus.
The circadian pacemaker is influenced by factors that are intrinsic or extrinsic to the body, among which the most potent is light. Other factors that regulate the endogenous rhythm are the availability of food, social commitments, and interaction.12 Under normal conditions, behavioral and physiological events such as changes in body temperature, sleep phases, or hormonal secretion occur at regular times during the days, synchronizing the circadian rhythms between day and night, allowing the maintenance of homeostasis.13,14
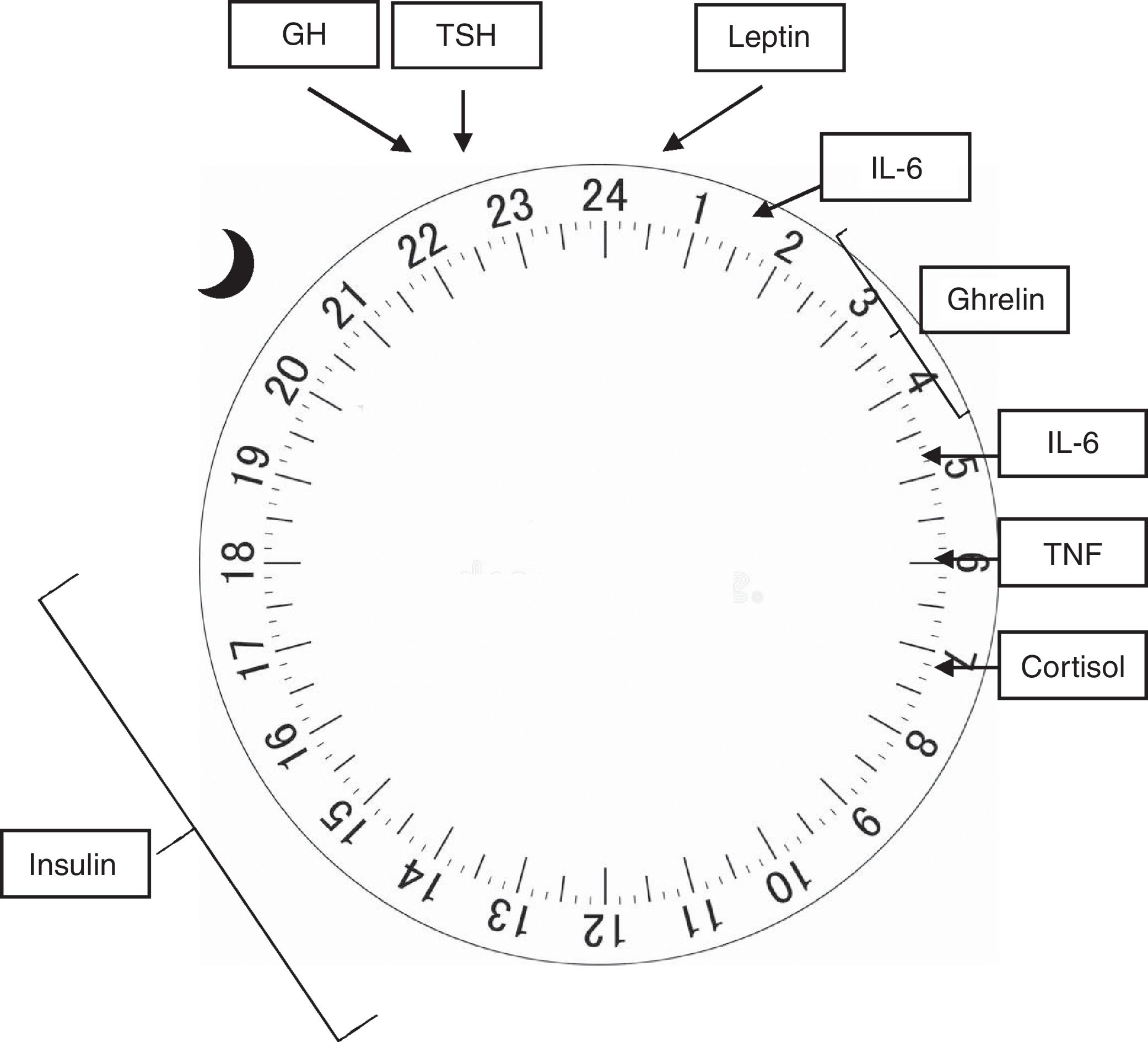
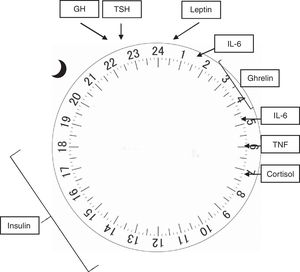
In humans (and other mammals), the concentration of several hormones varies throughout the 24h of the day, and its regulation is influenced by both sleep and activity.12 The adrenocorticotropic hormone (ACTH) is released in pulses at the end of the day, reaching its peak before awakening, with a concomitant cortisol peak occurring early in the morning, whereas its lowest point occurs at around 3 AM. The increase in cortisol acts on insulin sensitivity, which peaks between 12 PM and 6 PM, and its lowest point between midnight and 6 AM, which peripherally lowers blood glucose and stimulates appetite.14 Both the growth hormone (GH) and the thyroid stimulating hormone (TSH) have their plasma concentrations increased at sleep onset, reaching their maximum concentration 24h later, and decreasing in 24h before awakening.15 In electrophysiological terms, evidence suggests that the activity density of slow waves that occur during NREM sleep is closely related to the release of GH and TSH, whereas REM sleep is associated with cortisol release.16
Hormones responsible for regulating appetite are also influenced by the circadian rhythm. Leptin, secreted by the adipose tissue in a pulsatile manner, is elevated after meals and at night, while ghrelin, produced in the stomach, increases during fasting and sleep.16 Both act on the arcuate nucleus of the hypothalamus, where the appetite regulation center is believed to be located. In it, leptin prolongs the postprandial response to glucose and reduces appetite, whereas ghrelin, in its acetylated (active) form, acts on GH release, increasing appetite and promoting gastric emptying. The hypothalamic neuropeptides that stimulate appetite, orexin A and B, have their release stimulated by ghrelin and inhibited by leptin and glucose.14
The central nervous system and the immune system are mutually influential. Thus, sleep alterations favor immune modifications and exposure of the body to infections, as well as the immunological response may also induce sleep modifications.15 Interleukins 1 (IL-1) and 6 (IL-6), as well as tumor necrosis factor (TNF), have a circadian release. IL-1 decreases during the night to a lowest point at about 8 AM, whereas IL-6 reaches its peak at 2 and 5 AM, and the peak of release of TNF occurs at around 6 AM, with its lowest point occurring at around 3 PM. Both interleukins and TNF act by stimulating cortisol secretion, which in turn acts on weight maintenance or gain, with its peak occurring at night and lowest point upon awakening. Sleep deprivation increases the release of these cytokines and TNF, which are also increased in patients with obstructive sleep apnea/hypopnea syndrome.15
Fig. 2 shows the physiological release peaks of the several factors mentioned.
Sleep duration and overweight or obesityOverweight and obesity are considered to be pandemics related to the modern lifestyle, affecting more than 107 million children worldwide, according to a survey carried out with data obtained up to the year 2015.17 Although sedentary lifestyle and diet rich in processed foods are recognized as the main determinants of the progressive weight gain observed in the global population, another common characteristic to most modern societies is the progressive decrease in the total sleep time observed since childhood.18 A European study, observing sleep characteristics obtained between the 1970s and 1990s in population-based cohorts carried out in Switzerland, has shown that the reduction in total sleep time in the last decades is observed since the first six months of life.19 In these populations, the reduction in total sleep time appears to be related to later times of sleep onset combined with a wake-up time that has remained practically unchanged over the decades.
The association between insufficient sleep duration and weight gain has been the object of study in recent years, initially among the adult population and, more recently, in the adolescent, school, and preschool age groups. Most evidence suggests a positive association between sleep deprivation and the risk of overweight and/or obesity in all age groups studied.20 The most recent published meta-analysis, which included data from more than 55,000 children aged 0–16, found that participants with a shorter sleep duration were 76% more likely to be overweight/obese than those with longer sleep duration, in addition to presenting a greater gain in their annual body mass index (BMI).21 In turn, an increase of 1h in total daily sleep represented a reduction in the risk of overweight or obesity by 21%.
In pre-adolescents aged 10 years or older, followed-up for 3 years, sleep duration of less than 10.5h a day was associated with a 55% greater risk of overweight than those sleeping at least 11h.22 Similarly, a study that followed-up over 900 children aged 7–12 found an increase in BMI with a reduction in total sleep time, so that the difference in BMI at the end of the follow-up among children that slept persistently less during the study period averaged 1 point in comparison to children who slept more and consistently throughout follow-up.23 Among school-age children (8–10 years) whose sleep was assessed through objective data (actigraphy), sleep duration of less than 10h was associated with a two-fold increased risk of being overweight.24
The variability between sleep duration on weekdays when compared to weekends was also shown to be a risk factor for overweight/obesity. An American study with children aged 4–10 years using an objective home method of sleep evaluation found that sleep duration on weekends was more variable among obese children when compared to children with normal weight or who were overweight. In the same study, children who had significant variations in sleep duration between weekdays and weekends or those who slept little every night of the week were at increased risk for plasma insulin and LDL-cholesterol alterations.25
The bigger risk for weight gain among children with shorter sleep duration appears to be present at a very early age, even at preschool age. A Brazilian longitudinal study found that sleep duration of less than 10h a day at any time between 1 and 4 years of age was associated with a risk more than 30% greater of overweight or obesity at 4 years.26 Similar results were found in studies carried out in developed countries, one of which followed children from 6 months to 7 years of age, and concluded that the higher the exposure to periods of insufficient sleep during the follow-up period, the bigger the risk of overweight/obesity at 7 years.27 An even earlier onset of shorter sleep duration, at 2 months after birth, was also associated with an increased risk of overweight 6 years later.20
Sleep duration and growth in statureThe longitudinal growth in childhood is influenced by genetic and environmental factors, mediated by metabolic factors via GH. Short stature at 2 years of age was associated with a higher risk of chronic diseases and lower schooling and income in adulthood.28 Nevertheless, in addition to the association between GH release and sleep, the association between sleep pattern changes and height growth has been, until now, little explored in the literature.
The studies developed to date show conflicting results; however, they assess heterogeneous populations in terms of both age range and ethnicity – two factors associated with both sleep patterns and anthropometric measures – as well as the definition used for changes in the sleep pattern.29
A longitudinal study carried out in Singapore with approximately 900 children found a positive association between lower sleep duration at three months (12h a day or less) and shorter stature at 24 months, similar to that observed in a North-American study of 23 preschoolers, followed for 4–17 months.30,31 In that study, longer duration of nocturnal sleep, as well as daytime sleep episodes were associated with higher height growth.31 The cohort study of Jenni et al., however, which followed 300 children between 1 and 10 years of age every 6 months between 12 and 24 months and yearly afterwards, failed to observe an association between sleep duration and longitudinal growth in any of the evaluated periods.32 Among children aged 5–11 years born in the United Kingdom, a negative association was found between sleep duration and height.33
Among the studies evaluating individuals between pre-adolescence and adolescence, one of them, carried out in China, observed that a one-hour difference in usual sleep duration (less than 9h or 10h or more) in 143 children between 10 and 11 years was associated with greater height growth.34 Sleep duration, however, was not associated with differences between growth rates in a period of one year in adolescents between 12 and 16 years of age.35 It is important to note that, in that study, height was only objectively measured at one of the moments, and was reported by the adolescent when considering the initial height.
DiscussionSleep plays a crucial role in the body's homeostasis. Its association with weight–height development in childhood and adolescence, especially in its association with weight gain, has been the subject of great interest. Most of the available evidence speaks in favor of an influence of insufficient sleep on weight gain. The meta-analysis conducted by Chen et al. found a 58% greater aggregate risk of overweight or obesity in participants with shorter sleep duration in the age group of 0–18 years. Furthermore, among the individuals at the highest extreme of short sleep duration, the aggregate risk was 92% higher.36
It is estimated that sleep deprivation acts directly on the risk of overweight/obesity through its role on sympathetic activation and increase of catecholamines, elevation of cortisol by activation of the hypothalamic–pituitary–adrenal axis, and increase of interleukins and TNF through an activation of the inflammatory cascade. Therefore, there is an increase in insulin resistance and pancreatic β-cell dysfunction, favoring glucose tolerance, weight gain, and the development of type 2 diabetes.37 Another mechanism by which sleep deprivation could be associated with weight gain would be through appetite regulation, probably mediated by increased ghrelin and reduced leptin levels.20 Studies carried out in adult populations subjected to acute and intense sleep deprivation provide most evidence for metabolic alterations related to reduction of sleep time. One study, assessing 24-h total sleep deprivation in healthy men, found a significant reduction in energy expenditure on the following day, elevated plasma ghrelin levels in the morning, and elevated plasma levels of thyrotropin, cortisol, and norepinephrine.38 Elevations of IL-6 and TNF levels have also been observed after partial sleep deprivation among adults.39 However, the effect of sleep deprivation, especially moderate deprivations of the ideal sleep time, may not be immediate. In the study by Brondel et al., a 4-h sleep deprivation in one night led to an intake increase only 36h later and, in the study by St-Onge et al., 5 days after sleep deprivation.40,41 The association between shorter sleep duration and higher caloric intake among preschool children was demonstrated in a longitudinal evaluation in the pediatric age range.42
Few studies have evaluated the effect of interventions aimed at increasing sleep time on weight loss or maintenance or on height growth. An intervention performed with mothers of infants in the second week of life, based on sleep hygiene, feeding and emotional regulation, and sporadically reinforced up to 40 weeks postnatal, found a lower risk of overweight at 1 year of age in the intervention group.43 However, there is no reference to sleep duration between the groups at the time of the outcome assessment, and since interventions were also made on other behavioral issues (including food), one cannot affirm that the lower weight gain was due to the intervention on sleep. Another study that evaluated the effect of an intervention on sleep duration, exposure to television media and feeding, developed with children aged 2–5 years from low-income families of a developed country, found an increase in sleep duration of almost 1h a day, a reduction in television time on weekends and 0.4 points in BMI 6 months later.44 In turn, Wake et al.’s sleep intervention in 7-month-old children found no association with overweight 6 years later.45 In the intervention performed by Hart et al., children between 8 and 11 years of age were assessed through actigraphy.46 In the first week, sleep was evaluated in its usual pattern, followed by a week sleeping 1.5h more or less and, in the third week, changing patterns between the groups in relation to the restriction or increase of habitual sleep duration. In the sleep restriction week, the participants showed greater caloric intake and weight gain of, on average, 0.22kg.
Studies evaluating the possible role of sleep on height growth have an observational design, and their results suggest a positive association between longer duration of sleep and height. In the study by Lampl and Johnson, the probability of episodic growth, the so-called “growth spurt”, increased by 20% for each additional hour of sleep and 43% for each daytime sleep episode in children aged 4–17 months.31 Another study, carried out in China, including children between 10 and 11 years of age with BMI below the 15th percentile, found that children with longer sleep duration were taller.34 An interesting result of the manuscript is the association found between longer sleep duration and higher BMI, suggesting that sleep may play a regulatory role in both overweight and underweight situations. This result is similar to that found in a longitudinal study that followed-up 10,000 adolescents between the ages of 16 and 19 years.47 In that study, both adolescents with obesity or overweight, as well as those who were underweight, slept less than those with normal weight, with the lowest mean sleep duration being found among those who were underweight. The authors suggest as a possible mechanism of association in a context of low caloric intake, the elevation of hormones that favor wakefulness, such as orexins.
The most recent recommendations from organizations that study factors in favor of obesity prevention and management have recently started including sleep quality and duration optimization in childhood as a measure associated with dietary and physical activity modifications.48–50
ConclusionsThe association between shorter sleep duration and risk of overweight and obesity is well established for all pediatric age groups. However, further evidence is needed to establish an association between insufficient sleep duration and height growth deficit.
Pediatricians should include the encouragement of healthy sleep habits in their routine guidelines as an adjuvant to the prevention and management of excess weight.
FundingMLN is a CNPq PQ researcher.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: El Halal CS, Nunes ML. Sleep and weight-height development. J Pediatr (Rio J). 2019;95:S2–S9.