On the one hand, sepsis is the leading cause of non-trauma related death in pediatric patients across the world, in both developed and developing nations.1 On the other hand, epidemiologic data demonstrate the independent contribution of acute kidney injury (AKI) to morbidity and mortality in both adults and children.2,3 The mortality, morbidity, and financial cost of AKI is significant and has led to dedicated global initiatives to eliminate preventable AKI and mitigate the effects of existent AKI.4 Together, unfortunately, sepsis and AKI synergize into the “worst of both worlds” – inciting a litany of negative host responses and ultimately leading to poor patient outcome. In this issue of Jornal de Pediatria, Riyuzo et al.5 report data on the predictive factors of death in patients with sepsis associated AKI. In their retrospective evaluation of 77 children with sepsis and AKI, the rate of severe AKI (pRIFLE stage I–F and/or stage 2–3 were both over 75%) and the overall mortality rate was substantial (33.7%).
The drivers of injury and progression in sepsis and AKI are similar. Sepsis is propagated by the cardinal mediators of ischemia, hypoxia, inflammation, and cell death. The multi-dimensional pathophysiology of sepsis exerts perturbations from the cellular level to overall host homeostasis. Data from both animal and human models supports dysregulation of the innate immune system, imbalance of pro- and anti-inflammatory cytokine production/degradation, destabilization of apoptotic pathways, and disruption of endothelial stability. In a parallel fashion, AKI is typified by a set of host responses including ischemia, dysregulated inflammation, hypoxia, and renal tubular injury.6 Additionally, AKI results in deleterious autocrine, paracrine, and endocrine cytokine effects on extra-renal vital structures such as the brain, heart, lungs, and liver.7 Therefore, sepsis and AKI, individually, are more appropriately characterized as syndromes (versus “diseases” or “injuries”) as they lead to destabilization of homeostasis by a variety of global mechanisms.
Severe sepsis-associated acute kidney injury (SSAKI) is common, costly, and harmful. Sepsis is the leading cause of AKI in adults and children, accounting for 33–50% of all AKI in adults and 25–50% of those in children.8 As a unified syndrome, SSAKI contributes to high mortality, greater resource utilization (ventilatory support, dialysis), and increased morbidity for patients (increased length of stay). Additionally, the long-term sequelae of SSAKI are significant; a notable proportion of surviving patients (adults and children) suffer chronic kidney disease, early end-stage kidney disease, and earlier death.9,10 Unfortunately, the pathophysiology of SSAKI is poorly understood. Similar to both sepsis and AKI (taken independently), the drivers of SSAKI include intrarenal hemodynamic changes, endothelial dysfunction, dysregulation of inflammatory homeostasis, necrosis/apoptosis, and ischemic-hypoxic injury.11 On a host level, sepsis and AKI concurrently propagate one another by independent effects on systemic vascular tone, interstitial volume, altered distribution of serum albumin, and induction of nitric oxide production. Separation of the independent effects of AKI from sepsis has proven to be difficult, as the two share common pathways involving circulatory dysfunction, a disconnection of bio-energetics, and stresses on the nitrosative and oxidative pathways.12 Limitations in the ability of animal models to replicate human disease, and a paucity of human tissue data, discredit the assumptions of sepsis automatically decreasing renal blood flow and leading to tubular necrosis.13 In both “mice and men”, the multidimensional injury is potentiated in the very young and very old, and those with chronic illness.3 For these reasons, sepsis and AKI go “hand-in-hand” – and likely synergistically contribute to the quick, downward spiral of patients suffering both injury syndromes. Riyuzo et al.5 in their study of children with SSAKI, observed a clinical parallel to these pathophysiologic findings. In their study, the children with SSAKI were young (median 4 months), were diagnosed early (first day of admission to the intensive care unit), and suffered severe injury (>75% pRIFLE class I–F and stage 2–3). The independent risk factors associated with death included characteristics of severely ill patients (mechanical ventilation, dialytic therapy, and hypoalbuminemia). In their study, AKI was not independently associated with mortality, an unsurprising finding in the context of the overall severe illness of patients included for study. Importantly, no severity of illness metric (Pediatric Risk of Mortality, Pediatric Index of Mortality) was included in their study to compare surviving patients from those who died. An obvious association exists, however, between advanced kidney injury in patients with sepsis (stage 2–3 and/or the requirement of dialysis) and death – supported by the significant decrease demonstrated in the Kaplan–Meier survival estimates.
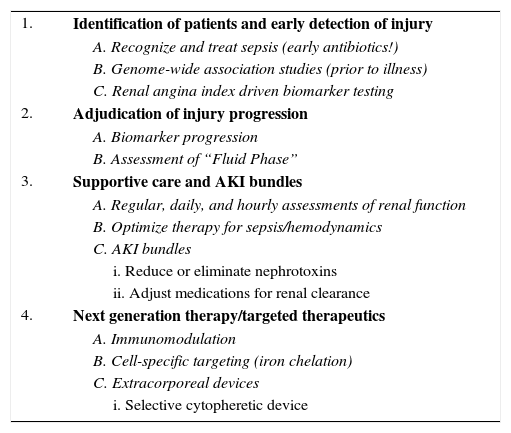
How then, given the synergistic contributions of sepsis and AKI, do we move the needle to improve outcomes for patients with SSAKI? A multi-faceted, timely, rational, and systematic approach is needed (Table 1). The first step is the timely identification of patients at-risk for SSAKI. Obviously – treat the sepsis! Early initiation of antibiotics is associated with reduced mortality – a finding that is only slowly being appreciated and incorporated into routine care of critically ill patients.14 Incidental population studies identified putative biomarkers for the purpose of identifying patients at-risk of developing AKI (and the progression of AKI) in the context of sepsis.15 Genome-wide association studies (GWAS) carry the potential to identify patients at high risk of SSAKI.16 Meanwhile, ongoing animal studies identify newer biomarkers for SSAKI, specifically those with potential for therapeutic targeting. Appropriate utilization of biomarkers in the context of SSAKI is paramount for the inclusion of this next-wave of precision medicine into routine practice. To mitigate capricious use of novel diagnostic biomarkers and to optimize the post-test probability of prediction, the renal angina methodology of risk stratification has been derived and validated in multiple pediatric populations. This methodology, as cited by Riyuzo et al.,5 stratifies patients by risk and is a facile system for prediction of AKI at a time after ICU admission (72h) carrying significant ramifications for patient management.17–19 The second step is early adjudication of the progression of injury. Biomarkers for the progression of SSAKI may be an important facet of management, particularly in the early stabilization phase of patients.14 Attention to fluid accumulation is paramount. Increasing evidence from both adults and children supports the finding of the deleterious independent contribution of fluid accumulation in critically ill patients.20 To this end, fluid management in the SSAKI patient population, inclusive of renal replacement therapy, is important to mitigate the effects of end-organ tissue edema.21 An important delineation must be made with regards to the “stage” of fluid resuscitation for a given patient; treating all patients the same and with the same approach to fluid resuscitation, stabilization, and maintenance is nonsensical.22,23 Third, consistent and systematic supportive care is paramount. Early goal directed therapy for sepsis has become commonplace in critically ill patients, but the notion of supportive care for AKI (regular assessments of creatinine, attention to urine output and weight, limitations of nephrotoxins, renal dosing of medications, avoidance of contrast, etc.) is not consistent across the globe. Early evidence of the introduction of AKI care bundles into management is promising.24 Finally, targeted therapy is on the horizon. Attenuation of early inflammation using endocannabinoids and cell-based therapies, cellular-specific targeting using iron chelation agents, and heme-oxygenase mediators are in advanced stages of clinical study.25 Extracorporeal therapies such as the selective cytopheretic device for attenuation of inflammation may be the next wave of therapy for the most critically ill patients with SSAKI.26
Moving the needle for SSAKI management.
| 1. | Identification of patients and early detection of injury |
| A. Recognize and treat sepsis (early antibiotics!) | |
| B. Genome-wide association studies (prior to illness) | |
| C. Renal angina index driven biomarker testing | |
| 2. | Adjudication of injury progression |
| A. Biomarker progression | |
| B. Assessment of “Fluid Phase” | |
| 3. | Supportive care and AKI bundles |
| A. Regular, daily, and hourly assessments of renal function | |
| B. Optimize therapy for sepsis/hemodynamics | |
| C. AKI bundles | |
| i. Reduce or eliminate nephrotoxins | |
| ii. Adjust medications for renal clearance | |
| 4. | Next generation therapy/targeted therapeutics |
| A. Immunomodulation | |
| B. Cell-specific targeting (iron chelation) | |
| C. Extracorporeal devices | |
| i. Selective cytopheretic device |
Severe sepsis associated AKI is a combination of independent and synergistic disease syndromes with notable negative effects on patient outcome. In this edition of the Jornal de Pediatria, Riyuzo et al.5 report just how significant of a problem SSAKI is, particularly in children. The intertwined pathophysiology makes proper identification and risk mitigation for these patients paramount. Caring for these patients requires an understanding of the importance of a timely, consistent, and multi-dimensional approach to management.
Conflicts of interestThe authors declare no conflicts of interest.