To investigate the occurrence of sarcopenia in children and adolescents with chronic liver disease.
MethodsA series of cases, with patients aged 6–19 years of both genders, who were treated in Liver Outpatient Clinics. Weight, height, muscle strength (assessed by manual grip strength), and muscle mass (estimated through dual-energy X-ray absorptiometry) were measured. Sarcopenia was diagnosed based on the simultaneous presence of muscle mass and muscle strength déficits, defined as the values below the mean for muscle mass and strength of the studied population, according to gender. A descriptive analysis (mean and standard deviation) was performed, and the difference of means was calculated by Student's t-test.
ResultsA total of 85 patients were studied, mostly females (64.7%), with a mean age of 11.7 (SD=3.4) years. Sarcopenia was identified in 40% of the patients. Muscle strength déficit was found in 54.1% of the subjects, and 50.6% showed muscle mass déficit. The mean muscle mass for males was higher than that for females (6.07; SD=1.22kg/m2vs. 5.42; SD=1.10kg/m2; p=0.016). However, there was no significant difference in sex-related muscle strength (male=0.85; SD=0.52kgf/kgm2 and female=0.68; SD=0.30kgf/kgm2; p=0.113).
ConclusionThe research findings identified that sarcopenia is a condition found in pediatric patients treated at a public referral institution for chronic liver disease.
Investigar a ocorrência de sarcopenia em crianças e adolescentes com hepatopatias crônicas.
MétodosSérie de casos, constituído por pacientes entre 6 e 19 anos, de ambos os sexos, acompanhados em ambulatórios de especialidade em hepatopatias. Foram feitas medidas de peso, altura, força muscular (avaliada pela força de preensão manual) e a massa muscular estimada a partir da absorciometria por dupla emissão de raios X. O diagnóstico de sarcopenia baseou-se na presença simultânea de déficit de massa muscular e de força muscular. Adotaram-se como déficit, os valores abaixo da média para massa e força muscular da população estudada, segundo sexo. Realizou-se análise descritiva (média e desvio-padrão), bem como a diferença de médias com o teste do t de Student.
ResultadosForam estudados 85 pacientes, a maioria do sexo feminino (64,7%), com média de 11,7 (DP=3,4) anos. A sarcopenia foi identificada em 40% dos pacientes, 54,1% apresentaram déficit de força muscular e 50,6% déficit de massa muscular. A média da massa muscular para o sexo masculino foi maior do que no feminino (6,07; DP=1,22kg/m2 vs 5,42; DP=1,10kg/m2; p=0,016). No entanto, não houve diferença significante para força muscular com relação aos sexos (masculino=0,85; DP=0,52 kgf/kgm2 e feminino=0,68; DP=0,30 kgf/kgm2; p=0,113).
ConclusãoOs achados da pesquisa identificaram que a sarcopenia é uma condição presente em pacientes pediátricos atendidos em uma instituição pública de referência para doença hepática crônica.
Chronic liver disease (CLD) in pediatric patients is accompanied by a progressive structural and functional decline in liver function due to fibrosis and subsequent hepatocellular necrosis.1 Therefore, the control and treatment of complications associated with CLD, especially at advanced stages of the disease, remain the pillars of therapy in the pediatric population, since it is not always possible to perform a liver transplant, which is a more effective treatment.2
Thus, in addition to monitoring liver function parameters, the evaluation of the nutritional status, mainly by body composition, represents an extremely important measure. This evaluation allows the measurement of adiposity and skeletal muscle mass,3 identifying nutritional alterations, such as sarcopenia and sarcopenic obesity.4,5
Sarcopenia is a syndrome characterized by progressive and generalized reduction of skeletal muscle mass and muscle strength.6 Although it is traditionally an aging-related condition, researchers7,8 have demonstrated that pediatric patients may develop this condition. Thus, its diagnosis is important for the evaluation and follow-up of patients with CLD, since the loss of lean mass is associated with poor prognosis, both in the pre- and post-transplantation periods.9,10
The pathogenesis of sarcopenia is multifactorial, but may be related to decreased protein synthesis, accelerated catabolism in liver failure, and reduced physical activity that is observed in individuals with chronic diseases.11
For the diagnosis of sarcopenia, it is recommended that the muscle mass deficit6 be measured by computed tomography (CT), magnetic resonance imaging (MRI), or dual X-ray absorptiometry (DXA). The latter is a method that is especially useful in pediatric patients.6
Other methods, such as bioimpedance4,6 and anthropometry,3,6 can be used as an alternative to measure muscle mass, since the gold standard methods are more expensive. On the other hand, in order to measure muscle strength, recent studies in cirrhotic adults12,13 and in healthy and hospitalized children14,15 have shown that the maximal grip strength (MGS), as measured by manual dynamometry (MD), is considered a useful marker of skeletal muscle mass, with a prognostic value in these populations.
Sarcopenia is a new concept in pediatrics and this topic is little explored in the literature, especially in children and adolescents with chronic liver disease. Few studies in the literature7,8 have evaluated sarcopenia in the pediatric population and the authors used only the muscle mass deficit assessed by CT as the diagnostic criterion. The aim of this study was to investigate the occurrence of sarcopenia by assessing muscle mass and strength in pediatric patients treated at CLD referral outpatient clinics in the city of Salvador, state of Bahia, Brazil.
MethodsThis is a series of cases, part of a larger study, entitled “Chronic liver disease in children and adolescents: parameters of nutritional assessment and body composition,” carried out in pediatric patients with CLD, followed at clinical treatment outpatient clinics (ATC) and transplanted patients (AT), from the Department of Gastroenterology and Pediatric Hepatology, of the Complexo Hospitalar Professor Edgard Santos (C-HUPES), Universidade Federal da Bahia (UFBA, Brazil), from February 2016 to March 2017.
A total of 194 patients participated in the study; those who were between 6 and 19 years of age, of both genders, with or without cirrhosis, who met the necessary criteria to undergo whole-body DXA scan and perform MD assessment were eligible for the present study. Children younger than 6 years of age have difficulty staying in decubitus for the time required to undergo DXA16 and also have difficulty identifying their dominant hand,17 so they were not included in the present study. Parents and/or guardians who agreed to their children's participation signed the Informed Consent, whereas the adolescents signed the Term of Assent. Patients with the following etiologies were included: biliary atresia, choledochal cyst, sclerosing cholangitis, Alagille's syndrome, autoimmune hepatitis/AIH, viral hepatitis, alpha-1 antitrypsin, Wilson's disease, lysosomal acid lipase (LAL-D), Niemann-Pick disease, tyrosinemia, and glycogenosis. Patients with genetic syndromes, cerebral palsy, neurological or osteoarticular diseases, other chronic concomitant diseases, those with amputated limbs, previous or present pathology in the limbs, history of previous trauma, or delayed motor development were excluded.
The study was approved by the Research Ethics Committee of UFBA C-HUPES, under Opinion No. 1,360,091/2015, and carried out in accordance with Resolution No. 466/2012/CONEP/CNS/MS.
Information on age, gender, origin, clinical data – such as CLD diagnosis, liver biopsy, and corticoid use were collected from the patient's chart. For adolescents, the stage of sexual maturation was evaluated by pediatricians and later classified into prepubertal (male: stages of sexual maturation 1 and 2, female: stage 1), pubertal (male: stage 3, female: stage 2) and postpubertal (stages 4 and 5 in both genders), according to the stage of sexual maturation proposed by Tanner.18
Anthropometric measurements and handgrip strength assessment were performed during the consultation, in outpatient clinics by trained nutritionists. Participants were categorized into the following age groups: <10 years; ≥10 and ≤14 years, and >14 years.
As for the anthropometric data, the techniques used to measure weight and height were those suggested by the Guideline Manual of the Brazilian Society of Pediatrics (SBP).19 The body mass index (BMI) was calculated20 and the classification of anthropometric nutritional status was used, according to recommendations of Brazil's Technical Standards of the Food and Nutrition Surveillance System (SISVAN) 2011,21 adapted from the World Health Organization (2007),20 considering BMI for age (BMI/A): low weight (z-score <−2); normal weight (z-score ≥−2 and ≤+1), overweight (z-score ≥+1 and <+2), and obesity (z-score ≥+2).
Manual grip strength was assessed using a manual dynamometer (Jamar© Dynamometer, Patterson Medical, IL, USA) and expressed in kilograms–force (kgF). The participant sat in a chair, without armrests, with his/her feet positioned flat on the floor, and the hip touching the back of the chair. The arm remained parallel to the body, with adducted shoulder, elbow flexed at 90° and forearm in the neutral position, and wrist between 0° to 30° of extension and 0° to 15° of ulnar deviation.22 Due to its ergonomic characteristics, it was possible to adjust the dynamometer to the hand size of the children and the adolescents. Three consecutive measurements were performed, alternating between the dominant and non-dominant sides, with a minimum interval of one minute between them; the highest value was used as the maximum value of MGS, regardless of dominance. In order to adjust for changes in maturation and body size, the relative manual grip strength was calculated as: relative manual grip strength (rMGS)=final MGS (FMGS)/BMI.14
Dual-energy X-ray absorptiometry (DXA) analysis: This test was performed in a specialized clinic by a physician experienced in the procedure. Body composition was estimated by DXA (GE Lunar Prodigy DPX-NT, GE Healthcare, IL, USA), using GE software, adjusted to determine the body composition index. The participants were in the supine position, with an empty bladder, and were not wearing metal objects and other items that could interfere in the analysis (inspection, reading, scanning), according to the procedure recommended by the International Society for Clinical Densitometry (ISCD).16
Whole-body DXA was performed to measure the appendicular skeletal muscle mass (ASM), together with information on gender, age, and height. The muscle mass of the four limbs (arms+legs), defined as ASM,23 was provided by GE software from DXA. Based on the ASM, the skeletal muscle index (SMI) was calculated, adjusted for the height squared (SMI=ASM/height2).23
Sarcopenia diagnosisFor the diagnosis of sarcopenia, the simultaneous presence of muscle mass (MM) and muscle strength (MG) deficit was considered.6 For the muscle mass and muscle strength deficit, values of SMI and rMGS that were lower than the mean of the study population were considered.24
Statistical analysisThe data were entered in the EpiData software (EpiData – Comprehensive Data Management and Basic Statistical Analysis System. Version 3.1; Odense Denmark) and the analyses were performed using the statistical package R (The R Foundation v. 3.5.1, USA). A descriptive analysis (mean and standard deviation) was performed, as well as the difference of means, using the Student's t-test. The normality of the SMI and rMGS variables, according to gender, were verified by the Shapiro–Wilk test. For categorical variables, the relative and absolute frequencies were calculated, and Pearson's chi-squared test or Fisher's exact test was performed. A significance level of 5% (p<0.05) was considered.
ResultsA total of 85 patients were included in the study; most were females 64.7% (55/85), with a mean age of 11.7 (SD=3.4) years, and 70.6% (60/85) were adolescents. Of these, 71.7% (43/60) were in the pubertal stage of sexual maturation and 15% (9/60) were in the post-pubertal stage.
Of the etiologies observed in the patients, biliary atresia 28.2% (24/85) was the most frequent cause of CLD, followed by autoimmune hepatitis in 21.2% (18/85). Of the 28 patients (32.9%) who had cirrhosis at the histopathological examination, 23 were classified as Child–Pugh A (82.1%), four as B (14.3%), and one as C (3.6%), and none of the patients had edema and/or ascites at the time of evaluation.
The mean SMI for males was significantly higher than for females (6.07, SD=1.22kg/m2vs. 5.42, SD=1.10kg/m2, p=0.016). However, there was no statistically significant difference in relation to rMGS between the genders (0.85, SD=0.52kgf/kgm2vs. 0.68, SD=0.30kgf/kgm2, p=0.113).
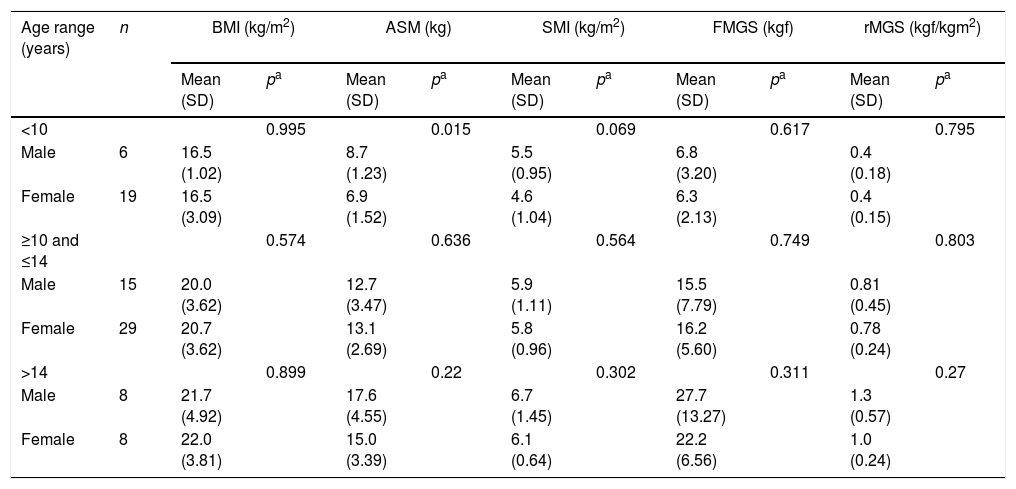
Table 1 shows the difference in means according to age group and gender, for BMI, muscle mass, and absolute and relative manual grip strength of the patients. It was observed that ASM and FMGS increased with advancing age in both genders. However, for males, the difference in means in both ASM and FMGS was significant only when comparing the age groups <10 with ≥10 and ≤14 years (p=0.001 and p=0.002, respectively). Regarding the age groups ≥10 and ≤14 years compared with >14 years, there was a statistically significant difference only for FMGS (p=0.011). In females, a significant difference was found for ASM between the age ranges <10 with ≥10 and ≤14 years (p=0.000). For FMGS, there was a significant difference between the age groups <10 with ≥10 and ≤14 years (p=0.000) and between ≥10 and ≤14 with >14 years (p=0.013).
Difference of means by gender of BMI, muscle mass, and handgrip strength, according to the age group, in pediatric patients treated in referral outpatient clinics for liver disease in Salvador, Bahia, 2017.
| Age range (years) | n | BMI (kg/m2) | ASM (kg) | SMI (kg/m2) | FMGS (kgf) | rMGS (kgf/kgm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | pa | Mean (SD) | pa | Mean (SD) | pa | Mean (SD) | pa | Mean (SD) | pa | ||
| <10 | 0.995 | 0.015 | 0.069 | 0.617 | 0.795 | ||||||
| Male | 6 | 16.5 (1.02) | 8.7 (1.23) | 5.5 (0.95) | 6.8 (3.20) | 0.4 (0.18) | |||||
| Female | 19 | 16.5 (3.09) | 6.9 (1.52) | 4.6 (1.04) | 6.3 (2.13) | 0.4 (0.15) | |||||
| ≥10 and ≤14 | 0.574 | 0.636 | 0.564 | 0.749 | 0.803 | ||||||
| Male | 15 | 20.0 (3.62) | 12.7 (3.47) | 5.9 (1.11) | 15.5 (7.79) | 0.81 (0.45) | |||||
| Female | 29 | 20.7 (3.62) | 13.1 (2.69) | 5.8 (0.96) | 16.2 (5.60) | 0.78 (0.24) | |||||
| >14 | 0.899 | 0.22 | 0.302 | 0.311 | 0.27 | ||||||
| Male | 8 | 21.7 (4.92) | 17.6 (4.55) | 6.7 (1.45) | 27.7 (13.27) | 1.3 (0.57) | |||||
| Female | 8 | 22.0 (3.81) | 15.0 (3.39) | 6.1 (0.64) | 22.2 (6.56) | 1.0 (0.24) | |||||
Data expressed as mean (SD); BMI, body mass index; ASM, appendicular skeletal muscle mass; SMI, skeletal muscle index; FMGS, final manual grip strength; rMGS, relative manual grip strength index.
The frequency of sarcopenia was identified in 40% (34/85) of the patients. When assessing the variables that characterize sarcopenia individually, 54.1% (46/85) of the patients had muscle strength deficit (rMGS) and 50.6% (43/85), muscle mass deficit (SMI).
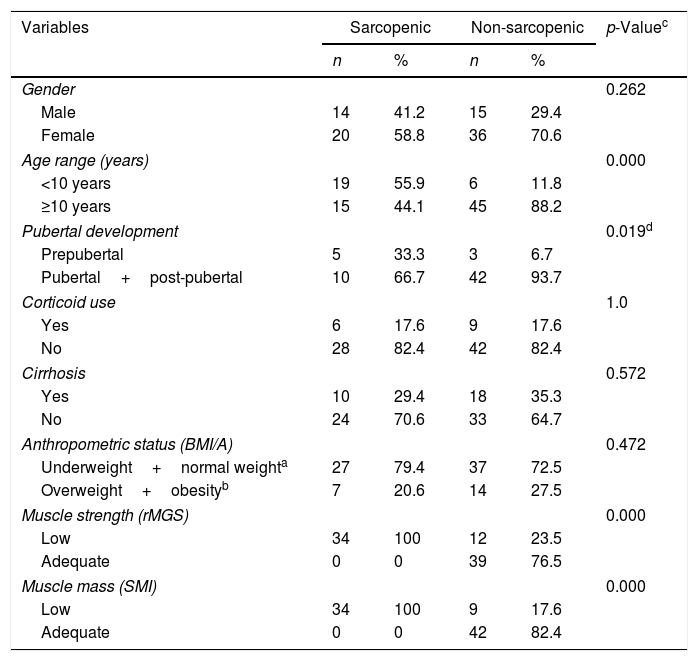
Table 2 shows the demographic, clinical, anthropometric, and body composition characteristics of patients according to the presence or absence of sarcopenia. It is noteworthy that in patients with sarcopenia receiving corticoids, none were overweight or obese (Data not shown in the table).
Demographic, clinical, anthropometric, and body composition characteristics according to the presence or absence of sarcopenia in pediatric patients treated at referral outpatient clinics for liver disease in Salvador, Bahia, 2017.
| Variables | Sarcopenic | Non-sarcopenic | p-Valuec | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 0.262 | ||||
| Male | 14 | 41.2 | 15 | 29.4 | |
| Female | 20 | 58.8 | 36 | 70.6 | |
| Age range (years) | 0.000 | ||||
| <10 years | 19 | 55.9 | 6 | 11.8 | |
| ≥10 years | 15 | 44.1 | 45 | 88.2 | |
| Pubertal development | 0.019d | ||||
| Prepubertal | 5 | 33.3 | 3 | 6.7 | |
| Pubertal+post-pubertal | 10 | 66.7 | 42 | 93.7 | |
| Corticoid use | 1.0 | ||||
| Yes | 6 | 17.6 | 9 | 17.6 | |
| No | 28 | 82.4 | 42 | 82.4 | |
| Cirrhosis | 0.572 | ||||
| Yes | 10 | 29.4 | 18 | 35.3 | |
| No | 24 | 70.6 | 33 | 64.7 | |
| Anthropometric status (BMI/A) | 0.472 | ||||
| Underweight+normal weighta | 27 | 79.4 | 37 | 72.5 | |
| Overweight+obesityb | 7 | 20.6 | 14 | 27.5 | |
| Muscle strength (rMGS) | 0.000 | ||||
| Low | 34 | 100 | 12 | 23.5 | |
| Adequate | 0 | 0 | 39 | 76.5 | |
| Muscle mass (SMI) | 0.000 | ||||
| Low | 34 | 100 | 9 | 17.6 | |
| Adequate | 0 | 0 | 42 | 82.4 | |
BMI/A, body mass index for age.
This article identified that sarcopenia is a condition identified in children and adolescents with CLD, treated at a referral public institution for this disease, in the city of Salvador, state of Bahia, Brazil. It is worth mentioning that CLD patients from the entire state of Bahia and neighboring states are treated in the clinical treatment and transplant outpatient clinics, and these patients are at an age range during which growth, development and, above all, adequate nutritional status and muscle mass are very important.
Few studies7,8 were identified that addressed the treatment of sarcopenia in children and adolescents with liver disease, and the respective investigators demonstrated that sarcopenia was one of the most common complications in this population, presumably with worse clinical and nutritional results.
The results of the present study showed that pediatric patients with CLD showed significant loss of muscle mass and strength. This condition, therefore, can result in the impairment of multiple physiological systems, as well as adversely affecting the quality of life of children and adolescents with CLD and impair post-transplant survival, as mass reserves and skeletal muscle strength are lost.7,10,14
A study carried out by Mangus et al.,7 in the city of Indianapolis, IN, quantified the muscle mass and body fat reserves of 81 pediatric patients with end-stage chronic diseases using computed tomography. Among the 35 patients with liver failure, 23.0% showed a reduction in muscle mass, a lower frequency than that found in the present study (50.6%). This difference can be justified because the cutoff points used by Mangus et al.7 are shown based on the SMI in cm2/m2, whereas in the present study, the SMI was shown in kg/m2; also, possibly because the diagnosis of sarcopenia involves other criteria, such as decreased muscle strength and/or physical performance rather than only muscle mass.6 Additionally, the prevalence of sarcopenia can vary widely between studies in the elderly, adult, and pediatric populations, depending on the gender of the patient, stage of the disease, criteria used to diagnose sarcopenia, and cut-off points used.2,4–6,11,14,24–26
In the sample assessed in the present study, most patients diagnosed with sarcopenia were females. This higher frequency can be justified by the fact that most patients have normal weight and are Child–Pugh A, and thus without significant impairment of hormone metabolism (testosterone),11 and possibly because males also have a greater amount of testosterone, the hormone responsible for muscle growth.27 Moreover, culturally, boys have more vigorous physical activity than girls.28 However, it is noteworthy that testosterone levels decline with liver dysfunction and may not be associated with sarcopenia in this population.11
Regarding the anthropometric status, it was observed that most patients with sarcopenia had normal weight and low weight was observed in few patients, according to BMI/A index. This index is not a good parameter to investigate the anthropometric status, since it evaluates the total body mass without considering the distribution of body compartments, which may not reflect the real anthropometric status of the pediatric patients with CLD.1,7,8
However, although the nutritional deficit impairs muscle mass and strength, the high frequency of sarcopenia identified in the present study can be justified by the gain of adipose tissue, a process that can happen as a compensatory physiological mechanism of loss of lean mass.7 It is worth mentioning that a condition called sarcopenic obesity, i.e. the simultaneous presence of excess weight and sarcopenia, has been the object of investigation, especially in individuals with chronic diseases.7
It was also observed that the increase in ASM in relation to the age group was more pronounced for males, especially in those age groups consistent with the rapid accumulation of muscle mass that occurs at puberty.27,28 These findings were also identified in studies with healthy children and/or adolescents.4,5,25
The increase in FMGS identified in the patients also disclosed significant differences between the genders and some age groups, especially in males, corroborating the results of studies carried out in Brazilian children17 and in the Czech Republic.14 This finding may have an effect, as it is associated with puberty onset, when the increase in the maximal manual grip strength between the genders can be different due to the androgenic action of testosterone28 and also by the difference in the increase of muscular strength that occurs in males during the growth spurt in relation to females.28
The values found for absolute and relative muscle mass and strength in the patients assessed in the present study were lower than those identified in the studies performed in healthy children and adolescents,5,14,25,29 mainly regarding relative muscular strength.14,17 These findings reinforce the contribution of the present study, as it provides additional informative data on the values of muscle mass and skeletal muscle strength of pediatric patients with CLD.
In the present study, it was decided to use the relative measures of muscle mass (SMI) and muscle strength (rMGS) for the diagnosis of sarcopenia, instead of the absolute measures (ASM and FMGS), aiming to adjust to the changes related to the maturation process and body size that occur in the assessed age range.14
Due to the lack of consensus regarding the evaluation of sarcopenia and the lack of criteria for the pediatric population, especially that with CLD, the present study used the recommendation of the European Working Group on Sarcopenia in Older People (EWGSOP),6 which considers the simultaneous presence of muscle mass and skeletal muscle strength deficit to diagnose sarcopenia. It is also noteworthy that there is also no standard cut-off point for evaluation of the variables necessary for the diagnosis of sarcopenia in pediatric patients with liver disease, and that the available studies in different populations used several different cutoff points. Therefore, the present study used the protocol proposed by Amparo et al.,24 who studied adult individuals with chronic disease and adopted values below the mean of the study population to consider muscle mass and skeletal muscle strength deficit.
Although sarcopenia is a condition that is mainly associated with the elderly population, study results have shown that this condition can be identified in the adult2 and pediatric populations,7,8 especially when occurring simultaneously with chronic diseases.7,8 The present study contributed data to the literature of the area, by identifying that sarcopenia is a frequent condition in pediatric patients with CLD undergoing outpatient follow-up.
In this sense, the evaluation of sarcopenia may represent a useful tool for the evaluation of the nutritional status of pediatric patients with liver disease. Thus, muscle mass and strength deficits may possibly contribute to the assessment of protein reserve used during catabolic periods, aiming to maintain the nutritional needs and body function of CLD patients, especially at the advanced stages of the disease.30
It is believed that muscle impairment can also be an important indicator of liver disease severity and, therefore, the development of future studies in this population with the purpose of evaluating the possibility of adding sarcopenia to the conventional prognostic criteria will be important, as a marker of nutritional status.
Some limitations must be taken into consideration. First, the type and size of the sample did not allow the results to be extrapolated to other populations. Therefore, it is recommended that further studies be carried out with larger, probabilistic samples. Another limitation is the absence of a cutoff point to classify muscle mass and muscle strength deficit in pediatric patients with CLD.
However, the contribution provided by the present study is noteworthy, emphasizing that the results are the first data on muscle mass and skeletal muscle strength derived from DXA and MD that illustrate the variation related to gender and age group for ASM, SMI, FMGS, and rMGS, as well as the first to establish the diagnosis of sarcopenia in pediatric patients with liver disease.
To conclude, sarcopenia was identified in the patients of the present study, and that its evaluation is important.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rezende IF, Conceição-Machado ME, Souza VS, Santos EM, Silva LR. Sarcopenia in children and adolescents with chronic liver disease. J Pediatr (Rio J). 2020;96:439–46.
Study conducted at Complexo Hospitalar Professor Edgar Santos (C-HUPES), Serviço de Gastroenterologia e Hepatologia Pediátricas; and Universidade Federal da Bahia (UFBA), Instituto de Ciências da Saúde, Programa de Pós-graduação em Processos Interativos dos Órgãos e Sistemas, Salvador, BA, Brazil.